ABSTRACT
This study evaluated the toxicological effects of chronic treatment with ethanolic extract of Waltheria indica aerial portion on body weight, hematological and biochemical parameters in albino rats using standard methods. Rats treated with 400 mg/kg body weight (bw)/day of the extract showed no behavioural changes. However, there was general reduction of activity in rats given 800 and 1,600 mg/kg bw/day of the extract. Also, the LD50 treated rats exhibited hypoactivity, grooming, prostration and irritation during treatment in the third and fourth weeks of treatment period. The data on body weight changes indicated that there were no significant differences in body weight between the control group and groups that received different doses of the extract (p<0.50). Haematological results for the red blood cell, haemoglobin, packed cell volume, mean corpuscular volume, mean corpuscular haemoglobin and mean corpuscular haemoglobin concentration in extract treated rats showed no significant changes at all doses of treatments when compared with controls in female rats. However, data on mean corpuscular haemoglobin in the male rats treated with 1,600 mg/kg showed a significantly decreased value when compared with controls. On the other hands, white blood cell counts decreased significantly after treatment with 400 and 800 mg/kg bw/day extract in the male rats. Lymphocytes count was also decreased significantly in males treated with 800 mg/kg bw/day of extract. Alanine transaminase (ALT), total bilirubin and creatinine increased significantly, respectively at 800 and 1,600 mg/kg bw/day doses of extract when compared with the control rats (p<0.05). In conclusion, the overall data of this study suggest that the oral administration of W. indica extract did not induce any toxic effects, especially when administered at low doses; however, further investigation is needed to evaluate its chronic toxicity.
Key words: Waltheria indica, Wistar rats, evaluation, toxicological, biochemical, haematological, parameters.
Abbreviation:
ALT, Alanine transaminase; AST, aspartate transaminase; DLC, differential leucocyte count; Hb, haemoglobin; MCV, mean corpuscular volume; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; PVC, packed cell volume; RBC, red blood cell; WBC, white blood cell.
Waltheria indica (Synonymn Waltheria americana) is a plant widespread throughout the tropics (Burkill et al., 2000). It belongs to the family Sterculiaceae and it is commonly called sleeping morning. Locally, the plant is called “hankufa” in Northern Nigeria and “Konkodi” in the south. The plant has been used in traditional medicine for treatment of several pathologies (Olajuyugbe et al., 2011). In Nigeria, W. indica roots and aerial parts have been used mainly against pain, inflammation, conditions of inflammation, diarrhea, dysentery, wounds, anaemia, epilepsy, convulsion, and asthma (Heinrich et al., 1992; Hamidu et al., 2008). Whole plant is used to treat peptic ulcer (Oluranti et al., 2012), while decoction of aerial parts may be taken to treat anaemia (Gbadamosi et al., 2012). The use of medicinal plants has received great attention in the world as an alternative to conventional drugs partly due to perceived therapeutic efficacy and low side effect profile of natural products from plants (Aluko, 2016) and the demand for these remedies has recently increased (Mhuji et al., 2016). The World Health Organization (WHO) estimated that about 80% of the population of most developing countries relies on herbal medicines for their primary health care (Dharm and Pramod, 2017). Some of these traditional medicines involve the use of crude plant extracts in the form of infusion, decoction or tincture which may contain an extensive diversity of molecules often with indefinite biological effects (Olowa and Nuneza, 2013; Brenda et al., 2016). Novel clinically active drugs are been isolated from higher plants; however, there are limited scientific evidence as to the efficacy and safety to support the continued therapeutic application of these medications (Amrit et al., 2014; Taiwo and Joel, 2015).
Studies providing an evidence for local and traditional uses of W. indica have been documented. Yerra et al. (2005) reported that flavonoids isolated from W. indica effectively inhibited the production of nitric oxide (NO), turmor necrosis factor alpha (TNF-α) and interleukin 12 (IL 12); this supports the use of the plant for the treatment of inflammatory diseases in traditional medicine. Hamidu et al. (2013) also reported that the ethyl acetate fraction of the plants significantly reduced oedema size, suggesting anti-inflammatory activities. Also reported are anti-microbial activities of the plant (Zailani et al., 2010) and analgesic effects (Yaro et al., 2007). Although, there are diverse potentially clinical utility and scientific studies published on W. indica, toxicity reports on this plant are sparse in literature; and it has been suggested that the safety of plants-based medicine needs to be evaluated essentially before recommending for human consumption (Poonam et al., 2014; Oloro et al., 2016). Toxicological studies in form of acute, sub-acute and sub-chronic are requirements for many products used as medicines (Oloro et al., 2016). The liver is the site of detoxification and deamination; the determination of the activity of certain enzymes was employed in knowing the toxic effects or level of plants extracts used as medicines (Asadu et al., 2015). This work reports the effects of ethanolic extract of the plant on haematologial and biochemical parameters in rats.
