ABSTRACT
Invertase (β-fructofuranosidase) finds major applications in food, cosmetics, and pharmaceutical industries. In the present study, fermentation conditions for invertase production from Saccharomyces cerevisiae Angel using date syrup in shake flask were investigated. The effect of time period (0-96 h), carbon sources (sucrose, date syrup, maltose, galactose, and glucose), nitrogen sources including yeast extract, peptone, urea, meat extract, and ammonium sulphate, pH (4 to 6.5), temperature (30 to 40°C), date syrup concentration (5 to 50 g/L), and inoculum size (1 to 10% v/v) were optimized. Optimum conditions for yeast growth and invertase yield were 20 g/L date syrup, 10 g/L yeast extract, temperature of 37°C, pH 5.5, and 60 h fermentation time. In addition, effect of date syrup concentration and size of inoculum were also optimized. Highest invertase titer of ~190 U/mL were obtained when 40 g/L of date syrup and 10% v/v inoculum were used. These results show great practical potential for invertase production by saving enzyme production cost.
Key words: Saccharomyces cerevisiae Angel, invertase, date syrup, β-fructofuranosidase.
Invertase (β-fructofuranosidase, EC 3.2.1.26) breakdown sucrose and releases equimolar mixture of glucose and fructose (Alegre et al., 2009; Qureshi et al., 2012b). Invertase finds potential applications in several industries, for instance in food (for confectionery products, infant food products, and beverages), in production of artificial honey, in cosmetics as plasticizing agent, in pharmaceutical products, in paper industry, and in manufacturing of sucrose biosensors (Alegre et al., 2009; Kotwal and Shankar, 2009; Uma et al., 2010a). Sucrose hydrolysis could be performed either by conventional method, for example, acid hydrolysis that produce invert sugar but, this method has certain limitations, including lower conversion efficiency, higher energy consumption, and high production cost. However, invertase applications show 100% conversion efficiency of sucrose into glucose and fructose (Kumar et al., 2008).
Invertase are produced from several microorganisms, including Mucor geophillus (Qureshi et al., 2012b), Aspergillus caespitosus (Alegre et al., 2009), A. niveus (Guimarães et al., 2009), A. niger (Rubio and Navarro, 2006), Rhodotorula glutinis (Rubio et al., 2002), Saccharomyces cerevisiae (Ali et al., 2008). However, S. cerevisiae is considered as best microorganism for invertase production, largely due to its high efficiency for sucrose consumption (Ali et al., 2008). Therefore, S. cerevisiae Angel baker yeast was purchased from local market and its invertase production capability was determined. Invertase production from Angel yeast using date syrup as a carbon source has never been investigated. So, this research work could provide new insight in fermentation processes.
Increasing invertase production demands, high nutrients cost, and environmental pollution problems has urged to utilize agro-industrial residues as cost effective nutrients for production of invertase and other value added products. Several studies have evaluated efficiencies of various agro-industrial residues as cost effective carbon sources, for example, date syrup for pectinase production (Qureshi et al., 2012a), oilcake for invertase production (Qureshi et al., 2012b), and protease production from molasses (Qureshi and Dahot, 2009). In the present study, efficiency of date syrup as a cost effective carbon source for invertase production from Saccharomyces cerevisiae Angel was investigated. Highest invertase titer of ~190 U/mL were obtained when 40 g/L of date syrup and 10% v/v inoculum were used. These results show great practical potential for invertase production from date syrup medium at industrial scale.
Microorganism and cultivation conditions
S. cerevisiae Angel was purchased from local market, Shanghai, China. Strain was incubated in YPD medium containing 20 g/L of glucose, 20 g/L of peptone, and 10 g/L of yeast extract for 24 h at 37°C in shaking incubator. After 24 h, 10% v/v culture was transferred into seeds culture medium (sucrose 20 g/L, yeast extract 10 g/L, MgSO4×7H2O 1.0 g/L, KH2PO4 2 g/L) and incubated at 37°C for 12 h in shaking incubator. Mature seeds culture (5.0% v/v) was inoculated in the fermentation medium (sucrose 20 g/L, yeast extract 10 g/L, MgSO4×7H2O 1.0 g/L, KH2PO4 2 g/L), and culture was incubated at 37°C for 96 h in shaking incubator. Samples were collected at regular interval of 12 h, and growth (OD) was spectrophotometrically measured. Culture broth was centrifuged at 12,100 g for 10 min to collect supernatant that was used for invertase activity. All media were sterilized at 115°C for 20 min prior inoculation.
Effect of carbon and nitrogen sources
Effect of different carbon sources (20 g/L) on microbial growth and invertase production was determined by replacing sucrose in the above mentioned fermentation medium with date syrup, maltose, galactose and glucose. Culture was incubated at 37°C for 60 h. For evaluating the effect of nitrogen source, various nitrogen sources, including yeast extract, peptone, urea, meat extract, and ammonium sulphate at a concentration of 10 g/L were supplemented in synthetic medium.
