ABSTRACT
Among the factors that may cause decreases in soybean yield there are the foliar diseases that despite control it can limit grain yield. The Asian soybean rust (Phakopsora pachyrhizi) is considered the most important disease of soybean in Brazil. The aim of this work was to simulate the progress of Asian rust in Brazilian soybean cultivars by removal of trifoliate leaves from the bottom to the top. The experimental design was a randomized complete block, in a 3x3x5 factorial scheme, with four replications. It was observed that there was significant interaction between cultivars and defoliation for all yield components and consequently in seed yield. Increase in the defoliation intensity towards the bottom to the top during the reproductive stages studied (R3, R5, R6) there is a linear decrease in grain productivity, reaching in the highest defoliation level, loss of 79.6, 77.7 and 38.6% in R3, R5 and R6 stages, respectively. The simulation of damage by soybean rust through defoliation cultivars showed severity in leaf area reduction and its consequent effect in grain seed yield.
Key words: Yield components, leaf area, reproductive stage, biotic stress.
In Brazil, soybean has the highest seed yield, and in the season 2012/2013, the average yield was 2938 kg ha-1 (CONAB, 2014). Among the factors that could cause losses to this crop, there are the foliar diseases that despite control management can limit yield. Asian rust (Phakopsora pachyrhizi) is considered the most significant disease of soybean in Brazil. Its biggest loss is caused by the premature abscission of leaves and their higher incidence and severity is mainly in the reproductive stages of the crop.
The soybean rust was first recorded in Brazil in 2000/2001 season and from there it spread throughout the country. In 2004, the losses caused by the disease (sum of seed losses, control expenses and reduced government revenues) were of US$ 2.28 billion (Yorinori and Lazzarotto, 2004). In more severe cases, without proper control, soybean rust can cause yield losses of about a 100% (Navarini et al., 2007; Oliveira, 2004; Barros et al., 2008; Yorinori, 2002). The temperature ideal for disease development is 15 to 28°C, with 6-12
hours of moisture on the leaf needed for spore germination (Dorrance et al., 2007). The disease starts from the bottom leaves where there is more moisture after the closure of plants in the area, providing favorable conditions. Without control, the disease progresses to the upper leaves and consequently accelerates abscission of leaves and reduces the effective leaf area of the plant.
Understanding the physiology of soybean production is important to understand the disease effect on yield. The yield is defined as a function of radiation absorbed by the crop canopy (leaves), the conversion of solar radiation absorbed by the plant in dry matter (that is, the efficiency use of the radiation) and the proportion of total plant dry matter accumulated during the growth period that is allocated to the seed (harvest index) (Hay and Porter, 2006). The main soybean yield components are the number of pods/plant, number of seed/plant (product of the number of pods x number of seeds/pod) and the seed weight. Understanding the influence of each component in yield may reveal answers on how to improve the yield in this legume.
The early defoliation in soybeans causes yield loss by interference in physiological processes such as photosynthesis, resulting in fewer pods, fewer seeds per plant, seeds viable per pod and lower seed weight (Ribeiro and Costa, 2000). Artificial defoliation made ??between R5 and R6 stages showed lower seed filling (Peluzío et al., 2002) and leaf removal in R4 stage caused yield loss of up to 93.4% (Barros et al., 2002; Peluzío et al., 2002). At R3 and R4 stages, the defoliation causes pods abortion at a time when the plant has peak photosynthetic activity for forming and filling it.
The quantification of defoliation can be used as a parameter for estimating damage, to evaluate treatments for disease control and tests of genotype to resistance to Asian rust as well (Hirano et al., 2010). Researches aimed at quantification and progress of Asian rust in soybean is important to establish better control strategies. The study of damage levels of soybean rust can be simulated through artificial defoliation of plants from the bottom to the top.
Most papers of defoliate simulation in soybean were conducted to simulate the attack by insects (Bahry et al., 2013; Bueno et al., 2010; Gregorutti et al., 2012; Timisina et al., 2007; Fontoura et al., 2006) being few studies that were performed to simulate defoliation diseases (Aqeel, 2011). Thus, the objective of this research was to simulate the progress of Asian soybean rust cultivars by removal of trifoliate leaves from the bottom to the top.