Plant
Fresh samples of aerial parts (flowers, leaves and stems) of W. indica were collected from the vicinity of the university dam, Ahmadu Bello University, Zaria in the month of July 2016. The plant material was identified by taxanomic means through comparison with the herbarium specimen and authenticated by Dr. Mujtaba Abubakar of Department of Pharmacognosy and Drug Development, Ahmadu Bello University, Zaria, Nigeria. A voucher specimen (NPR 2006) was prepared and deposited in the herbarium of the same department.
Preparation for extraction
Fresh plant material was washed, dirt removed, air-dried and then oven-dried for 2 h in the Department of Pharmacognosy and Drug Development, Ahmadu Bello University, Zaria. It was pounded into powder and sieved.
Extraction
Powdered material (500 g) was exhaustively extracted with aqueous ethanol (60% v/v) using Soxhlet apparatus. The aqueous ethanolic extract upon concentration yielded a yellowish green residue hitherto called the extract (15.3% w/w). The extract was suspended in water and defatted with petroleum ether. The solvents were then removed at 52°C under reduced pressure in a rotavapour. The solid sample was stored in a refrigerator until needed for experimentation.
Animals
The animals used in this study were young adult albino mice weighing 21.5 to 27.0 g and Wistar albino rats (191 to 215 g) of both sexes obtained from the animal house of the Department of Pharmacy, Ahmadu Bello University, Zaria, Nigeria. The animals were maintained under standard nutritional and environmental conditions, having access to water and food ad libitum. Feeding was withdrawn 12 h before experimentation.
Acute toxicity test
The acute toxicity (oral LD50) of the extract of W. indica aerial portion was earlier established in previous communications in 25 albino mice using standard method of Lorke (1983).
Sub-acute toxicity study
For the purpose of this study, adult Wistar rats of both sexes were allotted randomly to 4 groups, each consists of 6 rats. The extract was administered by gavage to three groups (II, III and IV, respectively) at 400, 800, and 1,600 mg/kg doses on alternate days for 28 days (4 weeks) between 08:00 and 09:00 h each day. Animals in group I (control) were given normal saline (2 ml/kg) orally. Animals were observed for clinical signs and symptoms, behaviour alterations, food and water intake and body weight changes. All experimental animals were observed twice daily for mortality during the 28 days period of study. Weight of each rat was recorded at day zero and at weekly intervals throughout the duration of the study. The group mean weights were calculated and recorded. At the end of the 28 days period, the animals were fasted overnight; then the following morning each animal was heparinized and blood samples collected from orbital sinusis. Samples were collected after 24 h of the last doses of the extract.
Haematology and blood chemistry examination: Hematological analysis
The hematological parameters (red blood cell [RBC], haemoglobin [HB], packed cell volume [PVC], white blood cell [WBC], differential leucocyte count [DLC], mean corpuscular volume [MCV], mean corpuscular haemoglobin [MCH], and mean corpuscular haemoglobin concentration [MCHC]) were measured using standard methods. Analysis was performed on all samples immediately after collection (TO). RBC counts were done using Neubauer haemocytometer (Shah and Altindag, 2005). 20 µl of each whole blood was diluted with 0.98 ml of Dacie’s fluid (1 ml of 40% formaldehyde that is full strength, 3.13 g trisodium citrate, 0.1 g brilliant cresyl blue, dissolved in 100 ml of distilled water). The solution was gently mixed to dispense the cells; this provided a 1:5 dilution of the blood. The mixed solution was drawn into a disposable plastic pipette. The first few drops were discarded and one drop touched the edge of a Neubauer haemocytometer between the cover slip and counting chamber. Capillary action draws the sample under the cover slip (Handy and Depledge, 1999). RBCs were counted on microscope in 5 of the secondary squares (model DM750, Leica Microsystems GmbH-wetzlar, Germany) at ×640. RBC was expressed as 106 mm-3. WBC was counted by using a neubaver laemocytometer (Shah, 2010). Blood was diluted 1:20 with Turk’s diluting fluid (1% glacial acetate solution and Gentian violet 0.3% w/v dissolved in distilled water). Total number of WBC expressed as 103 mm-3 (Wintrobe and Lee, 1967). PCV was determined by microhaematocrit centrifugation. The length of columns containing packed red cells and the packed red cells plus supernatant were measured and PVC was expressed as percentage. Haemoglobin concentration (Hb) was measured with haemoglobin test kit (Roach GmbH mannhein, Germany) using the cyanmethemoglobin method (Larsen and Snieszko, 1961). MCV, MCH and MCHC were indirectly calculated from the obtained values of Hb, PCV and RBC as described by Francesco et al. (2012).