Influence of initial pH and temperature
Initial pH of the enzyme production medium was adjusted in the range of 4 to 6.5 pH with 2.0 M H2SO4/2.0 M NaOH. Effect of temperature on cell growth and invertase production was checked at various temperature ranges from 30 to 40°C. Culture was incubated for 60 h at different conditions.
Effect of inoculum size and date syrup concentration
Effect of inoculum size (1-10 % v/v) was determined on invertase production. For optimizing the date syrup concentration, separate set of experiments at different date syrup concentrations (5-50 g/L) were performed under optimized cultural conditions.
Invertase assay
Invertase activity was assayed from culture supernatant collected after centrifugation 12,100 g for 10 min, according to method adapted from (Qureshi et al., 2012b). 1 mL of crude sample was taken, then 1 mL of substrate (sucrose solution prepared in 20 mM acetate buffer pH 5.5) was added. Reaction contents were mixed thoroughly and incubated at 37°C for 15 min. Then, 2 mL of dinitrosalicylic acid (DNS) reagent was added for color formation and to stop the reaction, and then reaction mixture was boiled for 5 min. The absorbance of samples was read at 540 nm against blank, according to reducing sugar determination method (Miller, 1959). Enzyme and substrate blank were prepared by replacing substrate or enzyme with 1 mL of double distilled water. One unit of invertase activity was defined as, the amount of crude enzyme required for releasing 1 µg of reducing sugar under assay conditions. All experiments were performed in triplicate and average of results are shown in figures as well in text, standard deviation was calculated in MS excel shown as error bars in all figures.
For obtaining highest possible invertase yield, cultural conditions must be optimized in terms of carbon and nitrogen sources, pH, temperature, and size and age of inoculum. There were two main reasons of this study, first was to optimize cultural conditions for obtaining the highest possible invertase titer. Second was the evaluation of cost effective carbon source for invertase production from S. cerevisiae Angel. Date syrup, a liquid containing high nutrient has been evaluated as carbon source for pectinase production (Qureshi et al., 2012a), but never used as a carbon source for invertase production. Utilization of agro-industrial waste as substrate not only reduce the enzyme production cost but also reduce the pollution problems occurring due to accumulation of waste material.
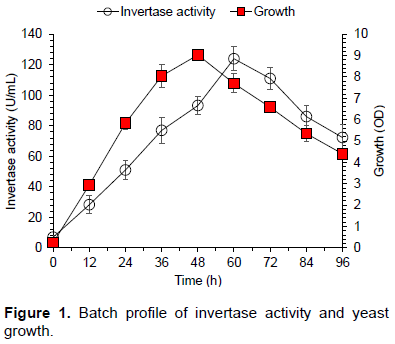
Effect of time of incubation on synthesis of invertase by S. cerevisiae Angel is shown in Figure 1. Cell growth and enzyme concentration increased with time of incubation, and reached to maximum after 60 h (124 U/mL). Similar results for invertase production are reported in literature (Ali et al., 2008). Enzyme production decreased on further incubation perhaps due to decrease of nutrients, cell death, carbon catabolite repression, and inhibitors accumulation in the fermentation medium (Ali et al., 2008; Qureshi et al., 2012b).
The effect of different carbon sources (sucrose, date syrup, maltose, galactose, and glucose) on yeast growth and enzyme production was determined; results are shown in Figure 2. S. cerevisiae Angel growth and invertase yield were highest when 20 g/L of date syrup was used as carbon source (156 U/mL) followed by sucrose (124 U/mL) and glucose (109 U/mL). Most of researchers have performed invertase production from pure sugars, that is increasing the fermentation medium cost. However, agro-industrial residue could be attractive carbon source for production of enzymes and other valuable products to solve the pollution problem and save the fermentation medium cost (Pandey et al., 2000). In this study, date syrup medium secreted highest invertase yield probably due to growth promoting substances present in date syrup (Qureshi et al., 2012a).
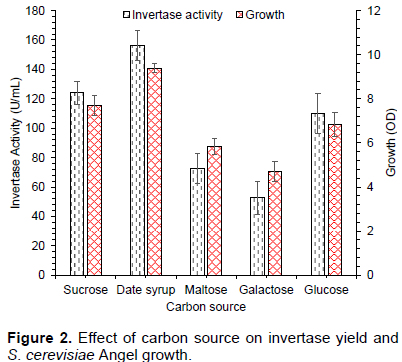
Figure 3 shows the effect of nitrogen sources (10 g/L) on S. cerevisiae growth and invertase yield. Several nitrogen sources including yeast extract, peptone, urea, meat extract, and ammonium sulphate were tested. Highest invertase yield was attained when 10 g/L of yeast extract was used. Our results were similar to results presented in literature (Qureshi et al., 2012b) for invertase production. By contrast, other researchers obtained maximal invertase yield when urea was used as nitrogen source (Ali et al., 2008).
