ABSTRACT
The present research aimed to evaluate the free and immobilized cell of Candida tropicalis NPD1401 for phenol degradation. Immobilized cell of C. tropicalis degraded efficiently up to 98% at a concentration of 1000 mg/l of phenol whereas free cells degraded up to 63% of the same concentration under 9 days of incubation. Stored immobilized beads were reused after 15 days and found to have successfully degraded 62.1% of phenol in the mineral salt medium (MSM). Growth of C. tropicalis was observed in the phenol containing medium by measuring the dry weight of biomass (0.89 g/l at concentration 1000 mg/l) and the degradation was monitored using analytical techniques. Liquid chromatography-mass spectroscopy (LC-MS) analysis confirmed that phenol was degraded by ortho-pathways by the finding of metabolite cis, cis-muconic acid, phenyl phosphate and catechol. Next, isolated strain was identified on the basis of PCR amplification of sequence D2 region of the large subunit of 28S rDNA and it was confirmed as C. tropicalis. By observing the efficiency of the isolate it may be used for the further bioremediation purpose of the phenol contaminated site in the environments.
Key words: Candida tropicalis, phenol, ortho-pathway, Cis-cis-muconic acid, immobilized cell.
Phenol is one of the major toxic aromatic compound discharges from industry and enters into the natural ecosystem. Phenol and phenol derivatives are released from petrochemical, chemical, pharmaceuticals, wood processing plants, paper and pulp, coke manufacturing and pesticide industries. Phenol is included as one of the most hazardous pollutants in the list of Environmental Protection Agency (EPA) (Pishgar et al., 2011). Phenol is also known as carbonic acid, phenic acid, phenylic acid, phenyl hydroxide and or oxybenzene (Nair et al., 2008). Inhalation and dermal exposure of phenol cause irritation, anorexia, progressive weight loss, diarrhea, vertigo, salivation, and a dark coloration of the urine (EPA, 2002). Repeated phenol exposure also causes renal damage, cardiovascular diseases and fatal for adult and children (ASTDR, 2014).
Considering the toxicity of phenol, it must be removed or its load must be reduced considerably from waste before its disposal in the environment. Treatment of waste containing phenol includes both physico-chemical and biological methods. Physico-chemical methods include adsorption, solvent extraction and chemical oxidation by ozone and chlorination (Molva, 2004). Nowadays, biological method is preferable compared to physico-chemical methods due to its expensiveness, and also generates toxic and non-biodegradable intermediate compounds. Microbial treatment of organic recalcitrant compounds is widely studied due to its potential of mineralization of toxic organic compounds. Various studied are carried out to degrade or metabolize the phenol and its derivatives into non-toxic, biodegradable compounds by using microorganisms such as bacteria, fungi and algae (Lika and Papadakis, 2009).
Microorganisms have been isolated and studied for phenol and chlorophenols degradation capability such as Acinetobacter, Alcaligenes, Corynebacterium glutamicum, Pseudomonas sp., Bacillus sp., Kocuria sp., Enterobacter sp. and Vibrio sp. (Field and Sierra-Alvarez, 2008; Karn et al., 2010a,b, 2011, 2017, Karn and Geetanjali, 2014). Fungi are effective in degrading a wide range of organic molecules due to their release of extra-cellular enzymes and high biomass formation. Earlier, Chang et al. (1998) isolated and observed Candida tropicalis for the degradation of high concentration of phenolic and chlorinated derivatives compounds. Lika and Papadakis (2009) and Basha et al. (2010) also reported yeast sp. such as C. tropicalis, Trichosporon and Rhodotorula species; and mycelia as Aspergillus niger, Phaenarochaetes chrysoporium were used for the bioremediation of phenol.
To achieve the successful remediation of particular compounds, selection of fungal species is important for degradation of phenol (Matsubara et al., 2006). Both aerobic and anaerobic processes were used to degrade phenol and its derivative, but aerobic process and microorganisms were found to be more effective in the treatment of phenolic pollutants (Al-Khalid and El-Naas, 2012). In the last two decades, there have been exhaustive researches on the use of immobilized microbial cells as biocatalysts, Bacterial cells immobilized on various matrices have been used extensively for degradation of various toxic (Qi et al., 2012; Mulla et al., 2013). One of the limiting factors for phenol biodegradation is the concentration of phenol. Moreover, enzymes are accompanied by many other enzymes in microorganisms, sometimes with activities against the same substrate.
