ABSTRACT
A cross-sectional study was carried out from November 2014 to March 2015 to determine the prevalence of Dictyocaulus arnfieldi and to identify associated risk factors in equines in Sudie district, south eastern Ethiopia. A total of 384 faecal samples were collected randomly from horses (n = 128), donkeys (n = 217) and mules (n = 39) for coprological examination. Isolation of D. arnfieldi was performed using a modified Baermann technique. The overall prevalence of D. arnfieldi was 164 (42.7%) with infection rates of 22.7, 57.6 and 22.7% in horses, donkeys and mules, respectively, with statistically significant (P<0.05) variation. High prevalence of lungworm infection was recorded in the age group of ≤4 years (50.9%) followed by the age group of 4-10 years (42.3%) and ≥10 years (40.46%), however, statistically non significant. Observed prevalence of lungworms in female equines was 37.1% and in males was 47.0% with no statistically significant difference (P>0.05). In this study, animals with poor body conditions were found to be highly infested (50.9%) compared to medium (41.6%) and good body conditions (21.3%) with statistically significant difference (P<0.05). The prevalence in non-dewormed equines was 53.2% and dewormed equines were 26.2% with significant difference (P<0.05). From this study, it can be concluded that body condition can be considered as one of the important factors which influence the occurrence of lung worm parasite in equines. It is recommended that owners should be trained to improve the management system, especially in terms of the level of nutrition so that the animal can have good body condition that confers some level of resistance against lung worm infection. In addition, strategic deworming should be implemented using broad spectrum anthelmintic drugs in the study area.
Key words: Equine, lung worm infection, prevalence, risk factors, Sudie.
Ethiopia is one of the developing countries in Africa, which is predominantly an agricultural country with over 85% of its population engaged in agricultural activity (FAO, 1999; EARO, 1999). The country has the highest equine population probably with the highest density per square kilometer in the world (Alemayehu, 2004) and it has a total of 6.9 and 42.4% in the world and Africa equine population, respectively (Wilson, 1991).
Ethiopia has 21.7 million horses, 5.57 million donkeys (second largest in the world next to China) and 380 thousand mules (CSA, 2009). Equines are one of the most important and mostly intimately associated with man. They have enormous contribution through their involvement in different social and economic sectors. Equines play an important role as working animals in many parts of the world, for packing, riding, carting and ploughing. Equine power is very crucial in both rural and urban transport system. This is because of its cheapness and availability and so provides the best alternative transport means in places where the road network is insufficiently developed and the landscape is rugged and mountainous and in the cities where narrow streets prevent easy delivery of merchandise (Feseha et al., 1991).
In Ethiopia equines have been as animals of burden for long period of time and still render valuable services mostly as pack animals throughout the country particularly in areas where modern means of transportation are absent, unaffordable or inaccessible (Abayneh et al., 2002).
In some areas of North West Kenya and Southern Ethiopia, donkey meat is a delicacy and the milk believed to treat whooping cough (Fred and Pascal, 2006).
Even though mules and donkeys have often been described as sturdy animals, they succumb to a variety of diseases and a number of other unhealthy circumstances. Among these, parasitic infection is a major cause of illness (Sapakota, 2009). Lungworms are widely distributed throughout the world providing nearly perfect conditions for their survival and development but are particularly common in countries with temperate climates, and in the highlands of tropical and subtropical countries. Dictyocaulidae are known to exist in East Africa and South Africa (Hansen and Perry, 1996).
Dictyocaulus arnfieldi is the true lungworm affecting donkeys, horses, ponies and zebras and is found throughout the world (Smith, 2009). Donkeys and their crosses (Mules) are the natural hosts for lungworm and the condition in horses is usually found in those that have been in the company of donkeys and mules (Rose and Hodgson, 2000).
The pathogenic effects of lungworm depend on their location within the respiratory tract, the number of infective larvae ingested, the animal immune status, the nutritional status and age of the host (Fraser, 2000; Blood and Radostits, 1989). Larvae migrating through the alveoli and bronchioles produce an inflammatory response, which may block small bronchi and bronchioles with inflammatory exudates. The bronchi contain fluid and immature, latter adult worms and the exudates they produce also block the bronchi. Secondary bacterial pneumonia and concurrent viral infections are of the complication of Dictyocaulosis (Howard, 1993).
