ABSTRACT
The Lablab bean (Lablab purpureus) has potential of being an outstanding resource for human food and animal feed in tropical agricultural systems. The bean is however grossly underutilized due to anti-nutritional factors, which may affect its nutritive value and organoleptic properties. In this study, twenty-four (24) lablab bean accessions were assayed for sensory flavor characteristics and volatile compounds to identify acceptable selections for adoption and incorporation into a rationalized breeding program. Sensory tests were carried out by a panel of 11 trained evaluators. Volatile compounds were extracted using hexane and separated using gas chromatography. Sensory tests showed significant differences for the bitter taste (p≤0.05), with accession 10706 showing the highest odour and bitter taste levels, while accession 13096 had the lowest. Two hundred and sixty two (262) volatile compounds were identified and grouped into 12 classes. The major compounds were esters (46), terpenes and terpenoids (59), hydrocarbons (57), and alcohols (28). The retention times of the volatile compounds revealed an overall 89% similarity of the lablab bean accessions. Accessions showing lower levels of bitter taste are recommended for inclusion in the participatory evaluation stage of the breeding process.
Key words: Lablab purpureus, odour, flavour, taste, volatile compounds, underutilized crops.
Lablab, Lablab purpureus (L. Sweet) is a legume species that grows in the tropic and the subtropical regions of Asia and Africa. It grows in a diverse range of environments and is drought tolerant (Maass et al., 2010; Ravinaik et al., 2015). The species however has remained a minor crop in most regions where it is grown (Engle and Altoveris, 2000; Maass et al., 2010). Lablab is cultivated as a cover crop since its dense green cover
reduces soil erosion by wind or rain (Mureithi et al., 2003). It has multiple uses as human food and fodder crop for livestock (Maass et al., 2010). As human food, it is eaten as green pods, mature seeds and its leaves are used as vegetables. In spite of these qualities, lablab has not been utilised extensively.
Lablab has the potential of being an outstanding resource for tropical agricultural systems and in improving human food and animal feed as a vegetable, pulse and/or forage crop (Pengelly and Lisson, 2003). The crop has high protein quality (Mortuza and Tzen, 2009). Like other legumes, it is however reported to contain anti-nutritional factors, which may affect its nutritive value and organoleptic properties. Among the important characteristics considered in selecting dry bean varieties for production and consumption is good flavour quality (Scott and Maiden, 1998), where flavour comprises of odour and taste. In India, specific lablab cultivars are preferred and valued for their nutritional and sensory attributes (Venkatachalam and Sathe, 2007). Some dark seeded types of lablab beans have been reported to have a bitter taste that may persist even after prolonged cooking (Wanjekeche et al., 2000). Factors that have been reported to contribute to bitter taste in beans include cyanogenic glycosides (Seigler et al., 1989), polyphenols such as tannins (Bressani and Elias, 1980), minerals for example iron (Yang and Lawless, 2005); saponins (Heng et al., 2004; Shi et al., 2004) and the malliard reaction (Martins et al., 2001).
The volatile components of cooked lablab beans have particularly been associated with odour which may affect the beans overall acceptance by consumers (Kim and Chung, 2008). Seed colour and quality of lablab bean are also major selection criteria in breeding programs, in conjunction with other minor criteria, particularly where human consumption is being considered (Pengelly and Maass, 2001, Spence et al., 2010). However, there has been limited study on the quality aspects of the lablab bean. This study therefore aimed to determine the sensory characteristics and the volatile compounds of lablab bean accessions from Kenya.
Twenty-two (22) accessions of L. purpureus from the Kenyan National Gene Bank and Repository Centre at the Kenya Agricultural and Livestock Research Organization (KALRO-Muguga, Kenya) and two varieties from farmers’ fields were used in the analysis of volatile compounds and sensory tests (Table 1). The accessions were bulked at the KALRO field, Njoro, Kenya (0° 20'S; 35° 56'E; 2166 m above sea level (asl)). The site receives an annual rainfall of about 960 mm with average maximum and minimum temperatures of 24 and 8°C with a mean of 14.9°C. The soils at this site are well drained, deep to very deep, dark reddish brown, friable and smeary, silt clay, with humic topsoil classified as mollic andosols (Jaetzold and Schmidt, 1983). The dry seeds were harvested, cleaned and dusted with insecticide (actellic super powder) and stored at room temperature. Prior to use, the seeds were washed and air-dried to remove the insecticide before the tests were carried out.
