ABSTRACT
This study was conducted to assess the ability of sunflower (Tithonia diversifolia) inoculated with Pseudomonas species to remediate soils contaminated with heavy metals (lead and zinc). The contaminants were added to 20 kg soil in pots as lead nitrate (Pb (NO3)2) and zinc nitrate (Zn (NO3)2); 400 mg/l metal concentrations were added to 20 kg soil in pots. The experimental design was a randomized complete block design (RCBD) with the two metal types (lead, zinc) and three species of Pseudomonas replicated thrice. The results reveal that all the Pseudomonas sp. (P. putida ATCC 29352, P. cepacia ATCC 29351 and P. fluorescens F113) used showed the potential for remediating zinc and lead. However, P. fluorescens inoculated sunflower remediate zinc (Zn) the best, followed by sunflower inoculated with P. putida ATCC 29352, and sunflower inoculated with P. cepacia ATCC 29351 at 8 Weeks After Planting (WAP). The order of remediation ability of sunflower inoculated with Pseudomonas spp. in soil polluted with lead (Pb) is in the order of sunflower inoculated with P. cepacia ATCC 29351 ˃ sunflower inoculated with P. putida ATCC 29352 > sunflower inoculated with P. fluorescens F113. The shoot and root of the plant were analysed for Zn and Pb uptake after 8 weeks. The bio-concentration factor (BCF) and translocation factor (TF) assessed at 8WAP showed that translocation of Zn from root to shoot by sunflower with Pseudomonas spp., was higher than Pb. In conclusion this research suggests that sunflower inoculated with Pseudomonas spp., has phytoextraction ability and could be used to remediate soil contaminated with Zn and Pb.
Key words: Bioremediation, phytoremediation, Pseudomonas spp., heavy metals.
Increase in the use of organic and inorganic materials as soil amendments have raised the concerns about their harmful effects on the environment. Modern agricultural practices pollute the soil to a large extent. With the advancing agro-technology, huge quantities of fertilizers, pesticides and herbicides added to increase the crop yield are reported to cause soil pollution (Olanrewaju et al., 2017; Önder et al., 2011). Heavy metals belong to the types of toxic substances that have adverse effects on health. The most common heavy metals are Cd, Cr, Cu, Hg, Pb, and Zn having their atomic number greater than 20 and with metallic properties. These metals cannot be easily degraded, and the clean-up usually requires their removal (Alori, 2015).
Lead is a metal which has been associated with human activities for several decades. It is an industrial metal that has become widespread in air, water, soil, and food. These pollutants affect and alter the chemical and biological properties of soil. Dixit et al. (2015), has reported herbicides and insecticides as one of the anthropogenic sources of lead in agricultural soil. More also in Nigeria, gasoline with an average Pb content of 0.66 g/dc3 remains in use (Aransiola et al., 2013). Zinc though occurs naturally in water, air and soil, it concentrations have tremendously increased through human activities (Subhashini and Swamy, 2013).
The remediation of metal contaminated sites often involves expensive and environmentally invasive and civil engineering-based practices (Olanrewaju et al., 2017). A range of technologies such as fixation, leaching, soil excavation, and landfill of the top contaminated soil ex situ have been used for the removal of metals. Many of these methods have high maintenance costs and may cause secondary pollution or adverse effect on biological activities, soil structure, and fertility (Abioye et al., 2013). The high cost of these approaches necessitated the need for less expensive clean up techniques. Bioremediation plays a major role in cleaning of polluted or contaminated site.
Bioremediation is the correction of soil polluted or contaminated with hazardous materials using living organisms such as microorganisms and green plants. Phytoremediation is a technology that uses specialized green plants together with associated soil microbes to remove, destroy, sequester or reduce the concentration or toxic effects of contaminants in polluted environment especially soil and water (Alori, 2015). Some microorganisms have developed and adopted various detoxifying mechanisms such as, bioaccumulation, biosorption, biomineralization, and biotransformation and hence, their ability to survival in heavy metal-polluted habitats. These organisms can be exploited for bioremediation either ex situ or in situ (Lin and Lin, 2005; Malik, 2004). Examples are Staurastrum sp., Bacillus sp., Paenibacillus sp., Synechococcus sp., Saccharomyces cerevisiae, Synechococcus sp., Pseudomonas sp. and fungi such as Aspergillus sp., and Corollospora sp. (Dixit et al., 2015). Pseudomonas spp. have been reported to have the ability to remediate polluted soils (Stamenov et al., 2015).