Despite the prevalence of patent D. arnifieldi infection in donkeys, overt clinical signs are rarely seen; however, on close examination, slight hyperpnoea and harsh lung sounds may be detected. Donkeys usually show no disease signs and can be silent carriers and shedders of this parasite, which causes clinical signs in horses (Johnson et al., 2003).
Despite the huge numbers of equine population and the increasing importance of equines in the Ethiopian economy, very little research relating to equine lungworm has been carried out in Ethiopia. And therefore, the aims of this study were to estimate the prevalence of lung worm infection in equines and to investigate the association between intensity of lung worm infection and risk factors area in Sudie district Oromia region, southeastern Ethiopia.
Description of the study area
This study was conducted in Oromia Regional State, Arsi zone, Sudie district which is located at a distance of 216 km south east of Addis Ababa and 93 km east of Asela (the capital of the zone) at altitude of 1500 to 2750 m.a.s.l. The area covers 112400 hectare in range lands. The area is comprised of twenty seven peasant associations and is bordered by different districts such as, Cholle by north, Robe by south, Diksis by west, and Seru and Cholle by east. Topographically, it has 7.41% highland, 70.37% midland and 22.27% lowland. It receives bimodal rainfall occurring from March to April (a short rainy season) and from July to October (long rainy season). It receives an annual range of rain fall from 880 to 1100 mm, and the annual range of temperature varies from 15 to 25°C. The equine population of the area is found to be 26560 (CSA, 2009).
Study animals
The study animals were horses, donkeys and mules in Sudie district, Arsi zone. Faecal samples were directly collected from the rectum of 384 equids of all age and sex groups. They were all local breeds, kept under extensive management system used for packing and transportation. The age of selected equines was determined using deciduous and the incisor teeth eruption times, wear, tear by Crane (1997) and Svendsen (1997) and by asking owners. Equines were grouped into three age categories namely equines under two years were classified as young (n=55), those in range of two to ten years were classified as adult (n=156) and those beyond ten years were classified as old (n=173). Body condition score was assessed subjectively using a scale from 1 (emaciated), 2 (thin), 3 (average), 4 (fat), to 5 (very fat) (Svendsen, 1997). The body condition of animals was classified as poor (emaciated and thin), medium (average) and good (fat and very fat) body condition scores. Equines those dewormed within 3 months interval and not dewormed within 3 months interval were selected for this study.
Study design and sampling procedure
A cross-sectional study design has been employed and the study animals were selected using simple random sampling method and the origin, age, sex, body condition scores and species of the animals were taken into consideration.
Sample size
The sample size required for the study was determined using the following formula given by Thrustfield (2005):
n= (1.96)2pexp (1-pexp)/d2 = (1.96)2 0.5(1-0.5)/(0.05)2
n =384
To calculate the sample size 95% confidence level, 50% expected prevalence and 5% of desired absolute precision (d=0.05) was used. Where n = required sample size, pexp = expected prevalence, d2= desired absolute precision at 95% confidence level. According to the formula, 384 equines were sampled.
Faecal sample collection and examination
Faecal samples were collected per-rectum or but some samples (especially from temperamental animals) were collected from fresh deposits using plastic gloves in clean plastic bags, labelled and kept in icebox and transported to Asela Regional Veterinary Laboratory and each sample was processed by Baerman and modified Baerman techniques as described by Charles and Robinson (2006). While collecting faecal sample, the species of the animals, sex, age, overt clinical signs of lungworm infection, and date of sampling were properly recorded. The larvae were then identified under lower power microscope (10X objective), based on the shape and number of gut cells, relative size and shape of larvae’s tail.
Modified Baerman procedure used in the laboratory
(1) The faecal samples were prepared with the necessary materials
(2) Place a double layer of cheese cloth or gauze on a disposable paper towel or equivalent on the bench. Using a spoon or spatula, weigh or measure approximately 5-10 g of faecal material. Place the faecal material in the center of cheesecloth. Form a pouch containing the faecal material by holding the four corners of the cheesecloth together and moulding around the faecal material.
(3) Using a rubber band or length of string close to the cheesecloth pouch. Push the sticks or short metal rod under the rubber band or string so that the pouch can be suspended.
(4) Place the pouch containing the faecal material in the plastic cone. Trim off the excess cheesecloth using scissor. Fill the plastic cone with lukewarm water. Make sure the faecal material is covered. Leave the apparatus to stand for 24 h.
(5) The supernatant was discarded and sediment was taken.