Differential leucocyte count
A blood smear was prepared dried and stained with Leishman’s stain slide placed on microscope and scanned at low power to find a distribution of cells. A drop of oil is placed on the slide and cells were examined with the oil immersion objective. Percentage of each type of white blood cell was determined and recorded.
Biochemical analysis
Serum alanine (ALT) and aspartate (AST) were colorimetrically assayed using the methods of Reitman and Frankel (1957). Total bilirubin, urea and creatinine were assayed using the sulphanilic reaction, diacetylmonoxine reaction and Jaff’s reaction, respectively as described by Kaplan et al. (1988). The biuret test (Henry et al., 1974) was used for total protein estimate, while chloride and bicarbonate were estimated by titrimetric method (Harold, 1988). Potassium and sodium levels were estimated by flame photometric method.
Statistical analysis
Data were expressed as mean±standard error of mean (SEM). The data were analyzed using the student’s t-test and the differences were considered significant when P<0.05.
The oral LD50 of W. indica extract (875 mg/kg) was as earlier reported by previous studies (Hamidu et al., 2013). Table 1 shows the body weight changes in the rats during the 28 days study periods. The data indicated that there were no significant differences in body weight between the control group and the groups that received different doses of the plant extract (p>0.05).
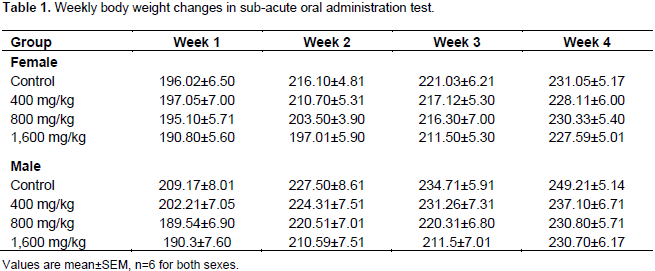
Sub-acute toxicity studies
The effects of W. indica aerial parts extract on haematological values of male and female rats in the sub-acute test are shown in Table 2. The table showed no significant difference in female rats treated with various doses as compared to the controls. Values in the table however showed that the MCV, significantly decreased (p<0.05) in the male rats treated with 1,600 mg/kg. DLC values are shown in Table 3. The table indicated that in female rats treated with 1,600 mg/kg, there was a significant increase (p<0.05) in neutrophil count. In the male rats, a significant increase in white blood cell was observed in the groups that received 800 and 1,600 mg/kg; and a significant decrease in lymphocyte in male rats treated with 1,600 mg/kg when compared with controls (p<0.05). The effects of administration of W. indica extracts on indices of liver and kidney function are shown in Tables 4 and 5, respectively. Indices of liver functions (AST, ALT and total bilirubin) did not show increases by the extract following 4 weeks of administration. The groups administered 800 and 1,600 mg/kg however produced higher values as compared to the controls, though not statistically significant.