The life cycle and luxuriant growth habits of sunflower, in particular, on soils with poor nutrient and on roadsides exposed to frequent Pb emission from automobiles necessitated the investigation of this plant for their metal accumulation potentials. Therefore, this study assessed the ability of sunflower and Pseudomonas spp. to remediate metal impacted soils.
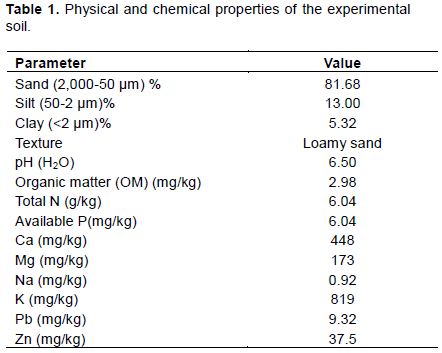
The soil sample used for this study was collected from a depth of 0-15 cm within Landmark Teaching and Research Farm, Omu-aran, Kwara State, Nigeria and transported to Crop and Soil Science Laboratory of Landmark University in plastic bags. The soil sample was air dried and sieved with 2 mm diameter mesh. The soil physical and chemical properties including particle size analysis, available phosphorus, total nitrogen, organic matter content, magnesium, calcium, potassium and soil pH were examined.
The pH of the soil samples was determined with a pH meter. Organic matter content was determined using the wet oxidation method, described by Shamshuddin et al. (1994). The hydrometer method of Gee and Or (2002), was employed in the determination of particle size. Exchangeable bases (K, Mg, Na, and Ca), were determined by ammonium acetate method of Chapman (1965). The total soil Nitrogen was determined by macroKjedahl analysis, (Bremner, 1965). The Bray1 method was used in the determination of available phosphorus, (Murphy and Riley, 1962).
The experimental soil was a loamy sand texture soil that is slightly acidic. Nutrients such as phosphorus (P), Nitrogen (N), and organic matter are available moderately available (Table 1).
Heavy metal contaminants preparation
The contaminants was added as lead nitrate (Pb (NO3)2) and zinc nitrate (Zn (NO3)2); 1.599 g of Pb (NO3)2 and 2.897 g of Zn (NO3)2 and was weighed and dissolved in 1,000 ml distilled water to make stock solutions. A 40 ml amount was measured out from the stock solutions into a 100 ml measuring cylinder and made up to mark to give 400 mg/dm3 metal concentrations. This therefore, was added to 20 kg soil in pots. The 400 mg/kg represents upper critical soil concentration for both Pb and Zn (Kabata-Pendias and Pendias, 1984).
Experimental layout
The experimental design was a Randomized Complete Block Design (RCBD) with the two metal type (lead and zinc), four species of Pseudomonas replicated thrice to make a total of 24 experimental pots. Each of the experimental pots was contaminated with the heavy metals at a concentration of 400 mg/kg. Ten seeds each of sunflowers inoculated with Pseudomonas were planted in each pot. The seeds in each pot started germinating 1 week after planting (WAP) which was monitored for 8 weeks. At 8 WAP, plants were harvested, separated into two compartments, viz. shoot and root and each compartment was then oven dried at 70°C. The soil on which plant were grown was also analysed for heavy metal content.
Determination of lead and zinc in plant material
Yusuf et al. (2003) acid digestion method was employed for the digestion of grounded plant samples. From each of this 1 g was weighed into 50 ml capacity beaker, followed by addition of 10 ml mixture of analytical grade acids: HNO3;H2SO4; HClO4 in the ratio 1:1:1. The beakers containing the samples were covered with watch glasses and left overnight. The digestion was carried out at temperature of 70°C until about 4 ml was left in the beaker. Then, a further 10 ml of the mixture of acids was added. This mixture was allowed to evaporate to a volume of about 4 ml. After cooling, the solution was filtered to remove small quantities of waxy solids and distilled water was added to make up to a final volume of 50 ml. Atomic Absorption Spectrometer (AAS) (Model number: AA320N) was used to determine the lead and zinc concentration.
Determination of Lead nd Zinc in soil
Five gram (5 g) of soil was weighed into 100 ml plastic bottle. 50 ml
of 0.1 m HCl was added and shaken for 30 min. Soil suspension was filtered. Pb, and Zn was determined AAS.
Determination of bioconcentration and translocation factor
Bioconcentration factor (BCF) and translocation factor (TF) were calculated using the formula of Yadav et al. (2009) as:
C aerial = Metal concentration in the aerial part of plant (shoot).