(6) Use a Pasteur pipette to transfer a small droplet of the sediment fluid from the petridish to a microscope slide. Add drop of iodine to fix the larvae and gently place a cover slip over the drop.
(7) Let examine under compound microscope at 10x magnification.
(8) Using the Pasteur pipette, remove a drop of sediment at the bottom of the tube and place it on microscope slide for examination. Be careful not to resuspend the sediment before you take a sample from it.
Data management and analysis
All raw data generated were entered into Microsoft excel spread sheet and statistical analyses were conducted using SPSS statistical software version 20.0 and multivariate logistic regression model. In all cases, 95% confidence intervals and P<0.05 were set for significance.
Over all field prevalence under coproscopic examination
Of the total 384 animals examined, the overall prevalence of D. arnfieldi was 164 (42.7%) in the study area.
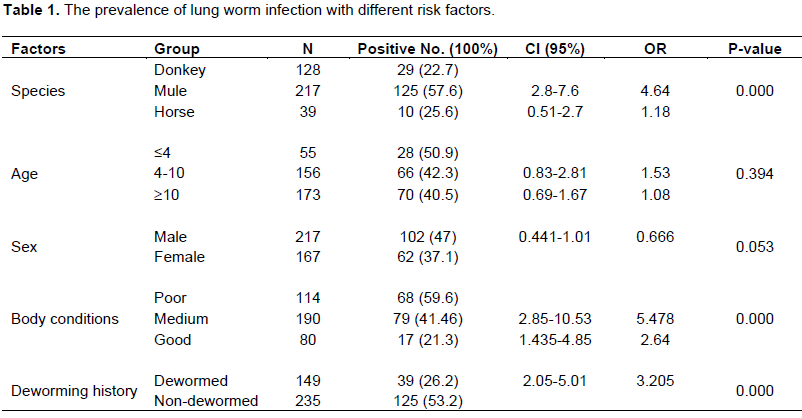
The prevalence of lung worm infection with different risk factors
Of the total horses (128), donkeys (217) and mules (39) examined, 22.66, 57.6, and 25.64 of horses (equus cabalus), donkeys (equus assinus) and mules were positive for D. arnfieldi, respectively and statistically with significant difference (P<0.05).
Age wise prevalence of the parasites was observed and its rate was 50.9, 42.3 and 40.5% in young, adult and old equines, respectively. And the prevalence was found to be statistically not significant (P>0.05). Out of 217 male equines, 102 (47.0%) of them were positive for D. arnfieldi and from 167 female equines, 37.1% were affected by D. arnfieldi. The sex of animals have no significant difference (P>0.05) on the prevalence of D. arnfieldi. Body condition scores of equines in Sudie district showed that the prevalence of D. arnfieldi were 59.6, 41.46 and 21.3% in poor, medium and good body condition scores, respectively with statistically significant difference (P<0.05).
Deworming history of animals with some types of anthelementics had a significant variation among the groups. Out of 235 equines specie without any deworming history 125 (53.2%) of them were found to be positive for D. arnfieldi and from 149 dewormed equines 39 (26.2%) were positive for D. arnfieldi statistically with significant (P<0.05) difference between the two groups (Table 1).
The overall prevalence of lungworm infection in this study was 42.7% that is higher than the previous findings of Yitna et al. (2015), who reported a prevalence of 37.5% in Lode Hetosa district, south eastern Ethiopia. This difference could be due to the differences in environmental conditions and management practice favouring the survival of the larvae of the parasite.
In the current study, the infection rate was compared among equine species and the highest prevalence was recorded in donkeys (57.6%). This result is in line with the reports of Yitna et al. (2015), Pandy (1980) and Lyons et al. (1985), who described prevalence of 57.81, 48 and 54% Lode Hetosa district, south eastern Ethiopia, in Morocco and Kentucky, USA, respectively. On the contrary, Feseha et al. (1991) and Hassan et al. (2004) had reported higher (83 and 70.5%) prevalences of D. arnfieldi in donkeys in Ethiopia and Sudan, respectively. And extremely higher prevalence (87.5 and 93%) of larvae of D. arnfieldi in Denmark and central Kentucky, USA were found in donkeys by Andersen and Fogh (2010) and Lyons et al. (1985), respectively. One study also indicated that, in an examination of fecal samples from part of a donkey herd, 85 of 90 donkeys were positive for lungworm larvae (Junquera, 2014).