A particular enzyme may be specific and selective, but if the contaminant enzymes have opposite (or just different) properties, this may reduce the apparent performance of the prepared biocatalyst (Palomo et al., 2002). To overcome this substrate limitation and increase the sustainability and reuse of microorganisms, immobilization of phenol degrading microorganisms was carried out in different immobilizing materials. Immobilization microorganism and enzymes are usual requirements for their large scale use (Santos et al., 2015). Considering these factors, the present work focused on the screening of efficient organism for phenol degradation by using free and immobilized culture to reduce the phenol concentration effectively.
Isolation and screening of phenol degrading microorganism
Isolation of organism was done by enrichment method of the sludge sample collected from industrial waste site of Punjab Chemical, Lalru, Punjab, India (30.79。N 75.85。E), in the mineral salt medium. Next, 3 g of industrial sludge was taken and dissolved in 100 ml of mineral salt medium supplemented with phenol up to 1200 mg/l. The mineral salt medium contained the following components at the specified concentrations (in g/l): K2HPO4, 0.4; KH2PO4,0.2; MgSO4.7H2O, 0.2; FeCl3 ,0.01; CaCl2.2H2O, 0.01; MnSO4.H2O, 0.01; Na2MoO4, 0.01; NaCl,0.1; glucose, 0.5; (NH4)2SO4, 0.5. Further organism was isolated by using dilution plate techniques from dilution 10-5 to 10-7 on solid mineral salt medium plates were prepared by adding 15 g/l bacteriological grade agar and incubated at 28°C. Further, resistant organism was selected by successive culturing up to five generations. Synthetic chlorophenol was purchased from Sigma Aldrich chemicals (USA) and other chemical reagents purchased were of analytical grade from Hi-Media, (India). All solutions were prepared in sterile Milli-Q water (Millipore direct Q3, Bangalore) India.
Growth, resistance and phenol degradation
The growth and phenol transformation response were conducted in 500-ml flasks, sealed with cotton stoppers, containing 100 ml of mineral salt medium (MSM) and inoculated with selected strains of C. tropicalis and were screened for their tolerance to phenol. Phenol was filter sterilized and added to the medium after autoclaving. One week old mycelia discs (2 x 5 mm inoculum) fungus disc cut from the actively growing mycelia were inoculated containing different concentrations (100, 200, 400, 800, 1000 mg/l) of phenol and incubated at 30°C with 120 RPM shaking. Control experiments using non-inoculated, sterile media with the same concentration and same conditions were also conducted. Phenol transformation was monitored at 10 days of incubation by collecting the 5 ml of sample. Growth was observed by means of biomass formation which was also harvested at 10 days of incubation, harvested biomass washed with distilled water, oven dried and the biomass was measured.
Immobilization of Candida tropicalis
2% (w/v) of the sodium alginate solution was dissolved in 25 mM Tris–acetate buffer (pH 7.5). The solution was stirred for 2 h at room temperature (25±2°C).The culture was centrifuged and the pellet was mixed into sodium alginate solution. The drops of this mixture were poured with the syringe into 100 ml of 3% (w/v) CaCl2 solution which initiated the formation of beads. The solution was stirred for 90 min during calcium alginate bead formation. The collected beads were washed with 25 mM Tris-acetate buffer (pH 7.5) to remove excess Ca2+ and stored in the same buffer at 4ºC (Sivasubramanian and Namasivayam, 2014). Biodegradation of phenol by immobilized and free cells was studied at 1000 mg/l concentration of phenol. Reusability of immobilized cells was also evaluated after 15 days. Beads were stored at 4°C Tris-acetate buffer.
Phenol estimation by analytical methods and LC-MS analysis
Further analytical methods used for phenol estimation by 4-amino antipyrine were used as substrate for quantitative estimation of phenol by the spectroscopic method. Absorbance was measured at 510 nm (EPA, 2007) and it was further confirmed by liquid chromatography analysis and metabolic product was analyzed by LC-MS details described. LC-MS analysis of the sample was done by using a Waters Micromass Q-Tof Micro (the mass spectrometer is coupled with Waters 2795 HPLC). Sample culture was centrifuged at 5000 rpm for 20 min. Supernatant has been taken in fresh vial. Separation was achieved with an LC column Waters X-Terra C18 column, eluted with a gradient of acetonitrile in water containing acetic acid (0.1% v/v); from 0 to 40% acetonitrile, using the following parameters- ionization: electro spray positive (ES+), acquisition: MRM unit resolution, Injection volume: 20 ïl and Flow rate: 0.15 ml/min. For mass spectrometer, the following parameters were used: desolvation gas: 550 L/h; cone gas: 30 L/h; desolvation temperature: 250°C; source temperature: 110ËšC; capillary voltage: 3000 V; cone voltage: 30 V; collision energy: 4 eV; nebulize gas: nitrogen 30 ml/min; collision gas: argon 0.5 µl /min (Comte et al., 2013; and Glish and Vachet, 2003).