C root = Metal concentration in root of plant.
Statistical analysis
Data were subjected to analysis of variance using SPSS (version 21). Means were separated using Duncan multiple Range Tests at significant level of P<0.05.
Effects of sunflower inoculated with Pseudomonas spp. on the concentration of heavy metals in polluted soil at 8 weeks after planting
Figure 1 shows the concentration of Zn and lead in polluted soil remediated with sunflower inoculated with Pseudomonas spp. At 8 WAP, zinc concentration in the soil has been significantly reduced by sunflower. All the Pseudomonas sp. used showed the potential for remediation of zinc polluted soil. However, sunflower inoculated with P. cepacia ATTC 29351 remediates the best followed by sunflower inoculated with P. putida ATTC 29352, and sunflower inoculated with P. fluorescens F113 (Figure 1a).
Figure 1b shows the concentration of Pb in polluted soil remediated with sunflower inoculated with Pseudomonas spp. at 8 WAP. The concentration of Pb in the polluted soil remediated with sunflower without Pseudomonas was higher than the concentration of Pb in polluted soil remediated with sunflower inoculated with Pseudomonas sp. The degree of remediation is in the order of sunflower inoculated with P. putida ATTC 29352 > sunflower inoculated with P. fluorescen F113> sunflower inoculated with P. cepacia ATTC 29351.
The concentration of Pb and Zn in the root of T. diversifolia inoculated with Pseudomonas spp.
The concentration of Pb and Zn in the root of sunflower inoculated with Pseudomonas spp. is revealed in Figure 2. The root of sunflower without Pseudomonas spp accumulates more Pb compared with the root of sunflower inoculated with Pseudomonas spp. The concentration of Zn at the root of sunflower un-inoculated with Pseudomonas spp. was lower than the root of sunflower inoculated with Pseudomonas spp.
Bio-concentration factor (BCF) and translocation factor of metals in sunflower inoculated with Pseudomonas spp.
This study evaluated the ability of sunflower inoculated with Pseudomonas spp. to accumulate metals from contaminated soil by BCF in accordance to Yadav et al. (2009). Table 2 reveals that the highest BCF of zinc polluted soil was recorded in polluted soil remediated with sunflower inoculated with P. cepacia. The shoot (stem and leaves) of sunflower inoculated with P. fluorescens F113 had the highest TF of zinc followed by sunflower inoculated with P. putida ATTC 29352 and then sunflower inoculated with P. cepacia ATTC 29351 while sunflower not inoculated with Pseudomonas spp. had the least. In the same vain, Table 2 shows the highest BCF of lead polluted soil was recorded in polluted soil remediated with sunflower inoculated with P. putida ATTC 29352 while the least was recorded in polluted soil remediated with sunflower not inoculated with Pseudomonas spp. The highest value of TF for soil polluted with lead was recorded in polluted soil remediated with sunflower inoculated with P. putida ATTC 29352 and then followed by sunflower inoculated with P. cepacia ATTC 29351 > sunflower inoculated with P. fluorescens F113> sunflower not inoculated with Pseudomonas spp.
In soil polluted with Zn, all the treatments (sunflower inoculated with P. putida ATTC 29352, sunflower inoculated with P.cepacia ATTC 29351, sunflower inoculated with P. fluorescens F113 and sunflower not inoculated with any Pseuomonas spp) had TF > 1 (Table 2a). In Pb polluted soil, all the treatment (sunflower inoculated with P. putida ATTC 29352, sunflower inoculated with P. cepacia ATTC 29351, sunflower inoculated with P. fluorescens F113 and sunflower not inoculated with any Pseuomonas spp) had TF < 1 (Table 2b).
Effects of sunflower inoculated with Pseudomonas spp. on the concentration of heavy metals in polluted soil at 8 weeks after planting
The reduction in Zn concentration by sunflower observed is in line with the study of Adesodun et al. (2010), who stated that sunflower demonstrated accumulative potential for zinc. Stamenov et al. (2015), also reported that Pseudomonas species show potential for bioremediation. Plants, in association with microbial inoculant, can remove or transform contaminants into harmless substance (Alori et al., 2017). Lower Pb concentration in polluted soil treated with sunflower inoculated with Pseudomonas spp. implies that the Pseudomonas spp. inoculants enhanced remediation potential of sunflower in lead polluted soil. Physalis minima Linn has potential to remediate soil polluted with Zinc (Subhashini and Swamy, 2013).