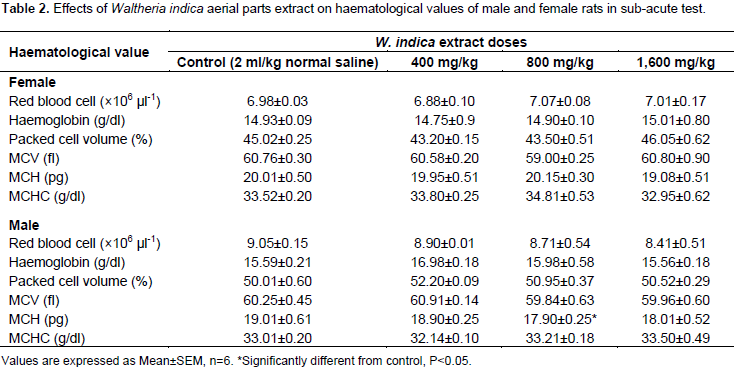
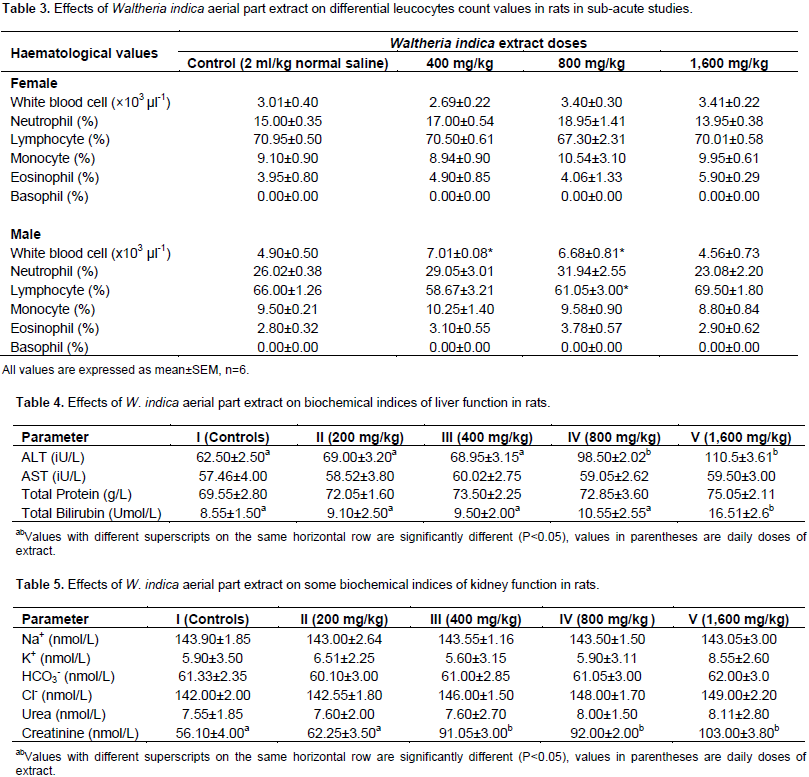
The oral LD50 of the ethanolic extract of W. indica aerial portion (stem, leave, flowers) was reported by our earlier studies (Hamidu et al., 2013). The study found that LD50 of the extract was 875 mg/kg; suggesting that the extract is relatively safe. In addition, no physical symptom of toxicity based on the oral LD50 value recommended by organization for economic co-operation and development (1998): very toxic ≤5 mg/kg, toxic >5≤50 mg/kg, harmful >50≤500 mg/kg and no label >500≤2000 mg/kg. Body weight changes after administration of various doses of the extract showed no significant difference from values obtained from control animals (p>0.05). Analysis of blood parameters is a relevant risk evaluation; since change in haematological and biochemical system has a higher predictive value for human toxicity in humans when data are translated from animal study (Raza et al., 2002). It has been demonstrated that W. indica contain various bioactive principles with pharmacological potential, which can cause beneficial and/or harmful effects on human health; furthermore, it was documented that the general concern of users for lack of scientific evidence has favored to conduct studies regarding the toxicity and harmful effects of plants used by people as natural drugs (Carlos et al., 2015). From the result of the haematological analysis, all the parameters analyzed: RBC, Hb, PCV, MCV, MCH, and MCHC showed no significant difference in female rats given various doses of the extract when compared with controls (p>0.05). However, MCH values significantly decreased (p>0.05) in male rats treated with 1,600 mg/kg for 28 days (Table 2).
MCH, MCHC and MCV are related to individual RBC, while parameters like Hb and PVC are associated with total population of RBCs (Mishra and Tandon, 2012). Therefore, significant decrease in these parameters as seen in this study after treatment with high doses (800 and 1,600 mg/kg) of the extract for 28 days may mean that either the incorporation of Hb into RBC or the morphology and osmotic fragility of RBCs were altered. DLC also showed significant increase (p<0.05) of neutrophils in female rats administered 1,600 mg/kg of the extract (Table 3). Results of all the parameters analyzed in the kidney function: sodium, potassium, bicarbonate, chloride, urea and creatinine, indicated no significant changes (p<0.05) in serum level of rats given various doses as compared to controls. Normally, urea and creatinine determine the general function of the kidney, whereas the electrolytes: sodium (Na+), potassium (K+), bicarbonate (HCo3-) and chloride (Cl-) are determinants of tubular function. The values of urea and creatinine showed no significant changes (p>0.05) in rats treated with various doses from the control groups; suggesting extract is not nephrotoxic at least in rats. Histomorphological studies of kidney tissues are being studied to confirm the biochemical results reported in this study. In conclusion, the overall data of this study suggest that the oral administration of W. indica extract did not induce any toxic effects, especially when administered at low doses; however, further investigation is needed to evaluate its chronic toxicity.