Identification of phenol degrading strain
Fungal gDNA Miniprep Kit (XcelGen, Gujarat, India) was used for the isolation of genomic DNA. The quality of DNA was evaluated by electrophoresis on 1.2% agarose gel. A fragment of D2 region of 28S rDNA gene was amplified by PCR from the isolated genomic DNA. Reaction mixture for the PCR contained 1X PCR buffer; 200 µM of dNTPs; 1.5 mM MgCl2, 0.1 µM of each primer and 2.5 units of Taq DNA polymerase (Invitrogen, USA) in a final volume of 100 µl sterile MQ water. The PCR was performed with initial denaturation carried out at 95°C for 4 min, followed by 30 cycles of denaturation at 95°C for 30 s, annealing at 55°C and extension at 72°C for 30 s. The thermal cycler was terminated by a final extension for 5 min at 72°C. The sequence for DF/DR primer was as follows: DF: 5’-ACCCGCTGAACTTAAGC-3’, and DR: 5’-GGTCCGTGTTTCAAGACGG-3’ (Fell, 1993). The PCR amplicon was purified and further processed for the sequencing. Forward and reverse DNA sequencing reaction of PCR amplicon was carried out with DF and DR primers using BDT v3.1 Cycle sequencing kit on ABI 3730xlgenetic analyzer. The D2 region of 28S rDNA gene sequence was used to carry out BLAST with the nr-database of NCBI Genbank database. Based on maximum identity score, 15 sequences were selected and the phylogenetic tree was constructed using MEGA 7.
Data analysis
Data were statistically analyzed by analysis of variance (ANOVA) and the mean differences were compared by Tukey-Kramer Multiple Comparison Test at p < 0.05. All the experiment was conducted with three replicates and the analyses were performed using GraphPad Prism (v 4.03) software.
Isolation, screening and identification of phenol degrading microorganism
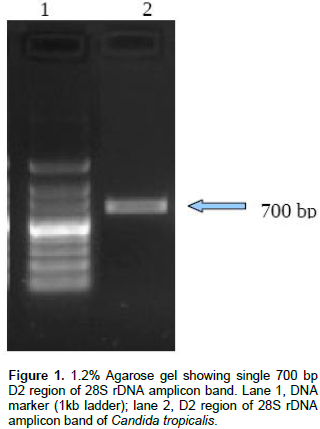
Resistant fungal strains was screened; out of seven isolates just only one isolate (NPD1401) was able to grow at 1200 mg/l of phenol on minimal salt agar plate therefore, this strain was selected for further study. The selected strain was identified by molecular technique, by D2 region of 28S rDNA gene using PCR. A single band of 700 bp of D2 region of large subunit 28S rDNA has been obtained as shown in Figure 1 and sequenced. Consensus sequence of 638 bp of D2 region of LSU gene was generated from forward and reverse sequence data using MultAlin Program (http://bioinfo.genotoul.fr/multalin/multalin.html) and alignment was manually corrected. Furthermore, based on the BLAST search analysis, strain NPD1401 showed 99% similarity with C. tropicalis. The obtained sequence of C. tropicalis was submitted to GenBank (NCBI) with accession number KT380847.
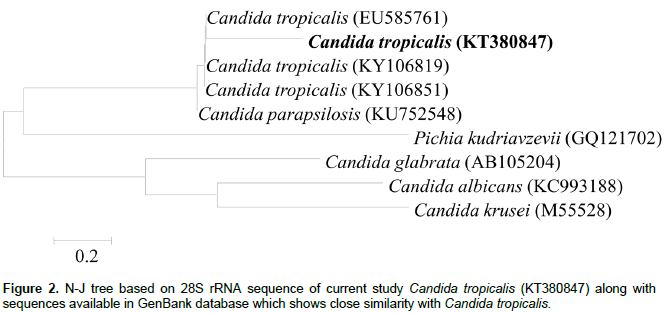
The evolutionary relationship was inferred using the Neighbor-Joining method. The bootstrap consensus tree was inferred from 1000 replicates (Kimura, 1980). The evolutionary distances were computed using the Kimura 2-parameter method and are in the units of the number of base substitutions per site. The analysis involved 16 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. All positions containing gaps and missing data were eliminated. There were a total of 583 positions in the final data-set. Further phylogenetic were constructed using MEGA7 software (Kumar et al., 2016) and shown in Figure 2. Recently, Karn et al. (2017) also isolated pentachlorophenol (PCP) degrading straion SK1 by enrichment method and identified by same method discussed in the current study.