However, extremely lower prevalence of 3.6 and 9.67% of D. arnfieldi were reported in donkeys by Nuraddis et al. (2011) and Inasi and Mustafa (2009) in Hawassa town, Ethiopia and in the Central Black Sea region, Turkey, respectively. In several studies, 50 to 80% of donkeys have been found infected with D. arnfieldi (Clayton and Duncan, 1981; klei, 1986).
The present prevalence of lungworm infection in mules (25.64%) is in agreement with previous finding of Tihitna et al. (2012) who reported 29.26% in and around Jimma town and Klei (1986), who reported prevalence of 29.3% in North America, respectively. The current prevalence is higher than finding of Ram (2009), who reported 14.10% in Nepal. On the contrary, higher prevalence of lungworm infection in mules (54%) was reported by Lyons et al. (1985) in central Kentucky, USA and 45.31% prevalence reported by Yitna et al. (2015) in Lode Hetosa district, south eastern Ethiopia. These differences may be due to agro-ecology of the study areas, management, season and sample size.
In this study area, the prevalence of D. arnfieldi in horses (22.66%) was higher than the previous findings of Saeed et al. (2010), Tihitna et al. (2012), Yacob and Ashenafi (2013), Tilahun et al. (2014) and Yitna et al. (2015) who reported 2.5, 4.26, 0.5 and 3.7% in Lahore (Pakistan), Jimma town, Arsi-Bale highlands of Oromia region, Hawassa town, Ethiopia and in Lode Hetosa district, south eastern Ethiopia, respectively. Before the advent of anthelmintic, Ivermectin, the prevalence of D. arnfieldi infection in horses in Kentucky at necropsy was approximately 11%, while it was 2% in live horses at the same time and at the same region, based on fecal examination (Lyons et al., 1985a, 1985b). This difference may suggest that the difficulty in antemortem diagnosis of D. arnfieldi in horses. However, patent infections have been found in horses, resulting in disease occurrence in closed herds with no exposure to donkey or mules (Lyons et al., 1985b; Claytons and Duncan, 1981).
In the present study, prevalences of 50.9, 42.3 and 40.5% were recorded in young, adult and old age groups, respectively and the prevalence was found to be statistically non-significant (P>0.05). This result was not in agreement with Tihitna et al. (2012) and Yitna et al. (2015).
Body condition scores were found to be a major risk factor (P<0.05) in the prevalence of equine lung worm infection was in agreement with finding of Yitna et al. (2015). The prevalence according to body condition grade was 59.6, 41.46, and 21.3% in poor, medium, good body condition scores of the equines, respectively. In addition, in different species of equids, (donkey, horse and mules) body condition scores were considered as risk factor and was statistically significant (P<0.05). This might be due to the fact that poorly nourished animal appears to be less competent in getting rid of infection, although, it is unusual for well fed animals to succumb to the disease provided the right environmental conditions are available (Kimberling, 1988).
Sex was not found as a major risk factors (P>0.05) in the prevalence of equine lungworm infection. A prevalence of 47.0 and 37.1% were recorded in male and female equines, respectively that match with the insignificant prevalence reported by Tihitna et al. (2012) and Yitna et al. (2015). However, the prevalence in male equine was higher as compared to female.
Deworming history of animals with anthelmintic usage was found as a major risk factors for statistical variation (p<0.05) in the prevalence of the parasite. Higher prevalence (53.2%) of the D. arnfieldi was recorded in equines with non-dewormed history than dewormed (26.2%). The reason why dewormed equines infected with D. arnfieldi might be either due to the anthelmintic used in the area for the treatment only temporarily suppress egg production of the adult worms or parasite may become resistance to anthelmintic used. It may also be related to the poor quality of anthelmintic used in the country. In contrast, 46.8% of none dewormed animals were not infected by lungworm; this might be due to development of acquired immunity from previous exposure (Blood and Radostits, 1999; Urquhart et al., 1996) and it may also be due to no exposure to the infective stages of D. arnfieldi. Similarly, at the time of examination, the adult parasite might not shed eggs because either it is in the prepatent period which lasts about 4 weeks or larvae in the lungs may become arrested (dormant, hypobiotic, inhibited) for up to 5 months when there is unfavorable condition.
The result of current study clearly indicated that equine lungworm is highly prevalent in donkeys. It also demonstrated the abundance and distribution of lungworm parasitism in different age groups and body condition scores of equines. The findings of current study suggest that emphasis be given to lungworm parasitism in the study area and in the country as whole.