Sensory evaluation
A panel of 11-trained evaluators tasted the dry cooked seeds from the lablab bean accessions. The samples were boiled in distilled water until cooked and five seeds from each sample were served to each panellist while warm (about 40°C) in identical containers. A complete random design (CRD) was used, with three tasting replicates, and the coded samples randomly presented. The sensory characteristics were evaluated through qualitative descriptive analyses where the bitter taste intensity of the accessions was ordered using five descriptive terms; trace, slightly intense, moderately intense, very intense and extremely intense; while the odour intensity was assayed by using a vertical mark on a 15 cm line scale as described by Quirien and Keith (2005).
For analysis of category scale for the bitter taste data, the categories were converted to numerical scores by assigning successive numbers to each category; 0 was assigned to the trace and 5 to extremely intense. For analysis of line scale odour intensity data, panelists’ marks were converted to numerical scores by measuring the distance in centimetres from the left or lowest intensity point on the scale to the panelists’ mark. The scores were converted using 0.5 cm = 1 unit score as suggested by Quirien and Keith (2005).
Analysis of volatile compounds
Extraction of volatile compounds
Dry lablab seeds of the 24 accessions were ground into fine powder using a mortar and pestle. The mortar and pestle were washed and sterilised between samples. The volatile compounds were then cold extracted from the powder using gas chromatography (GC) grade hexane (BDH, England) (Mestres et al., 2000). Fifty grams (50 g) of ground seed powder was put into a separating funnel and to it added 100 ml of hexane. The solution was mixed thoroughly before filtering into a 250 ml conical flask through a filter paper (Whatman 1, diameter 125 mm). The extracts were then concentrated by evaporating the hexane using a rotavapor (BÜCHI Rotavapor R-205 Labortechnik GmbH, Essen, Germany). The concentrated sample was poured into a 5 ml sample bottle and allowed to dry through evaporation at room temperature (in the dark). The samples were stored at -20°C before gas chromatography (GC) analysis.
Gas chromatography
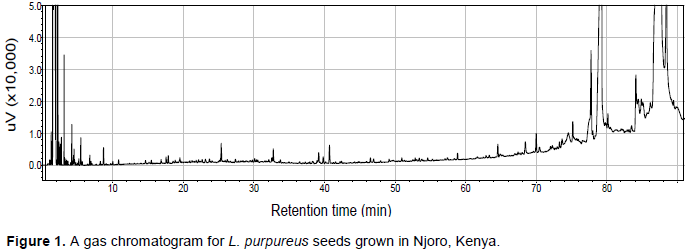
One hundred microliters (100 µl) of hexane were added to the sample bottle the night before GC analysis to dissolve the extracts. Five microliters of the sample was injected into the gas chromatography system (Shimadzu GC Model GC2010, Tokyo, Japan) which was fitted with a 30-m fused silica open-tubular column (ZB-5, 0.25 mm i.d., 0.25 mm film thickness, Phenomenex) with phase composition of 5% phenyl and 95% dimethylpolysiloxane. The following were the operating conditions: Initial and final temperatures with the holding times of 32°C for 5 min and 195°C for 5 min, respectively; the ramp rate was 2°C/min. Flame ionisation detector (FID) was used at 250°C and injector temperature was 220°C. The volatile compounds were identified from the chromatogram (Figure 1) obtained from each sample by comparing the peak retention (kovats) indices with those found in available literature (Adams, 1995) and online database, Pherobase (El-Sayed, 2005) as discussed by Babushok et al. (2007).