Biodegradation of phenol by free and immobilized cells
During degradation the well grown biomass in the MSM was observed and after completion of 9 days the dry weight given in Table 1 was observed. C. tropicalis has shown effective degradation up to 200 mg/l degradation was about 99%, at 400 mg/l it was 81%, at 800 it was about 72%, and 1000 mg/l it was about 63% using the free cell within ninth day of incubation (Table 1). By observing the efficiency of this isolate, we directly used 1000 mg/l for immobilized and the fresh immobilized cells degraded 98.01% of phenol within 8 days. The immobilized beads of isolates were stored on 25 mM Tris-acetate buffer at 4°C for 15 days. Further, it was re-used and an observed 62.3% of phenol was degraded by stored immobilized cells within 9 days of incubation (Figure 3). C. tropicalis NPD 1401 strain was shown as efficient phenol degrading strain. Previously, Rocha et al. (2007) isolated C. tropicalis, C. rugosa, and Pichia membranaefaciens strain; of these three strains, only C. tropicalis was capable of growing at higher phenol concentration, that is, 1000 mg/l in the minimal medium.
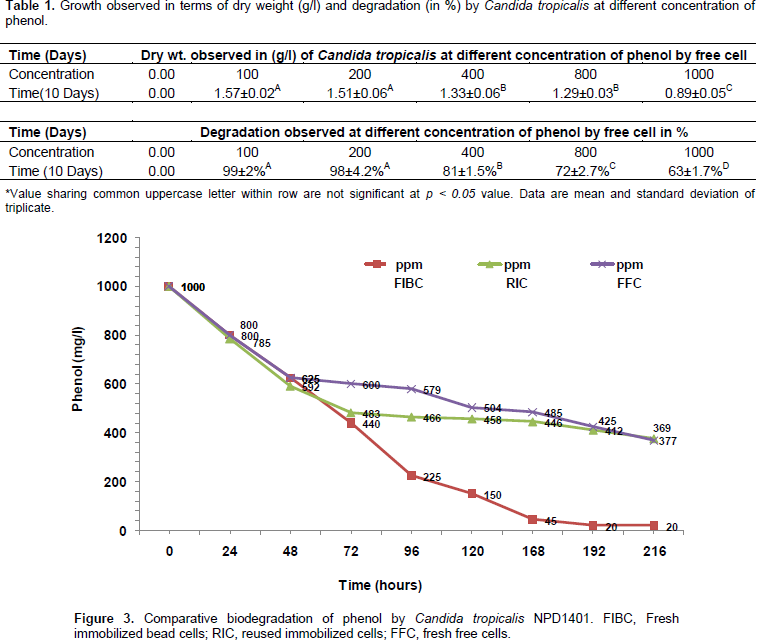
Zhou et al. (2011) designed statistical experiment and used optimized process of phenol degradation by C. tropicalis Z-04. The predicted results showed that the maximum removal efficiency of phenol (99.10%) could be obtained under the optimum conditions of yeast extract 0.41 g/l, phenol 1.03 g/l, inoculum size 1.43% (V/V) and temperature 30.04°C. These predicted values were further verified by validation experiments. Wang et al. (2012) used C. tropicalis W1 isolated from the sludge in the Yantai River of China by selective enrichment with phenol and investigated degraded phenol concentration of 900 mg/l in 30 h, but had no marked degradation activity in 4-chlorophenol. Sivasubramanian and Namasivayam (2014) also observed bioremediation of phenol using C. tropicalis SSK01 immobilized cells isolated from petroleum contaminated soil and observed maximum phenol degradation was 95.2% degradation at 34.20°C and pH 6.86 with a concentration of 610 mg/l. Due to the efficiency towards the degradation of C. tropicalis, various researchers focused on this species. By comparing the previous isolates for phenol degradation, it was observed that the current strain is a more efficient and degraded higher concentration of phenol.