The trial was conducted in a greenhouse at the Universidade Federal de Viçosa, Minas Gerais State, Brazil. The soil had the following chemical characteristics: pH (H2O) = 5.06; P (Mehlich 1) = 2.2 mg dm-3; K = 32 mg dm-3; Ca = 1.28 cmolc dm-3; Mg = 0.38 cmolc dm-3; Al = 0.49 cmolc dm-3; H + Al = 5.80 cmolc dm-3; Organic matter = 2.94 g dm-3; CTC (pH 7.0) = 7.54 cmolc dm-3; Base saturation (V%) = 23.1. It also had a clay texture. Based on these results were applied calcitic lime (1 g kg-1 of soil) and to fertilizer was used 300 and 150 mg kg-1 of soil of P and K, respectively.
The experimental design was a randomized complete block. Treatments resulted from the combination of three factors (3x5x3), with four replications. Factors consisted of defoliation stage (R3, R5 and R6), defoliation levels (no defoliation, 2, 4, 6 and 8 trifoliate leaves from the bottom to the top) and cultivars (TMG 1176 RR, M 7211 RR and TMG 7188 RR). Each plot consisted of a pot of 2.5 L of soil with two plants. The stages for performing defoliation were considered according to classification of Fehr and Caviness (1977):
R3: had pod with 5 mm length in one of the last four upper nodes on the main stem with a fully developed leaf.
R5: had seed with 3 mm length in a pod located in one of the last four upper nodes on the main stem with a fully developed leaf.
R6: had pod containing green seed that fills the pod cavity located in one of the last four upper nodes on the main stem with a fully developed leaf.
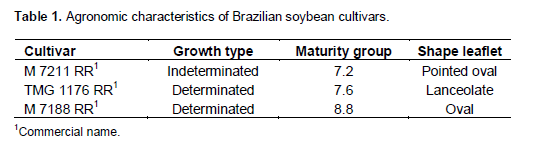
The defoliation levels studied were considered according to the disease in the field, because without proper control it progresses rapidly to the upper leaves. The goal of each level was to simulate that disease would be controlled at that particular point without progress to the upper leaves. The available cultivars have different characteristics in relation to maturity group; growth type and leaf shape (Table 1). This contrast, allows us to have more reliable results because the genetic variability within Brazilian soybean cultivars was considered.
The defoliation was artificially performed with scissors in their respective stages, removing the trifoliate leaves and keeping the petiole, as well as in disease occurrence in the field, where the leaflets first become detached from the petiole. In the case of the cultivar M 7211 RR, which had an indeterminate growth, with the removal of trefoil in R3, the plants did not have the largest number of trifoliate leaves of treatment (8 trifoliate), it was removed as soon as the next developed.
The sowing was carried out using five seeds/pot and at V1 stage. Thinning was done by keeping the two most vigorous plants. Number of pods/plant, number of seeds/plant, 100-seed weight and seed yield/plant were evaluated. The data were subjected to variance and regression analyses and mean comparisons in Genes software (Cruz, 2013).
In variance analysis (Table 2), it was observed that there was significant interaction between cultivars and defoliation levels for all yield components and consequently in the productivity. The interaction between defoliation stages time and defoliation levels only was not significant for 100-seed weight, which component had isolated influence of defoliation stage. Regarding the number of pods/plant (Table 3), it was observed that within all removal levels of trifoliate leaves, the TMG 7188 RR cultivar was outstanding. The M 7211 RR produced significant pod number smaller, regardless of the number of leaves removed. Through regression analysis, it was noted that all cultivars had a linear decrease in the pod number according to increase in the number of trifoliate leaves removed (Figure 1A).