Data analysis
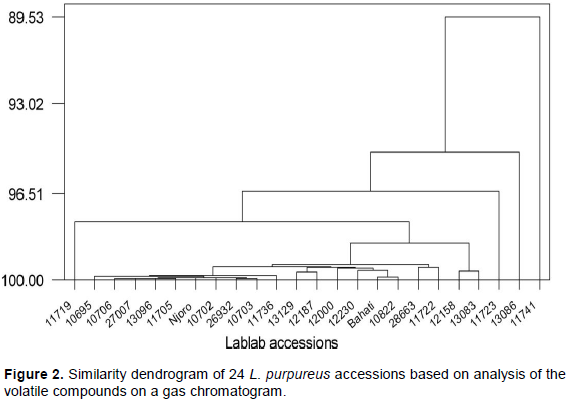
Analysis of variance (ANOVA) was done using general linear model (GLM) procedure using the SAS software Version 8.1 (SAS Institute, Inc., 2000) to determine the differences among the sensory characteristics and among the retention times of the volatile constituents. Means were separated using Fisher’s least significant difference (LSD) test. Correlation coefficients were determined to establish the relation between the quality variables (Bower, 2000). Pairwise similarity levels of the lablab accessions were calculated based on the retention times of the major peak areas of the volatile compounds and used to derive a dendrogram using MINITAB 11.12 statistical analysis software (MINITAB Inc, State College, Pennsylvania, USA, 1996).
Sensory evaluation
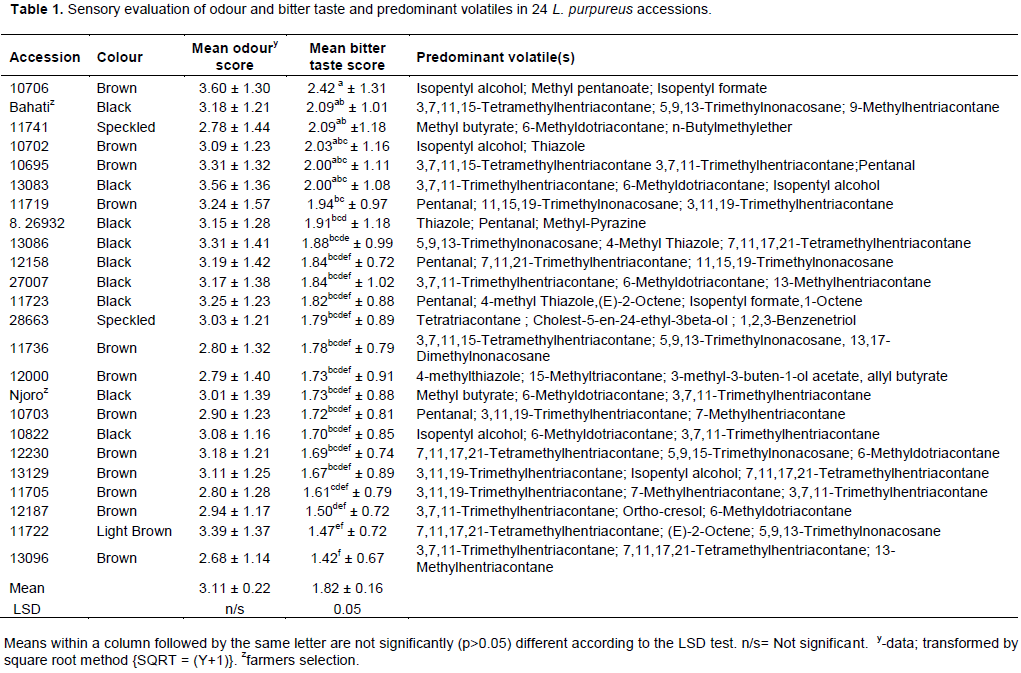
The odour score ranged from 2.68 to 3.60, with a mean of 3.12 while the score for bitterness ranged from 1.42 to 2.42, with a mean of 1.82 (Table 1). There was no significant difference (p>0.05) in the odour of the 24 samples, though a significant difference was observed (p≤0.05) for the bitter taste parameter. Accession 10706 exhibited the highest means for both bitter and odour taste, while accession 13096 had the least means for the same attributes. Significant positive correlation was observed between odour intensity and bitter taste of the 24 L. purpureus accessions (r = 0.510, p≤0.05) and an insignificant positive correlation between the colour of the seed and the odour intensity (r=0.046, p>0.05) and bitter taste (r=0.027, p>0.05). The black, brown and speckled (dark coloured seeds with black spots) coloured accessions had varying intensities (high, medium and low) of both odour and taste (Table 1), showing no relationship of the level of bitterness and odour intensity with colour of seed. Both accessions that had the highest and lowest intensity for odour and bitter taste, 10706 and 13096, were brown in colour.