Among the various species of yeast, C. tropicalis is the most studied yeast species for its potential for phenol degradation (Yan et al., 2005; Adav et al., 2007; Zhou et al., 2011; Ahmad et al., 2013; Basak et al., 2014a; Long et al., 2014). Besides C. tropicalis, other yeast such as C. lipolytica, Candida utilis, Candida albicans, Trichosporon montevideense, and Trichosporon cutaneum were also used to degrade the phenol and its derivatives (Chen et al., 2002; Vilimkova et al., 2008; Liu et al., 2011; Gerginovaa et al., 2014). Compared with free cells, immobilized cell was found more efficient for phenol degradation. Previously, polyacrylamide (PAA) gel beads, calcium alginate beads, sugarcane bagasse, agar-entrapment by Ramírez et al. (2001), Basak et al. (2014b) and Adav et al. (2007) were used to immobilize C. tropicalis and degrade different concentration of phenol. Aerobic granules of C. tropicalis were sufficient enough to degrade the phenol up to 1000 mg/l. The highest concentration of phenol (>1000 mg/l) was inhibitory for C. tropicalis present in the aerobic granule (Adav et al., 2007). Vilimkova et al. (2008) found NADPH-dependent phenol hydroxylase and catechol-1, 2-dioxygenase from C. tropicalis which helps in the degradation of phenol. The present strain successfully degraded phenolin both free as well as immobilized cell.
Radovich (1985) demonstrated mass transfer limitations caused by the transport resistance within the immobilization matrix affect the activity of the immobilized cells. A concentration gradient within the immobilized cell matrix (ICM) is established at steady state. Assuming that the distribution of cells is such that the fermentation reaction occurs throughout the ICM, the process must be modeled as simultaneous reaction and diffusion. The internal mass transfer effects are also traditionally accounted for by an effectiveness factor, which is defined as the ratio of the actual reaction rate to the reaction rate which would occur if all the interior of the biocatalyst particle was exposed to the same reactant concentration as the exterior of the particle. These mass transfer limitations may bring about an inhomogeneous distribution of viable cells within the immobilizing matrix, a change in the cell's growth kinetics or the cell's enzyme kinetics, and a change in the operational stability of the cells. Homaei et al. (2013) suggested that the heterogeneity of the immobilized enzyme systems allows an easy recovery of both enzymes and products, multiple re-use of enzymes, continuous operation of enzymatic processes, rapid termination of reactions, and greater variety of bioreactor designs.
LC-MS analysis
After LC-MS analysis, we determined that possibly phenol degradation is initiated in the presence of molecular oxygen and the aromatic ring is further hydrolyzed by the phenol hydroxylase into catechol. The aromatic ring of phenol breakdown by the ortho or meta- oxidation pathways was described previously by Jiang et al. (2006), and Lika and Papadakis (2009). Catechol 1, 2- dioxygenase and catechol 2, 3 dioxygenase are the enzymes involved in the breakdown of aromatic ring present in the phenol by ortho and meta pathway respectively, with the cleavage sites for both enzymes different (An et al., 2001; Cai et al., 2007; Nair et al., 2008; Agarry et al., 2008). Wang et al. (2007) reported on the Acinetobacter sp. PD12 metabolized phenol in the o-pathway and detected the presence of catechol 1, 2-dioxygenase. In the result, we found featured peak (Figure 4a) having m/z 143.42 which showed the presence of cis, cis - muconic acid.