The authors have not declared any conflict of interest
REFERENCES
|
Abayneh T, Gebreab F, Zekarias B, Tadesse G (2002). The potential role of Donkeys inland tillage in central Ethiopia. Bull. Anim. Health Prod. Afr. 50:172-178.
|
|
|
|
Alemayehu L (2004). Case study on reproductive activity of equines in relation to environmental factors in central Ethiopia, Berlin: humbold university of Berlin, PhD thesis pp. 8-13.
|
|
|
|
|
Andersen S, Fogh J (2010). Prevalence of lungworm (D. arnfieldi)in donkeys in Denmark and in horses in herds together with donkeys. Nord Vet. Med. 33:484-491.
|
|
|
|
|
Blood DC, Radostits OM (1989). Veterinary Medicine a text book of the disease of cattle, sheep, pigs, goats and horses 6th Ed., Balilliere Tindal pp. 1039-1044.
|
|
|
|
|
Blood DC, Radostits OM (1999). Veterinary Medicine, A text book of the disease of Cattle, Sheep, Pigs, Goats and Horses, 7th Ed., Bailliere Tindal, London, UK. pp. 1064-1066.
|
|
|
|
|
Central Stastical Authority (CSA) (2009). Federal Democratic Republic of Ethiopia, Central Stastical Authority (CSA), Agricultural Sample Survey 2008/2009). A Report on Livestock and Livestock characteristics (Private peasant holdings), Addis Ababa P. 183.
|
|
|
|
|
Charles MH, Robinson E (2006). Diagnostic veterinary parasitology for veterinary technicians, 3rd Ed., Mosby Inc. St. Louis, Missouri P. 243.
|
|
|
|
|
Clayton HM, Duncan JL (1981). Natural infection with Dictyocaulus arnfieldi in pony and donkey foals. Res. Vet. Sci. 31:278-280.
|
|
|
|
|
Crane M (1997). Medical, In: E.D. Svendsen, (Ed.). The professional Hand Books of the Donkey. 3rd Ed., Whittet Books Limited, London pp. 29-31.
|
|
|
|
|
EARO (1999). National animal health research programme strategy document, Ethiopia Agricultural research organization (EARO), Addis Ababa, Ethiopia pp. 35-61.
|
|
|
|
|
FAO (1999). Production year book. Food and agriculture organization of United Nations. Rome pp. 74-92.
|
|
|
|
|
Feseha G, Mohammed A, Yilma J (1991). Vermicular endoparasitism in donkeys of Debre Zeit and Menagesha, Ethiopia. Donkeys, mules and horses in tropical agricultural developement. Proc. Colloq. On donkeys, mules and horses. Eds. D. Fielding and R.A. Pearson, University of Edinburgh, Center for tropical veterinary medicine: UK. pp. 156-160.
|
|
|
|
|
Fraser CM (2000). The Merck Veterinary Manual. A hand book of Diagnosis Therapy and Disease prevention and control for the Veterinarians 8th Ed., Merck and Co; Inc, Rahaway, NIT, USA. pp. 851-852.
|
|
|
|
|
Fred O, Pascal K (2006). Extension approaches to improving the welfare of working equines. Kenya Network for Dissemination of Agricultural Technologies (KENDAT), Nairobi, Kenya pp. 1-28.
|
|
|
|
|
Hansen J, Perry B (1996). The Epidemiology, Diagnosis and control of Helminthes parasites of ruminants, ILRAD, Nairobi, Kenya P. 83.
|
|
|
|
|
Hassan T, Salih MM, Abakar AD (2004). A Survey of Gastrointestinal Nematodes of Donkeys (Equus asinus) in Khartoum State, Sudan. J. Anim. Vet. Adv. 3:736-739.
|
|
|
|
|
Howard J (1993). Current Veterinary therapy of food animal practice, 3rd Ed., W.B. Sunders Company Harcourt Brace Ivovanov pp. 673-675.
|
|
|
|
|
Johnson M, MacKintosh G, Labes E, Taylor J, Wharton A (2003). "Dictyocaulus species. Cross infection between cattle and red deer". New Zeal. Vet. J. 51(2):93-98.