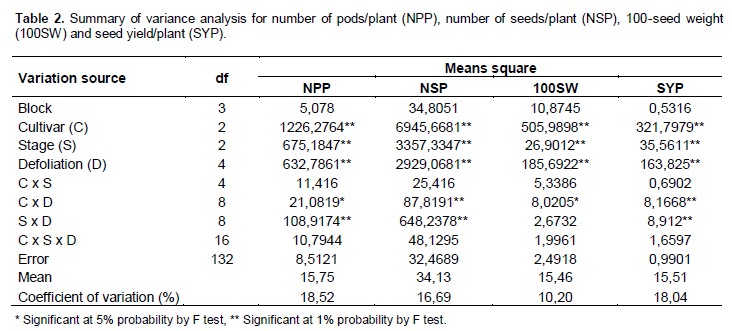
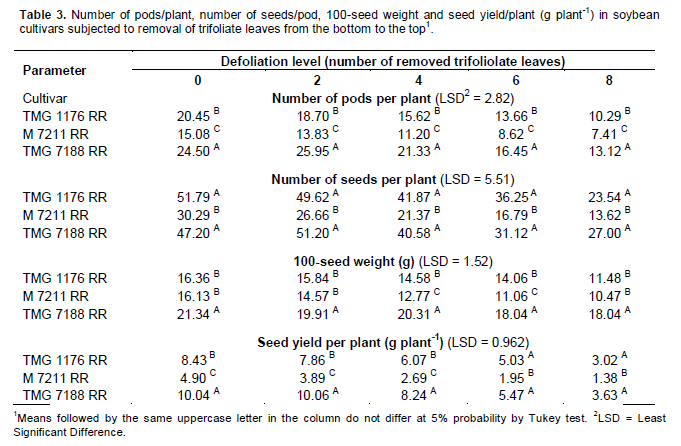
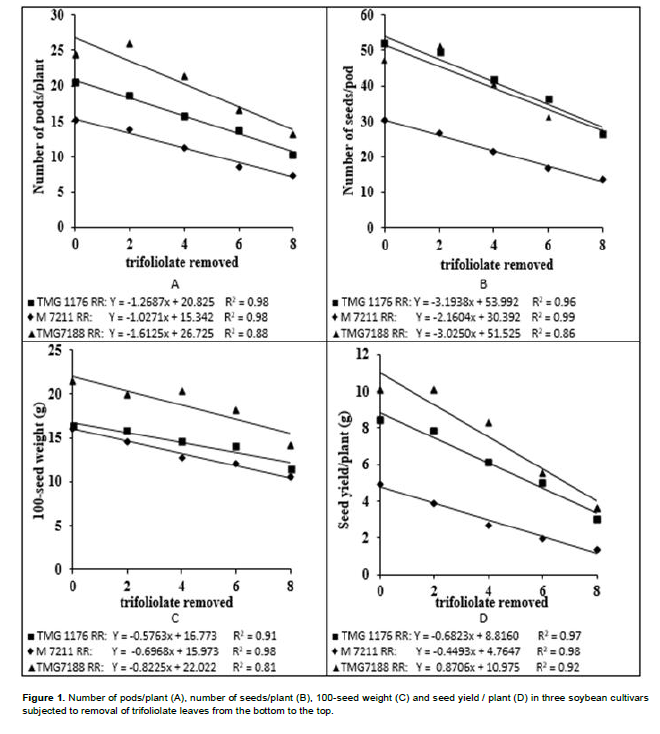
Regarding the number of seeds/plant (Table 3), the cultivar M 7211 RR produced significantly lower number than the others. This performance is due to the reduced pod number combined with low number of seeds per plant (approximately 1.80) produced by this cultivar. At all defoliation stages there was a linear decrease in the number of seeds/plant (Figure 1B). Differently the number of pods/plant, the number of seeds/plant produced by TMG 7188 RR cultivar was significantly higher. The number of seeds produced by TMG 7188 RR was very close to the TMG 1176 RR, because this last cultivar has the greater number of seeds/pod trait that is genetically linked to the lanceolate leaf character.
Comparing the 100-seed weight of cultivars within the levels of defoliation (Table 3) showed that the TMG 7188 RR cultivar had higher seed weight in comparison with the others, regardless of how many trifolioliate leaves was removed. In the M 7211 RR cultivar from the removal of four trifoliate had larger reductions in seed weight. The three cultivars had a significant linear decrease in seed weight with the increase in defoliation (Figure 1C). The decrease in this component is smaller than the others, in which case the decrease is mainly caused by leaf removal in R5, a critical moment the plant should not go through stress, because the number of seeds was already defined and it is starting filling.