Identification of volatile compounds in L. purpureus accessions
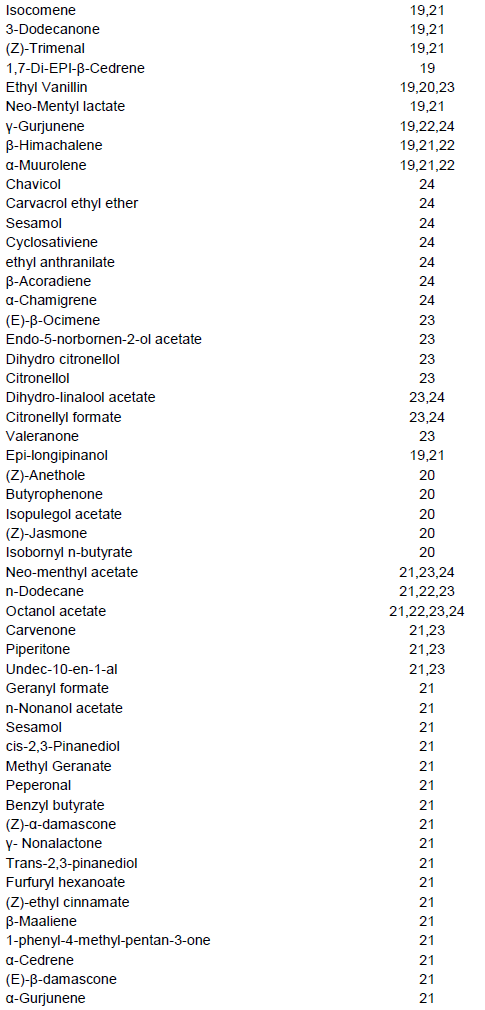
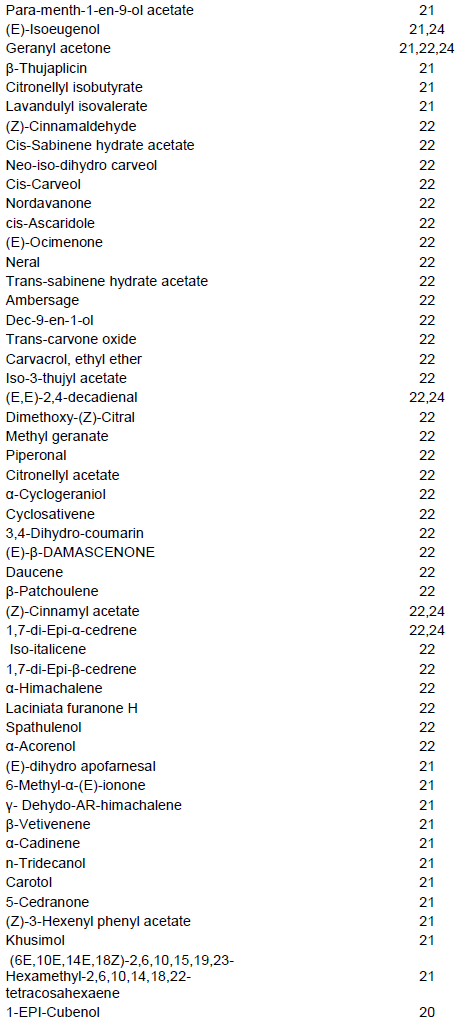
The volatile compounds in the assayed L. purpureus accessions were easily identified from the gas chromato-gram. A typical chromatogram from one of the assayed accessions is presented in Figure 1. The most common volatile constituents identified were terpenes and terpenoids, and their derivatives, which accounted for 46% of all the detected odour compounds (Table 1). A total of 262 major compounds were identified from the peaks with area measurement of above 100,000 in all the accessions (Table 2). The detected compounds were separated into 12 classes, namely alcohols (28), aldehydes (10), ketones (19), esters (46), acids (7), oxygen heterocycles (1), pyrazines (5), thiazoles (4), hydrocarbons (57), terpenes and terpenoids (59), phenols (5) and miscellaneous compounds. The most common compounds with characteristic odour description included; isopentyl alcohol (associated with fusel, alcoholic, pungent, etherial, fruity odour), 3,7,11-trime-thylhentriacontane, (E)-2-Octene, 7,11,17,21-tetramethyl-hentriacontane/7,11,17,25-tetramethylhentriacontane, 6-methyldotriacontane, norbornene, pentanol, 4-methyl thiazole (ripe, nutty, vegetable, tomato with a green tropical nuance odour), 5,9,13-trimethylnonacosane/ 5,9,15-trimethylnonacosane/5,9,19-trimethylnonacosane, methyl butyrate (pungent, etherial, fruity, perfumey and fusel with a fermented, cultured, creamy undernote odour), isopentyl formate (plum, dry earthy fruit green odour), 13,17-dimethylnonacosane, 13-methylhentriacontane, 9-methylhentriacontane, 7-methylhentriacontane, santene, heptanal/n-nonane, 5-methylnonacosane, 5-methylhentriacontane, 3,11,19-trimethylhentriacontane, 3,7-dimethylhentriacontane (Table 2) Some volatile compounds were unique to only one accession (Table 2). For example, 37 volatile compounds were unique to accession 28663. The following accessions also had volatile compounds that were unique to them: Bahati, 11741, 11719, 26932, 13086, 12158, 11736, 12000, Njoro, 12230, 13129, 11705, 12187, 11722 and 13096.