The authors have not declared any conflict of interests.
The authors sincerely appreciate the guidance and quality academic contributions of Professor M. Mabrouk. The technical assistance of Dr. Mujtaba Abubakar of the Department of Pharmacognosy and Drug Development, Ahmadu Bello University, Zaria and all his technical staff is acknowledged.
REFERENCES
|
Aluko BT (2016). Evaluation of the safety profile of the leaves of Gutenbergia nigritana Benth. E. J. Med. Plants 15(3):1-7.
Crossref
|
|
|
|
Amrit KS, Dharam PA, Prakash D, Suchita D, Tanveer N, Balgangashar R (2014). Acute and sub - cute toxicity studies of pharmacologically active seabuckthorn leaf extract. Int. J. Pharm. Pharm. Sci. 6(6):414-419.
|
|
|
|
Asadu CL, Abong O, Anosike CA, Robert U (2015). In vivo toxicological studies on methanolic leaf extract of Lantana camera. Am. Eurasian J. Toxicol. Sci. 7(2):115-122.
|
|
|
|
Brenda AB, Nadiah Z, Waisadin B, Sergio AG, Dounia K, Hateem F, Abdelhamid E (2016). Plants extracts: from encapsulation to application. Expert Opin. Drug Deliv. 13(8):1165-1175.
Crossref
|
|
|
|
Burkill HM, Corey SC, Moore AK (2000). The useful plants of West Tropical Africa. 2nd Edn., Royal Botanical Garden, Kew. pp. 293-294.
|
|
|
|
Carlos GE, Raphael PP, Alfonso AA, Luicita KR, Nancy AH, Eleazor CM (2015). Effects of aqueous and ethanolic extract of dried leaves of pseudocalymma allianceum (Bignonaceae) on hematological and biochemical parameters of wistar rats. Asian Pac. J. Reprod. 4(2):129-134.
Crossref
|
|
|
|
Dharm N, Pramod KS (2017). Ethnobotanical importance and herbal medicine in Vidhya region of Eastern Utter Pradesh, India. J. Med. Plant Res. 11(25):403-413.
Crossref
|
|
|
|
Francesco F, Satheeshkumar P, Kumar DS, Caterina F, Giuseppe P (2012). A Comparative study of haemoglobin and blood Chemistry of Indian and Italian Grey Mullet (Mugil Cephalus Linneaus 1758). HOAJ Biol. 1:1-5.
Crossref
|
|
|
|
Gbadamosi IT, Mood JO, Yekini AO (2012). Nutritional composition of ten ethnobotanicals used for treatment of anaemia in South West Nigeria. Eur. J. Med. Plants 2(2):140-150.
Crossref
|
|
|
|
Hamidu JL, Ayo JO, Adelaiye AB, Abubakar MS (2008). Sedative and anticonvulsant effects of ethyl acetate fraction of Waltheria indica in mice. J. Pharmacol. Toxicol. 3:261-266.
Crossref
|
|
|
|
Hamidu JL, Ayo JO, Adelaiye AB Abubakar MS (2013). Evaluation of Waltheria indica aerial parts extract for analgesic and anti-inflammatory activities. Eur. J. Sci. Res. 110(2):295-302.
|
|
|
|
Handy RD, Depledge MH (1999). Physiological responses: Their measurements and uses as environmental biomarkers in ecotoxicology. Ecotoxicology 8:329-349.
Crossref
|
|
|
|
Harold S (1988). Practical Clinical Biochemistry. CBS Publ. New Delhi, India. P 132.
|
|
|
|
Heinrich M, Kuht M, Write CW, Rimper H, Phillipson JD, Schandelmaer A, Warhist DC (1992). Parasitology and Microbiological evaluation of Mixed Indian Medicinal Plants. J. Ethnopharmacol. 36:81-85.