The seed yield was significantly different within all defoliation levels performed (Table 3). Until removal of four trifoliolate, the productivity remained the order of TMG 7188 RR> TMG 1176 RR> M 7211 RR. With the removal of 6 and 8 trefoils the TMG 7188 RR and TMG 1176 RR not statistically different, suggesting that this cultivar, even being of lower maturity group (7.6) can maintain certain yield in higher defoliation, when compared to maturity group 8.8. For yield in relation of trifoliolate leaves removed (Figure 1D), as well as all yield components, there was a linear decrease for all cultivars and despite the difference in the yield, the trend was similar for all.
To the number of pods on each level at different defoliation stages (Table 4), it was observed that after the removal of the two first trifoliate leaves, this yield component began to be affected in R3 and R5 stages, when compared to R6 stage, wherein the defoliation no more affects this component. This result can be seen best in Figure 2, which was found a linear decrease for the defoliation at R3 and R5 stages, reaching more than 50% of reduction in the number of pods with the removal of 8 trefoils.
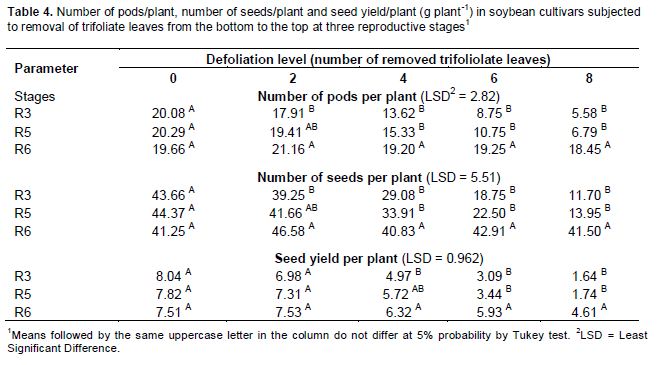
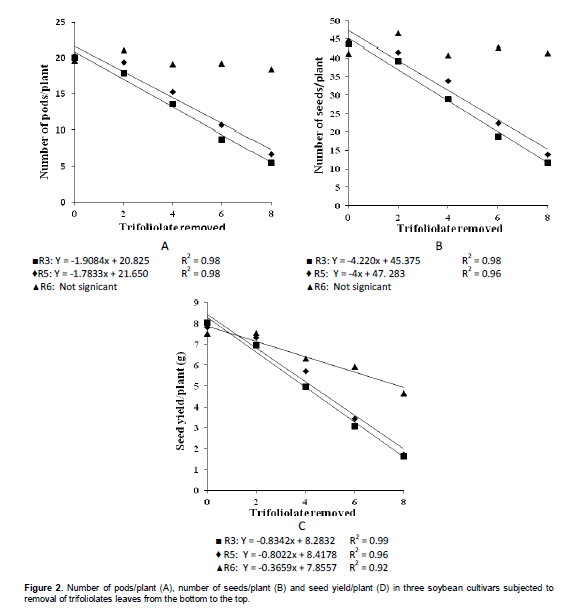
The influence of trifolioliate leaves removal at different stages in the number of seeds/plant can be seen in Table 4, where it was found the same behavior of the number of pods/plant. From the removal of two trefoils in R3 and R5 stages there was significant decrease in the number of seeds/plant, compared with defoliation in the R6 stage, which there is no more change to this trait. This is due to the abortion of pods resulting in lower total number of seeds/plant. In Figure 2 B it can be observed a linear decrease in the number of seeds/plant at R3 and R5 stages.