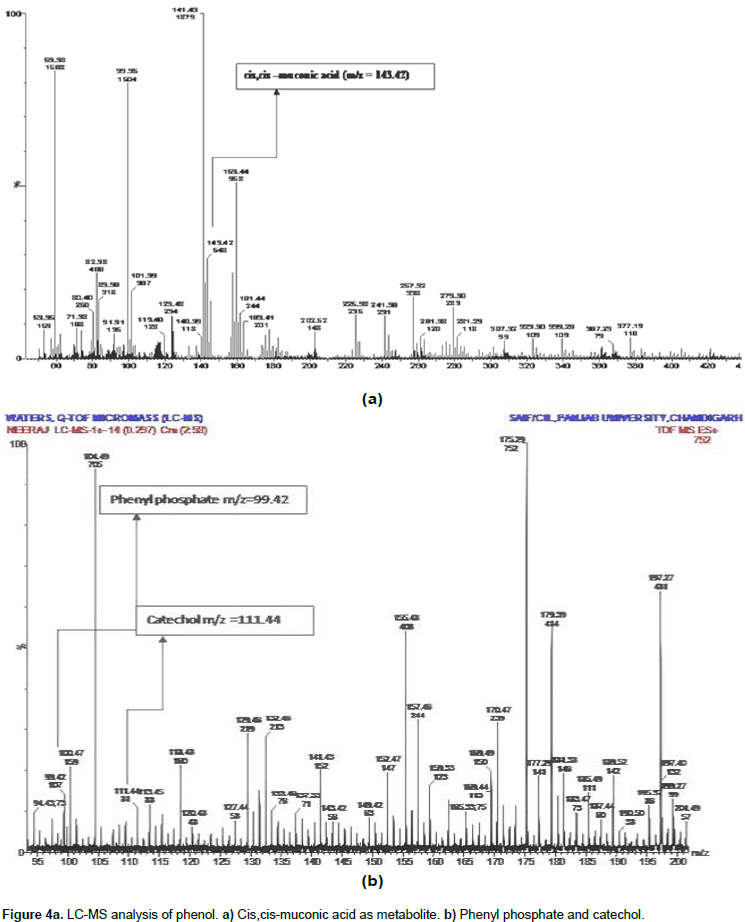
During phenol degradation cis, cis -muconic acid was prominently detected using LC-MS analysis (LC-MS of the sample was analyzed and outsourced at SAIF, Punjab University, Chandigarh, India). The cis,cis muconic acid belongs to aerobic degradation ortho-pathways for phenol biodegrdation. Due to the presence of cis, cis - muconic acid it has been predicted that C. tropicalis used ortho-metabolic pathway for phenol degradation. Presence of cis, cis-muconic acid indicates the isolated yeast strain and follows ortho-metabolic pathway for biodegradation of phenol. The present result was supported by the finding of Tuah (2006) who also confirmed that C. tropicalis strain follows ortho - metabolic pathway for phenol degradation. The enzymes involved in metabolic pathways are specific for substrate used. Other intermediate metabolite of degraded phenol like phenol pyrophosphate and catechol (Figure 4b) which provide evidence of the ability of Candida tropicalis for phenol degradation was also observed. Thus, we can clearly say that the present strain are efficient for phenol degradation and can be applied to the contaminated site in the environment.
The authors have not declared any conflict of interests.
Authors are thankful to the Ambala College of Engineering and Applied Research for providing necessary facility to complete this study.
REFERENCES
|
Adav SS, Chen MY, Lee DJ, Ren NQ (2007). Degradation of Phenol by Aerobic Granules and Isolated Yeast Candida tropicalis. Biotechnol. Bioeng. 96(5):844-852.
Crossref
|
|
|
|
Agarry SE, Durojaiye AO, Solomon BO (2008). Microbial degradation of phenols: A review. Int. J. Environ Pollut. 32:12-28.
Crossref
|
|
|
|
|
Ahmad MF, Haydar S, Quraishi TA (2013). Enhancement of biosorption of zinc ions from aqueous solution by immobilized Candida utilis and Candida tropicalis cells. Int. Biodeterior. Biodegrad. 83:119-128.
Crossref
|
|
|
|
|
Al-Khalid T, El-Naas MH (2012). Aerobic Biodegradation of Phenols: A Comprehensive Review. Crit. Rev. Environ. Sci. Technol. 42:1631-1690.
Crossref
|
|
|
|
|
An HR, Park HJ, Kim ES (2001). Cloning and expression of thermophilic catechol 1,2 dioxygenase gene (catA) from Streptomyces setonii. FEMS Microbiol. Lett. 195:17-22.
Crossref
|
|
|
|
|
ASTDR- Agency for Toxic Substances and Disease Registry (2014). Medical Management Guidelines for Phenol. Available at: https://www.atsdr.cdc.gov/mmg/mmg.asp?id=144&tid=27.
|
|
|
|
|
Basak B, Bhunia B, Dey A (2014b). Studies on the potential use of sugarcane bagasse as carrier matrix for immobilization of Candida tropicalis PHB5 for phenol biodegradation. Int. Biodeterior. Biodegrad. 93:107-117.
Crossref
|
|
|
|
|
Basak B, Bhunia B, Dutta S, Chakraborty S, Dey A (2014a). Kinetics of phenol biodegradation at high concentration by a metabolically versatile isolated yeast Candida tropicalis PHB5. Environ. Sci. Pollut. Res. 21:1444-1454.
Crossref
|
|
|
|
|
Basha KM, Rajendran A, Thangavelu V (2010). Recent Advances in the Biodegradation of Phenol: A review. Asian J. Exp. Biol. Sci. 1:219-234.
|
|
|
|
|
Cai W, Li J, Zhang Z (2007). The characteristics and mechanisms of phenol biodegradation by Fusarium sp. J. Hazard. Mat. 148:38-42.
Crossref
|
|
|
|
|
Chang YH, Li CT, Chang MC, Shieh WK (1998). Batch phenol degradation by Candida tropicalis and its fusant. Biotechnol. Bioeng. 60:391-395.