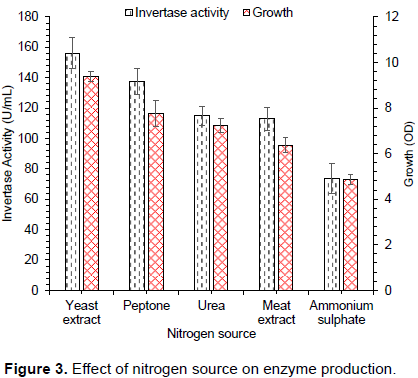
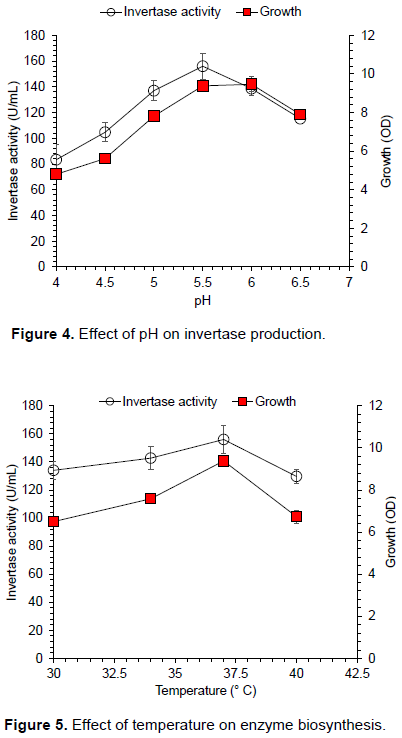
Figure 4 shows the influence of initial pH (4 to 6.5) on the invertase biosynthesis by S. cerevisiae using 20 g/L of date syrup and 10 g/L of yeast extract in mineral medium when incubated at 37°C for 60 h. Maximum growth was obtained at pH value of 6.0, whereas highest invertase yield was noted at 5.5. Yeast growth and enzyme secretion sharply decreased at pH 6.5. Although, there was no significant difference in the cell growth at the pH values of 5.5 and 6.0. Only 0.1 OD increase in cell growth was observed at pH 6 comparing to 5.5 pH. Therefore, pH 5.5 was selected for further experiments based on optimum enzyme activity and microbial growth. Our results are similar to results of (Uma et al., 2010) for invertase yield. Figure 5 shows the effect of incubation temperature on the invertase synthesis by yeast (30-40°C), when grown in fermentation medium containing 20 g/L of date syrup and 10 g/L of yeast extract, culture was incubated for 60 h at the initial pH 5.5. The maximum yield of invertase was noted at 37°C, on further increase of temperature, invertase titer decreased. Generally speaking, data presented in figure show almost similar invertase activity in the range of 30-37°C temperature with 134 U/mL (30°C) and 156 U/mL (37C°). But, looking at industrial production unit minute increase save enzyme production cost at large extent. At per milliliter there is only 22 Units difference at both temperature ranges, but this difference increases to 22,000 U/L and if production fermenter has 100 L medium than 220,000,0 U enzyme titer could be increased at 37°C operation instead of 30°C. This minor change at laboratory scale is of significant importance at commercial scale.
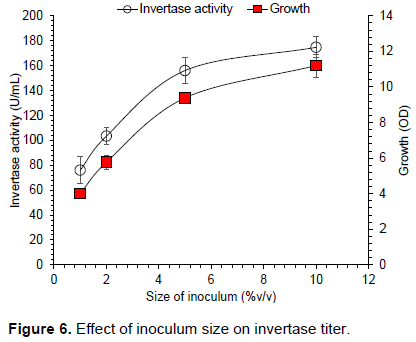
Among several factors that affect enzyme production, age and size of inoculum are of significant importance. Figure 6 shows the effect of inoculum size (1-10% v/v) on invertase production. Maximum invertase titer of 174.6 U/mL was obtained when (10% v/v) inoculum was used. Enzyme yield increased by increasing size of inoculum, this occurred due to increase of yeast growth (Figure 6). There was significant difference in enzyme activity when inoculum size was increased from 1 to 5% v/v and enzyme titer increased from 76 to 156 U/mL. However, further increase in size of inoculum from 5 to 10% v/v enhanced enzyme titer from 156 to 174 U/mL. This increase could be important achievement at commercial scale compared to laboratory stage. Previous studies have reported best enzyme titer at different size of inoculum; Ali et al. (2008) found 2% v/v to be optimal for invertase production. Gancedo (1998) obtained maximum invertase titer when 10% v/v inoculum was used. In the present study, lower invertase titer was observed when less than 10% v/v inoculum was added in the fermentation medium. This might be due to lower concentration of yeast cell that was not sufficient for higher invertase yield.