In the comparison of stages within each quantity of removed trefoil (Table 4) it was noted that there was a decrease from the loss of four trefoils, wherein in R3 stage, to decrease in yield was significantly greater than R6. However, with the loss of 6 and 8 trefoils, in both R3 and R5 stages had significant decrease in yield. In Figure 2C, it was observed that for defoliation in R3 and R5 stages there was a marked linear decrease in yield, reaching about 80% decrease with the removal of 8 trifoliate leaves. This is due to decrease in the number of pods, seeds and seed weight. Already a linear decrease in R6 stage is only resulting in the decrease of the seed weight. In this sense, should seek to control until R6 stage, because for many producers, at that stage the severity of soybean rust would not result in more lost in yield. However, depending on the crop situation, the control should be still performed. Gasparetto et al. (2011) observed that the fungicides application promoted gains in seed yield (kg ha-1), which ranged from 30 to 59% compared to control, generating revenue in excess of 77 to 210%.
Comparing the control regarding the removal of 8 trifoliate leaves in R6 stage, the decrease was about 38%, an unacceptable loss to a crop that is already ending the cycle and has received all necessary management. To the 100-seed weight, there was effect only ofn the defoliation stage (Table 5). In the R3 stage the 100-seed weight was significantly higher than in the R6 stage.
The soybean rust, in severe cases, when the disease reaches the soybean plant in pod formation stage or early seed filling can cause abortion and the drop pods, resulting in up to total yield loss (EMBRAPA, 2011). According to Egli (2010), the soybean plant has two mechanisms to adjust the number of pods and seeds with the same availability of assimilates:
i) The flower production varies with changes in environmental conditions and among cultivars; and ii) not all flowers produce pods and not all pods reach up to maturation. However, the abortion of pods is enhanced when plants undergo major environmental changes (like a stress period caused by defoliation) on flowering and seed filling.
The similarity between defoliation in the two different stages (R3 and R5) is because in the R3 stage the plant starts pod formation, and the stress by defoliation increases the abortion of these pods, however, the plant still has a specified period for forming and filling of some pods. In R5 stage, the pods are all already formed; however, because they are still at the beginning of photosynthate translocation to the seeds, increasing the defoliation also causes abortion, since the sources are removable. Thus, the plant just keeps the amount of pods that it will be able to fill the seeds with assimilates from the leaves (sources) that remain, unlike the defoliation caused by more advanced stage, where the seeds are reportedly in the process of filling and this negative effect would be in the weight of these seeds.
The soybean, as well as other species, has a strategy to leave progeny, in this case the stress caused by defoliation at R3, causes rearrangement in yield components, in order to maintain quality seed. Thus, the plant aborts most of their pods and retains only those which have the ability to translocate photoassimilates from the remaining leaves, which results in keeping the seed size. Especially in this case, where two of the three cultivars are determinate growth type, wherein R3 stage the total of leaves have been issued. In R5 stage it happens pretty much the same; however, as the pods are already in early seed filling, the abortion of pods can be fewer. In the R6 stage seeds are all formed, filling the entire pod cavity, however, it had not yet fully completed receipt of photoassimilates from the leaves, which results in the largest decrease in the seed weight. This proves by the recommendation of desiccation in soybean that is in R7, precisely for no loss in seed weight and consequently in yield (EMBRAPA, 2010).
It is known that the pods reaching its maximum length and seeds that underwent by cell division rarely abort. However, after this phase, environmental changes may result in improper pod filling, requiring a change in the size of the seed corresponding to the availability of assimilates (Egli, 2010), that is, the seeds cannot grow without assimilates availability and stress caused by loss of leaves in R5 and R6 stages interfering directly in seed filling.
There are two sources of assimilates for seed filling: current photosynthesis and remobilization of carbohydrate reserves. The contribution of carbohydrate reserves is apparently very low (<15% of the total seed weight) so, the maintenance of the leaves is essential to maintain the photosynthetic rate (current photosynthesis) during this phase (Egly, 2010).
The leaves removal interferes directly in leaf area index (LAI) of the plant. The soybean plant normally reaches a value higher than the critical LAI (95% absorption of sunlight). Thus, the plant may lose part of its leaves without seriously affecting its performance (Weber and Cadwell, 1966). However, it should be considered that a substantial loss always smacks a negative effect, especially if it occurs at a stage of advanced development, that is, during the reproductive phase, when it is no longer possible to replace the leaf area lost by the new vegetative growth. In this case, varieties with a tendency to have more branches, offer certain advantage (Miyasaka and Medina, 1981).