The concentration of Pb and Zn in the root of sunflower inoculated with Pseudomonas spp.
The Pseudomonas spp. used have the ability to translocate Zn from root to upper part of the plant while the reverse is the case with Pb. Inoculation of sunflower with Pseudomonas spp. aid translocation of Zn but not Pb from the root. The results indicate that sunflower inoculated with Pseudomonas spp. mopped up substantial concentration of Zn to above ground biomass as higher concentration was recorded in shoot compare to concentration in root.
Bio-concentration factor (BCF) and translocation factor of metals in sunflower inoculated with Pseudomonas spp.
Sunflower inoculated with P. cepacia ATTC 29351 accumulates more zinc than the other treatments.
Translocation factor which reveals the measure of the ability of plants to transfer accumulated metals from the roots to the shoots is given by the ratio of concentration of metal in the shoot to that in the roots (Cui et al., 2004). This result agrees with those of other researcher such as Aransiola et al. (2013) which shows that plant species may effectively and selectively act as accumulators and indicators.
This study assumed that plants with BCF value > 1 are accumulators while plants with BCF <1 are excluders (Aransiola, 2013). The results in this study show that remediating zinc polluted soil with sunflower inoculated P. putida ATTC 29352 and sunflower inoculated with P. fluorescens F113 had BCF values > 1, indicating that this plant when inoculated with these species of Pseudomonas, have the potential to be used as accumulators of lead while sunflower inoculated with P. cepacia ATTC 29351 had BCF < 1 for Pb and sunflower inoculated with P. putida ATTC 29352, sunflower inoculated with P. fluorescens F113 and sunflower inoculated with P. cepacia ATTC 29351 had BCF < 1 for Zn. According to Usman and Mohamed (2009), the success of phytoextraction process depends on heavy metal removal by the shoots. It is therefore suggested that plant species having the higher metal concentration in its shoots than in its roots can be considered as accumulator for phytoremediation. However, since P. cepacia had BCF < 1 for Pb and sunflower inoculated with P. putida ATTC 29352, sunflower inoculated with P. fluorescens F113 and sunflower inoculated with P. cepacia ATTC 29351 had BCF < 1 for Zn, they could also be an excluder in phytoremediation processes. This is in line with the report by Aransiola et al. (2013). Ma et al. (2001) classified plants with TF value > 1 as high efficiency plants for metal translocation from the roots to the shoots. Both sunflower and sunflower inoculated with these Pseudomonas spp. could be classified as high efficient plant for Zn translocation from roots to the above ground parts of the plant, hence could be good phytoremediators for Zn polluted soil as suggested by Wei and Chen (2006). However, sunflower stored the metal removed from the soil in the root. Ma et al. (2001) and Srivastava et al. 2006) explained that one of the factors that determine the efficacy of phytoextraction in metal contaminated soil is root uptake.
This study demonstrated that Pseudomonas spp. enhance bioremediation potential of sunflower and that the combination can be used as bioremediator in soil polluted with zinc and lead. Sunflower inoculated with P. cepacia ATTC 29351 best remediates soil polluted with Zn, whereas that inoculated with P. putida ATTC 29352 performed significantly better than the rest in soil polluted with Pb. It also recorded that sunflower inoculated with Pseudomonas spp. accumulates more zinc in the shoot and more lead in the root.
The authors have not declared any conflict of interests.
REFERENCES
|
Abioye OP, Ijah UJJ, Aransiola SA (2013). Remediation mechanisms of tropical plants for lead-contaminated environment, in: Gupta DK (Ed.), Plant-Based Remediation Processes, Soil Biology, Springer, Berlin, Germany pp. 59-77.
Crossref
|
|
|
|
Adesodun JK, Atayese MO, Agbaje TA, Osadiaye BA, Mafe OF, Soretire AA (2010). Phytoremediation potentials of Sunflowers (Tithonia diversifolia and Helianthus annuus) for metals in Soils Contaminated with Zinc and Lead Nitrates. Water Air Soil Pollution 207:195-201
Crossref
|
|
|
|
|
Alori ET (2015). Phytoremediation Using Microbial Community, In: A. A. Ansari, et al. (Eds.), Phytoremediation: Management of Environmental Contaminants, Springer International Publishing, Switzerland pp. 183-190.
|
|
|
|
|
Alori ET, Dare MO, Babalola OO (2017). Microbial inoculants for soil quality and plant fitness, in: Lichtfouse E. (Ed.), Sustainable Agriculture Review, springer pp. 181-308.