Effect of date syrup concentration (5-50 g/L) on cell growth and enzyme production was checked, results areshown in Figure 7. Invertase concentration increased withdate syrup concentration upto 40 g/L (~190 U/mL), on further increase of date syrup concentration, enzyme yield and microbial growth decreased. There was no major difference in invertase activity either of the 20 g/L (174 U/mL) or 40 g/L (189 U/mL) date syrup used. From economical point of view, 20 g/L date syrup could be applied at commercial scale invertase production from S. cerevisiae Angel. This was probably due to accumulation of inverted sugars that was responsible for catabolite repression (Ali et al., 2008).
Increasing invertase demands, high nutrient cost, and environmental pollution has compelled the utilization of agro-industrial residues as inexpensive nutrients for enzyme and other valuable products.
In the present study, cultural conditions were optimized for invertase production from S. cerevisiae Angel using date syrup in shake flask under submerged fermentation conditions. Highest invertase titer of ~190 U/mL was obtained when 40 g/L of date syrup and 10% v/v inoculum were used. These results show great practical potential for invertase production from date syrup medium at industrial scale.
The authors have not declared any conflict of interests.
REFERENCES
|
Alegre ACP, Polizeli MdLTd, Terenzi HF, Jorge JA, Guimarães LHS (2009). Production of thermostable invertases by Aspergillus caespitosus under submerged or solid state fermentation usingagroindustrial residues as carbon source. Braz. J. Microbiol. 40:612-622.
Crossref
|
|
|
|
Ali S, Aslam A, Qadeer M (2008). Characterization of a Saccharomyces cerevisiae mutant with enhanced production of β-d-fructofuranosidase. Bioresourc. Technol. 99:7-12.
Crossref
|
|
|
|
|
Gancedo JM (1998). Yeast carbon catabolite repression. Microbiol. Mol. Biol. R. 62:334-361.
|
|
|
|
|
Guimarães LHS, Somera AF, Terenzi HF, de Moraes MdLT, Jorge JA. (2009). Production of β-fructofuranosidases by Aspergillus niveus using agroindustrial residues as carbon sources: Characterization of an intracellular enzyme accumulated in the presence of glucose. Process Biochem. 44:237-241.
Crossref
|
|
|
|
|
Kotwal S, Shankar V (2009). Immobilized invertase. Biotechnol. Adv. 27:311-322.
Crossref
|
|
|
|
|
Kumar S, Chauhan VS, Nahar P (2008). Invertase embedded-PVC tubing as a flow-through reactor aimed at conversion of sucrose into inverted sugar. Enzym. Microb.Technol. 43:517-522.
Crossref
|
|
|
|
|
Miller GL (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31:426-428.
Crossref
|
|
|
|
|
Pandey A, Soccol CR, Nigam P, Brand D, Mohan R, Roussos S. (2000). Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem. Eng. J. 6:153-162.
Crossref
|
|
|
|
|
Qureshi AS, Bhutto MA, Chisti Y, Khushk I, Dahot MU, Bano S (2012a). Production of pectinase by Bacillus subtilis EFRL 01 in a date syrup medium. Afr. J. Biotechnol. 11:12563-12570.
|
|
|
|
|
Qureshi AS, Dahot MU (2009). Production of protease by Staphylococcus epidermidis EFRL 12 using cost effective substrate (molasses) as a carbon source. Pakistan J. Biotechnol. 6:55-60.
|
|
|
|
|
Qureshi AS, Khushk I, Bhutto MA, Dahot MU, Ul-Haq I, Bano S, Iqbal H (2012b). Production and partial characterization of invertase from Mucor geophillus EFRL 03. Afr. J. Biotechnol. 11:10736-10743.
|
|
|
|
|
Rubio M, Navarro A (2006). Regulation of invertase synthesis in Aspergillus niger. Enzym. Microb. Technol. 39:601-606.
Crossref
|
|
|
|
|
Rubio MaC, Runco R, Navarro AR (2002). Invertase from a strain of Rhodotorula glutinis. Phytochemistry 61:605-609.
Crossref
|
|
|
|
|
Uma C, Gomathi D, Muthulakshmi C, Gopalakrishnan V (2010a). Production, purification and characterization of invertase by Aspergillus flavus using fruit peel waste as substrate. Adv. Biol. Res. 4:31-36.
|
|
|
|
|
Uma C, Gomathi D, Muthulakshmi C, Gopalakrishnan V (2010b). Production, purification and characterization of invertase by Aspergillus flavus using fruit peel waste as substrate. Adv. Biol. Res. 4:31-36.
|
|