According to Carretero (2011), much of the variation in the cultivars cycle is associated with differences in the vegetative phase, which is not directly connected to yield. Increase in the leaf area does not increase canopy photosynthesis or the growth rate of the crop, after the radiation interception reaches a maximum, there is no benefit in production with higher rates of leaf area.
One explanation for a very marked loss is linked to the growth type of cultivar. It is known that the R6 stage is defined when at least one pod in the last four nodes is completely filled, but still green. However, it is also known that in the cultivars of determinate growth type, the maturation begin from the top to bottom, that is, when pods in the last four are already at R6, most pods in the middle and bottom part of the plant are still in a later filling stage, which causes further loss. In contrast, in cultivars with indeterminate growth type, which maturation occurs from the bottom up, in this case, when there are pods at R6 stage in the last 4 nodes, it is certain that the pods below are already in an advanced stage, that is, there will be no loss in the yield.
In this study, two of the three cultivars are determinate growth type, which may have influenced this linear decrease in R6. In practice, in crop visit, usually technicians and producers usually observe the pods in the top of plant, for decision making if it would or not, do one more application to disease control, which may be affecting the final seed weight and consequently seed yield.
After the pathogens infect the bottom leaves, there is acceleration of falling leaves. Oliveira and Antuniassi (2011) under curative control conditions, with evaluation one day before the application, noted that the average severity in the lower third was of 35.9% (between 28.9 and 42.8%) while the upper third was 4.57% (varying between 2 and 7%), whereas the 90% CI. Andrade and Andrade (2002) obtained results which showed that in the chemical control of soybean rust, just a delay of seven days in the fungicide application (after detection of the disease), was sufficient for the increase in defoliation at 82%. With a delay of 14 days, defoliation increased by 155%.
As defoliation intensity rises from the bottom to the top during the reproductive stages (R3, R5, R6) studied, there is a linear decrease in plant yield, reaching on the highest defoliation level, loss of 79.6, 77.7 and 38.6% in R3, R5 and R6, respectively. The damage simulation by soybean rust through defoliation showed the severity in the reduction in the leaf area and its consequent reflection in the yield.
The authors have not declared any conflict of interest.
REFERENCES
|
Andrade PJM, Andrade DFAA (2002). Ferrugem asiática: uma ameaça à sojicultura brasileira. EMBRAPA Agropecuária Oeste, P. 11. |
|
|
|
Aqeel AM (2011). Using manual defoliation to simulate soybean rust: effect on growth and yield formation. University of Kentucky, Doctoral Dissertation 223:128. |
|
|
|
Bahry CA, Venske E, Nardino M, Zimmer PD, Souza VQS, Caron BO (2013). Desempenho agronômico da soja em função da desfolha em diferentes estádios vegetativos. Tec. Cienc. Agropec. 7(4):19-24. |
|
|
Barros HB, Sediyama T, Reis MS, Cecon P (2008). Efeito do número de aplicações de fungicidas no controle da ferrugem asiática da soja. Acta Sci. Agron. 30(2):239-245.
Crossref |
|
|
|
Barros HB, Santos MM, Pelúzio JM, Rocha RNC, Silva RR, Vendrusco JB (2002). Desfolha na produção de soja (Glycine max 'M-SOY 109'), cultivada no cerrado, Gurupi-TO, Brasil. Biosci. J. 18:5-10.Bueno, AF, Batistela MJ, Moscardi F (2010). Níveis de desfolha tolerados na cultura da soja sem a ocorrência de prejuízos à produtividade. Embrapa soja. Circ. Técnica. 79:1-11. |
|
|
|
Carretero D (2011). Fisiologia da produção de soja: Princípios e processos na construção da produtividade. In: Siqueri F, Caju J, Moreira M (Eds). Boletim de pesquisa de soja. Fundação MT, Rondonópolis, Brazil. |
|
|
Cruz CD (2013). GENES - A software package for analysis in experimental statistics and quantitative genetics. Acta Sci. Agron. 35:271-276.