Crossref
|
|
|
|
|
Chen KC, Lin YH, Chen WH, Liu YC (2002). Degradation of phenol by PAA-immobilized Candida tropicalis. Enzyme Microb. Technol. 31:490-497.
Crossref
|
|
|
|
|
Comte A, Christen P, Davidson S, Pophillat M, Lorquin J, Auria R, Simon G, Casalot L (2013). Biochemical, Transcriptional and Translational Evidences of the Phenol-meta-Degradation Pathway by the Hyperthermophilic Sulfolobus solfataricus 98/2. PLoS One 8(12):1-7.
Crossref
|
|
|
|
|
EPA (2007). Phenolics (Spectrophotometric, manual 4-AAP with Distillation), Environmental Protection Agency, Method-9065. Available at:
View
|
|
|
|
|
EPA (2002). Toxicological Review of Phenol (CAS No. 108-95-2). EPA635/R-02/006., U.S. Environmental Protection Agency, Washington D.C.
|
|
|
|
|
Fell JW (1993). Rapid identification of yeast species using three primers in a polymerase chain reaction. Mol. Mar. Biol. Biotechnol. 2:174-180.
|
|
|
|
|
Field AJ, Sierra-Alvarez R (2008). Microbial degradation of chlorinated phenols. Rev. Environ. Sci. Biotechnol. 7:211-241.
Crossref
|
|
|
|
|
Gerginovaa M, Zlatevab P, Penevaa N, Alexievaa Z (2014). Influence of phenolic substrates utilised by yeast Trichosporon cutaneum on the degradation kinetics. Biotechnol. Biotechnol. Equip. 28:33-37.
Crossref
|
|
|
|
|
Glish GL, Vachet RW (2003). The basics of mass spectrometry in the twenty-first century. Nat. Rev. Drug Discov. 2:140-150.
Crossref
|
|
|
|
|
Homaei AA, Sariri R, Vianello F, Stevanato R (2013). Enzyme immobilization: an update. J. Chem. Biol. 6:185-205.
Crossref
|
|
|
|
|
Jiang HL, Tay ST, Maszenan AM, Tay JH (2006). Physiological traits of bacterial strains isolated from phenol-degrading aerobic granules. FEMS Microbiol. Ecol. 57:182-191.
Crossref
|
|
|
|
|
Karn SK, Chakrabarty SK, Reddy MS (2010a). Characterization of pentachlorophenol degrading Bacillus strains from secondary pulp-and-paper-industry sludge. Int. Biodeterior. Biodegrad. 64:609-613.
Crossref
|
|
|
|
|
Karn SK, Chakrabarty SK, Reddy MS (2010b). Pentachlorophenol degradation by Pseudomonas stutzeri CL7 in the secondary sludge of pulp and paper mill. J. Environ. Sci. 22:1608-1612.
Crossref
|
|
|
|
|
Karn SK, Chakrabarty SK, Reddy MS (2011). Degradation of pentachlorophenol by Kocuria sp. CL2 isolated from secondary sludge of pulp and paper mill. Biodegradation 22:63-69.
Crossref
|
|
|
|
|
Karn SK, Eswari SJ, Rajput VD, Kumar S, Kumar A (2017). Simultaneous application of Vibrio sp. (SK1), biochar amendment and modeling for removal of pentachlorophenol (PCP) in the farmland soil. Environ. Eng. Sci. doi:10.1089/ees.2016.0456.
Crossref
|
|
|
|
|
Karn SK, Geetanjali (2014). Pentachlorophenol remediation by Enterobacter sp. SG1 isolated from industrial dump site. Pak. J. Biol. Sci. 17:388-394.
Crossref
|
|
|
|
|
Kimura M (1980). A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120.
Crossref
|
|
|
|
|
Kumar S, Stecher G, Tamura K (2016). MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 33:1870-18074.
Crossref
|
|
|
|
|
Lika K, Papadakis IA (2009). Modeling the biodegradation of phenolic compounds by microalgae. J. Sea Res. 62:135-146.
Crossref
|
|
|
|
|
Liu H, Yu QJ, Wang G, Cong FYY (2011). Biodegradation of phenol at high concentration by a novel yeast Trichosporon montevideense PHE1. Process Biochem. 46:678-1681.
Crossref
|
|
|
|
|
Long Y, Yang S, Xie Z, Cheng L (2014). Identification and characterization of phenol hydroxylase from phenol-degrading Candida tropicalis strain JH8. Can. J. Microbiol. 60:585-591.
Crossref
|
|
|
|
|
Matsubara M, Lynch JM, De Leij FA (2006). A simple screening procedure for selecting fungi with potential for use in the bioremediation of contaminated land. Enzyme Microbiol. Technol. 39:1365-1372.