Crossref
|
|
|
|
|
Aransiola SA, Ijah UJJ, Abioye OP (2013). Phytoremediation of Lead Polluted Soil by Glycine max L. Applied and Environmental Soil Science pp. 1-7
Crossref
|
|
|
|
|
Bremner JM (1965). Total Nitrogen, in: C. A. Black (Ed.), Methods of soil analysis part 2, American Society of Agronomy Inc, Madison, Wisconsin, USA. pp. 1149-1178.
|
|
|
|
|
Chapman HD (1965). Cation exchange capacity, in: Black CA (Ed.), Methods of soil analysis part 2, American Society of Agronomy Inc, Madison Wisconsin USA. pp. 891-901.
|
|
|
|
|
Cui Y-J, Zhu Y-G, Zhaietal R-H (2004). Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environment International 30(6):785-791.
Crossref
|
|
|
|
|
Dixit R, Wasiullah, Malaviya D, Pandiyan K, Singh UB, Sahu A, Shukla R, Singh BP, Rai JP, Sharma PK, Lade H, Paul D (2015). Bioremediation of Heavy Metals from Soil and Aquatic Environment: An Overview of Principles and Criteria of Fundamental Processes Sustainability 7:2189-2212.
|
|
|
|
|
Gee WG, Or D (2002). Particle size analysis, in: J. Dane and G. C. Topp (Eds.), Methods of soil Analysis, Soil Science Society of America pp. 253-293.
|
|
|
|
|
Kabata-Pendias A, Pendias H (1984). Trace elements in soil and plants. Boca:CRC.
|
|
|
|
|
Lin CC, Lin HL (2005) Remediation of soil contaminated with the heavy metal (Cd2+). Journal Hazard Materials 122:7-15.
Crossref
|
|
|
|
|
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED (2001). A fern that accumulates arsenic. Nature 409:579
Crossref
|
|
|
|
|
Malik A (2004) Metal bioremediation through growing cells. Environment International 30:261-278.
Crossref
|
|
|
|
|
Murphy J, Riley JP (1962). Amodified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta. 27:31-36.
Crossref
|
|
|
|
|
Olanrewaju OS, Glick BR, Babalola OO (2017). Mechanisms of action of plant growth promoting bacteria. World Journal of Microbiology and Biotechnology 33:197.
Crossref
|
|
|
|
|
Önder M, Ceyhan E, Kahraman A (2011). Effects of Agricultural Practices on Environment International Conference on Biology, Environment and Chemistry, IACSIT Press, Singapoore. pp. 28-32.
|
|
|
|
|
Shamshuddin J, Jamilah I, Ogunwale JA (1994). Organic carbon determination in acid sulphate soils. Pertanika 17:197-200.
|
|
|
|
|
Srivastava M, Ma LQ, Santos JAG (2006). Three new arsenic hyperaccumulating ferns. Sci. Total Environ 364:24-31.
Crossref
|
|
|
|
|
Stamenov DR, Äurić SS, Hajnal-Jafari TI (2015). Bioremediation potential of five strains of Pseudomonas sp. Zbornik Matice srpske za prirodne nauke 128:41-46.
|
|
|
|
|
Subhashini V, Swamy AVVS (2013). Phytoremediation of Zinc Contaminated Soils by Physalis minima Linn. International Journal of Innovative Research in Science Engineering and Technology 2:4488-4492.
|
|
|
|
|
Usman ARA, Mohamed HM (2009). Effect of microbial inoculation and EDTA on the uptake and translocation of heavy metal by corn and sunflower. Chemosphere 76(7):893-899.
Crossref
|
|
|
|
|
Wei C-Y, Chen T-B (2006). Arsenic accumulation by two brake ferns growing on an arsenic mine and their potential in phytoremediation. Chemosphere 63:1048-1053.
Crossref
|
|
|
|
|
Yadav SK, Juwarkar AA, Kumar GP, Thawale PR, Singh SK, Chakrabarti T (2009). Bioaccumulation and phytotranslocation of arsenic, chromiumand zinc by Jatropha curcas L: impact of dairy sludge and biofertilizer. Bioresource Technology 100:4616-4622.
Crossref
|
|
|
|
|
Yusuf AA, Arowolo TA, Bamgbose O (2003). Cadmium, copper and nickel levels in vegetables from industrial and residential areas of Lagos City, Nigeria. Food and Chemical Toxicology 41(3):375-378.
Crossref
|
|