Crossref
|
|
|
|
|
Junquera P (2014). Dictyocaulus species, parasitic lungworms of Cattle, Sheep, Goats and other Livestock. Biology, prevention and control. Dictyocaulus filaria, Dictyocaulus viviparus, Dictyocaulus arnfieldi. In: Merck Veterinary Manual, Merck and Co, Inc, Whitehouse Station, N.J., USA, Last Updated on Friday, January 03, 14:28.
|
|
|
|
|
Kimberling CV (1988). Diseases of Sheep, 3rd Ed., Lea and Febiger, Philadelphia pp. 29-31.
|
|
|
|
|
Klei TR (1986). Other parasites: recent advances. Veterinary Clinics of North America. Equine Pract. 2:329-336.
|
|
|
|
|
Lyons ET, Tolliver SC, Drudge JH, Swerszek TW, Crowe MW (1985a). Parasites in lungs of dead equids in Kentucky. Emphasis on Dictyocaulus arnfieldi. Am. Vet. Res. 46:924-927.
|
|
|
|
|
Lyons ET, Tolliver SC, Drudge JH, Swerszek TW, Crowe MW (1985b). Lungworms (Dictyocaulus arnfieldi). Prevalence in live equids in Kentucky. Am. J. Vet. Res. 46:921-923.
|
|
|
|
|
Lyons ET, Tolliver SC, Drudge JH, Swerczek TW, Crowe MW (1985). Lungworms (Dictyocaulus arnfieldi): prevalence in live equids in Kentucky. Am. J. Vet. Res. 46:9-21.
|
|
|
|
|
Nuraddis I, Tilahun B, Benti D, Tadele T (2011). Survey of Prevalence
|
|
|
|
|
Pandy SV (1980). Epidemiological observations on lungworm, Dictyocaulus arnfieldi, in donkeys from Morocco. J. Helminthes 54:275-279.
Crossref
|
|
|
|
|
Ram CS (2009). A Report on Prevalence of Helminthes Parasites in Mules of Brick Kiln of Lalitpur District. Lalitpur, Nepal P 10.
|
|
|
|
|
Rose FR, Hodgson RDS (2000). Manual of Equine practice. 2nd Ed., USA, Saunders P 224.
|
|
|
|
|
Saeed K, Qadir Z, Ashraf K, Ahmad N (2010). Role of intrinsic and extrinsic epidemiological factors on Strongylosis in horses in Lahore. J. Anim. Plant Sci. 20(4):277-280.
|
|
|
|
|
Sapakota CR (2009). A Report on Prevalence of Helminthes Parasites in Mules of Brick Kiln P. 160.
|
|
|
|
|
Smith PB (2009). Large animal internal medicine, 4th Ed., USA, Mosby Elsevier of Lalitpur District pp. 232-321.
|
|
|
|
|
Svendsen ED (1997). Parasites abroad: The professional hand book of the donkey, 3rd Ed., Whitt et Books Limited, 18 Anley Road, London W14 OBY pp. 166-182.
|
|
|
|
|
Thrustfield M (2005). Veterinary epidemiology 3nd Ed., Veterinary Clinical Studies Royal (Dick) School of Veterinary Studies University of Edinburgh pp. 233.
|
|
|
|
|
Tihitna S, Basaznew B, Mersha C, Achenef M (2012). Ocuurrence of Lungworm Infection in Equines and their Associated Risk Factors. Glob. Vet. 8(1):35-38.
|
|
|
|
|
Tilahun B, Nuraddis I, Benti D, Tadele T (2014). Prevalence of Helminth Parasites of Horses in and Around Hawassa Town, Southern Ethiopia, Acta Parasitol. Glob. 5(1):07-11.
|
|
|
|
|
Urquhart GM, Armur JI, Dunn AM, Jenninngs FW (1996). Veterinary parasitology 2nd Ed., Ames, Iowa, U.S.A.: Iowa State Pr. pp. 39-40.
|
|
|
|
|
Wilson RT (1991). Equines in Ethiopia, In: Fielding, Pearson, R.A. (editorial): Donkeys, Mules and Horses in tropical agricultural development. Edinburgh, Scotland, Center for Tropical Veterinary Medicine, University of Edinburgh. pp. 33-47.
|
|
|
|
|
Yacob HT, Hagos A (2013). Epidemiological study on Gastrointestinal Helminthes of horses in Arsi-Bale highlands of Oromia Region, Ethiopia. Ethiop. Vet. J. 17(2):51-62.
|
|
|
|
|
Yitna T, Hailu D, Ketema B, Anwar S (2013). Prevalence and Associated Risk Factors of Equine Lungworm in Lode Hetosa District, South Eastern Ethiopia. Acad. J. Anim. Dis. 4(2):104-111.
|
|