Crossref
|
|
|
|
|
Molva M (2004). Removal of Phenol from Industrial Wastewaters Using Lignitic Coals. Izmir Institute Technology, Izmir, Turkey. Available at: http://library.iyte.edu.tr/tezler/master/cevremuh/T000458.pdf.
|
|
|
|
|
Mulla SI, Talwar MP, Bagewadi ZK, Hoskeri RS, Ninnekar HZ (2013). Enhanced degradation of 2-nitrotoluene by immobilized cells of Micrococcus sp. strain SMN-1. Chemosphere 90:1920-1924.
Crossref
|
|
|
|
|
Nair I, Jayachandran K, Shankar S (2008). Biodegradation of Phenol. Afr. J. Biotechnol. 7:4951-4958.
|
|
|
|
|
Palomo JM, Fernandez-Lorente G, Mateo C, Fuentes M, Guisan JM, Fernandez-Lafuente R (2002) Tetrahedron: Asymmetry 13:2653-2659.
Crossref
|
|
|
|
|
Pishgar R, Najafpour G, Neya BN, Mousavi N, Bakhshi Z (2011). Anaerobic Biodegradation of Phenol: Comparative Study of Free and Immobilized Growth. Iranica J. Eviron. Environ. 2:348-355.
Crossref
|
|
|
|
|
Qi Y, Zheng CL, Zhang YT (2012). Microbial degradation of nitrobenzene by immobilized cells of Micrococcus luteus. Adv. Mat. Res. 599:52-59.
Crossref
|
|
|
|
|
Radovich JM (1985). Mass transfer limitation in immobilized cells. Biotechnol. Adv. 3(1):1-12.
Crossref
|
|
|
|
|
Ramírez CJ, Ordaz NR, Urbina EC, Mayer JG (2001). Degradation kinetics of phenol by immobilized cells of Candida tropicalis in a fluidized bed reactor. World J. Microbiol. Biotechnol. 17:697-705.
Crossref
|
|
|
|
|
Rocha LL, deAguiar CR, Cavalcante RM, Nascimento RF, Martins SC, Santaella ST, Melo VM (2007). Isolation and characterization of phenol-degrading yeasts from an oil refinery wastewater in Brazil. Mycopathologia 164:183-188.
Crossref
|
|
|
|
|
Santos JCS. Dos, Barbosa O, Ortiz C, Murcia AB, Rafael CR, Fernandez-Lafuente R (2015). Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 7:2413-2432.
Crossref
|
|
|
|
|
Sivasubramanian S, Namasivayam SKR (2014). Optimization of Parameters for Phenol Degradation using Immobilized Candida Tropicalis SSK01 in Batch Reactor. J. Environ. Biol. 35:531-536.
|
|
|
|
|
Tuah MBP (2006). The Performance of Phenol Biodegradation by Candida tropicalis Retl-Cr1 using Batch and Fed-Batch Fermentation Techniques. Ph.D. Thesis, Universiti Teknologi Malaysia. Malaysia. http://eprints.utm.my/1306/.
|
|
|
|
|
Vilimkova L, Paca J, Kremlackova V, Jan P, Marie S (2008). Isolation of Cytoplasmic NADPH-Dependent Phenol Hydroxylase and Catechol-1,2 dioxygenase from Candida tropicalis Yeast. Interdiscipl. Toxicol. 1:225-230.
Crossref
|
|
|
|
|
Wang Jianhua, Xuanxuan Ma, Sujing Liu, Pengcheng Sun, Ping Fan, Chuanhai Xia (2012). Biodegradation of Phenol and 4-Chlorophenol by Candida tropicalis W1. Proc. Environ. Sci. 16:299-303.
Crossref
|
|
|
|
|
Wang Y, Tian Y, Han B, Zhaw HB, Bi JN, Cai BL (2007). Biodegradation of phenol by free and immobilized Acinetobacter sp. strain PD12. J. Environ. Sci. 19:222-225.
Crossref
|
|
|
|
|
Yan J, Jianping W, Hongmei L, Suliang Y, Zongding H (2005). The biodegradation of phenol at high initial concentration by the yeast Candida tropicalis. Biochem. Eng. J. 24:243-247.
Crossref
|
|
|
|
|
Zhou J, Yu X, Ding C, Wang Z, Zhou Q, Pao H, Cai W (2011). Optimization of phenol degradation by Candida tropicalis Z-04 using Plackett-Burman design and response surface methodology. J. Environ. Sci. 23:22-30.
Crossref
|
|