Crossref
|
|
|
|
Henry RJ, Cannon DC, Winkelman JW (1974). Clinical Chemistry; Principles and techniques, Harper and Row 2nd Edn. pp. 943-949.
|
|
|
|
Kaplan LA, Szabo LL, Opherin EK (1988). Clinical Chemistry: Interpretation and techniques, 3rd edn. Leo and Febinger, Philadelphia. pp. 112-231.
|
|
|
|
Larsen HN, Snieszko SF (1961). Comparison of various methods of determination of haemoglobin in trout blood. Prog. Fish-Culturist 23(1):8-17.
Crossref
|
|
|
|
Lorke D (1983). A new approach to Practical acute toxicity testing. Arch. Toxicol. 54:275-287.
Crossref
|
|
|
|
Mhuji K, Patrick A, Chacha M (2016). Acute oral toxicity study of Mystroxylon aethiopicum root bark aqueous extract in albino mice. J. Med. Plants Res. 10(37):656-661.
Crossref
|
|
|
|
Mishra N, Tandon VL (2012). Hematological effects of aqueous extract of ornamental plants in male swiss albino mice. Vet. World 5(I):19-23.
Crossref
|
|
|
|
Olajuyugbe OO, Babalola AE, Afolabi AJ (2011). Antibacterial and Phytochemical screening of crude ethanolic extract of Waltheria indica Linn. Afr. J. Microbiol. Res. 5:3760-3764.
Crossref
|
|
|
|
Oloro J, kuguli JM, Nabirunbi R, Tanayen JK, Laurence I, Francis B, Amon AG (2016). Acute and sub - acute toxicity evaluation in rats of PPOJ5 and ADOJS herbal remedies used traditionally in the management of HIV infection. E. J. Med. Plants 16(4):1-6.
Crossref
|
|
|
|
Olowa LF, Nunez OM (2013). Brime Shrimp Lethality assay of the ethanolic extracts of selected species of medicinal plants from Iligan City, Philippines. Int. Res. J. Biol. Sci. 2(II):74-77.
|
|
|
|
Oluranti OO, Olawuyi OJ, Jonathan SG (2012). Therapeutic properties of some Nigerian higher fungi. Nat. Sci. 10(10):135-143.
|
|
|
|
Poonam S, Priyanka B, Tasleem A, Imran K, Ramir S (2014). Pharmacology, phytochemistry and safety of aphrodisiac medicinal plants: A review. Res. Rev. J. Pharmacol. Toxicol. Stud. 2:1-8.
|
|
|
|
Raza M, Al-Shabanah OA, El-Hadiya TM, Al-majeed AA (2002). Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, livers and kidneys of swiss albino mice. J. Sci. Pharm. 70(2):135-145.
|
|
|
|
Reitman S, Frankel SA (1957). A calorimetric method for determination of Serum Glutamic Oxaloacetic and Glutamic Pyruvic transaminase. Am. J. Clin. Pathol. 28:56-60.
Crossref
|
|
|
|
Shah SL (2010). Haematological changes in Tinca tinca after exposure to lethal and sub -lethal doses of mercury, cadmium and lead. Iran. J. Fish Sci. 9:434-443.
|
|
|
|
Shah SL, Altindag A (2005). Alternations of immunological parameters of tench (Tinca tinca) after acute and chronic exposure to lethal and sub lethal treatment with mercury, cadmium and lead. Turks J. Vet. Anim. Sci. 29:1163-1168.
|
|
|
|
Taiwo OE, Joel OO (2015). Acute toxicity and histopathological assessment of methanolic extract of Cleome viscosa (Linn.) whole plant. J. Med. Plant Res. 9 (11):360-369.
Crossref
|
|
|
|
Wintrobe MM, Lee GR (1967). Clinical haematology, 6th edn. Philadelpia: Library of congress, Lea and Febinger. P 28.
|
|
|
|
Yaro AH, Mohammed Z, Shok, M, Ilyas N, Musa K Y (2007). Analgesic activity of Waltheria indica. Eur. J. Sci. Res. 16(1):1-6.
|
|
|
|
Yerra KR, Shih-Hua F, Yew-Min T (2005). Anti-inflammatory activities of flavonoids isolated from Caesalpina Pulcherrima. J. Ethnopharmacol. 100(3):249-253.
Crossref
|
|
|
|
Zailani AH, Jada SM, Wurochekke UA (2010). Antimicrobial activity of Waltheria indica. J. Am. Sci. 6(12):1590-1599.
|