Crossref |
|
|
|
Dorrance AE, Draper MA, Hershman DEH (2007). Using foliar fungicides to manage soybean rust. NC-504 Land Grant Universities Cooperating. Bulletin SR-2005. Hay RKM, Porter JR (2006). The Physiology of Crop Yield, 2nd ed. Blackwell Publishing Ltd, Oxford, UK. |
|
|
Egly DB (2010) Soybean yield physiology: principles and processes of yield production. In: G. SINGH (Ed.). The Soybean: botany, productions and uses. 1st ed. India, CABI. pp. 113-141.
Crossref |
|
|
|
EMBRAPA: Empresa Brasileira de Pesquisa Agropecuária (2010). Tecnologias de produção de soja região central do Brasil 2012 e 2013. Embrapa Soja: Embrapa Cerrados: Embrapa Agropecuária Oeste. P. 261. |
|
|
|
Fehr WR, Caviness CE (1977). Stage of soybean development. Ames: Iowa State University of Science and Technology, (Special report 80). |
|
|
|
Fontoura TB, Costa JA, Daros E (2006). Efeito de níveis e épocas e desfolhamento sobre o rendimento e os componentes do rendimento de grãos da soja. Sci. Agrar. 7:49-54. |
|
|
|
Gasparetto R, Fernandes CD, Marchi CE, Borges MF (2011). Eficiência e viabilidade econômica da aplicação de fungicidas no controle da ferrugem asiática da soja em Campo Grande, MS. Arq. Inst. Biol. 7:251-260. |
|
|
|
Gregorutti VC, Caviglia OP, Saluso A (2012). Defoliation affects soybean yield depending on time and level of light interception reduction. Aust. J. Crop Sci. 6:1166-1171. |
|
|
Hirano M, Hikishima M, Silva AJ, Xavier SA, Canteri MG (2010). Validação de escala diagramática para estimativa de desfolha provocada pela ferrugem asiática em soja. Summa Phytopathologica, 36:248-250.
Crossref |
|
|
|
Miyasaka S, Medina JC (1981). A soja no Brasil. ITAL, Campinas, Brazil. |
|
|
Navarini L, Dallagnol LJ, Balardin RS, Moreira MT, Meneghetti C, Madalosso MG (2007). Controle Químico da Ferrugem Asiática (Phakopsora pachyrhiziSidow) na cultura da soja. Summa Phytopathol. 33:182-186.
Crossref |
|
|
Oliveira MAP, Antuniassi UR (2011). Eficácia do flutriafol e do flutriafol + tiofanato metílico aplicados com gotas finas ou médias no controle da ferrugem asiática da soja. Revista Energ. na Agric. 26(1):94-112.
Crossref |
|
|
|
Oliveira SHF (2004). Época de aplicação de fungicidas no controle da ferrugem asiática (Phakopsora pachyrhizi) da soja. Fitopatol. Bras. 29:295. |
|
|
|
Peluzio JM, Barros HB, Rocha RNC, Silva RR, Nascimento IR (2002). Influência do desfolhamento artificial no rendimento de grãos e componentes de produção da soja [Glycine max (L.) Merrill]. Ciênc. Agrotec. 26:1197-1203. |
|
|
Ribeiro ALP, Costa EC (2000). Desfolhamento em estádios de desenvolvimento da soja, cultivar BR 16, no rendimento de grãos. Cienc. Rural. 30:767-771.
Crossref |
|
|
Timisina J, Boote KJ, Duffield S (2007). Evaluating the CROPGRO soybean model for predicting impacts of insect defoliation and depodding. Agron. J. 99:148-157.
Crossref |
|
|
Weber CR, Cadwell BE (1966) Effects of defoliation and stem bruising on soybeans. Crop Sci. 6:25-27.
Crossref |
|
|
|
Yorinori JT, Lazzarotto JJ (2004). Situação da ferrugem asiática na América do Sul. Embrapa Soja, Documents 236. Londrina, Brazil. |
|
|
|
Yorinori JT, Paiva WM (2002). Ferrugem da Soja: Phakopsora pachyrhizi Sidow. Londrina, Embrapa Soja, Folder. |