The utilization of the lablab bean for human consumption has been hampered by anti-nutritional factors that have impact on bean flavour besides the undesirable food colouring effect of the black variety (Waldmueller, 1992; Wanjekeche et al., 2000). In this study, it was established that the assayed lablab accessions had different bitter taste levels, with some being bitterer than others, similar to results obtained by Wanjekeche et al. (2000). Studies have shown that lablab accessions are highly diverse in most agro-morphological traits with diversity being greater in South East Asia than in Africa (Maass et al., 2010; Ravinaik et al., 2015). Based on the scores for bitter taste, the following accessions are recommended for inclusion in a rationalised breeding programme; 13096, 11722, 12187 and 11705. This study also established that the colour of the seeds did not have any relationship with the bitter taste and odour of the bean, with the accessions with highest and lowest bitter taste scores having a brown colour. This was contrary to findings from other studies that had shown a close relationship between intensity of seed colour and odour (Khah and Arvanitoyannis, 2003). This study however revealed a relationship between taste and odour (r=0.8). The correlation between the two quality parameters in this study is not unexpected and may be explained by the fact that both contribute to food flavour (Nursten, 1977). However, it should be noted that the correlation is not very strong, and further analysis on more accessions may need to be carried out to confirm the relationship.
Though the volatile profiles of the assayed accessions were different, there was a high level of similarity between accessions indicating a narrow genetic base for the lablab germplasm in Kenya. Analysis of molecular diversity of a larger gene pool of lablab accessions including those in this study had also revealed low genetic diversity of the accessions (Kimani et al., 2012). These findings suggest that any crop improvement efforts in Kenya are likely to be compromised by the low genetic diversity of progenitors in hybridization programmes and meaningful genetic gains can only be realised through deliberate germplasm enrichment programmes. Germplasm can be introduced into Kenya from South East Asia and other parts of Africa where some of the wild and domesticated lablab landraces have been established to be genetically diverse (Maass et al., 2005; Tefera, 2006). Some wild and undomesticated lablab can still be found to occur naturally in several African countries and these can be a rich source of genes for crop improvement (Verdcourt, 1979).
Data from the present study revealed that the volatile compounds identified in assayed accessions were slightly different from those identified in other studies. The compounds identified that were common in this study and that of Kim and Chung (2008) on lablab include pentanal, geranylacetone, heptanal, pentanol and (Z)-3-hexenol.
Differences in volatile compounds between the two studies may be due to the use of different extraction methodologies, growing conditions and the genetic differences of the accessions in the two studies. The compound 2,3,5-trimethylpyrazine that was also identified in the Kenyan lablab accessions is thought to be the characteristic compound in the fermented bean aroma (Seo et al., 1996) and has also been identified in soypaste (Zhang et al., 2010). (Z)-3-Hexenol was identified in French beans (Hinterholzer et al., 1998) using gas chromatography/olfactometry (GC/O). Among the compounds identified in this study, 2,3-butanediol, heptanal, pentanol and pentanal were found to be present in the volatile isolate of kidney beans and soybean (van Ruth et al., 2004, 2005) isolated in a model mouth system and sampling of the headspace (van Ruth and Roozen, 2000). The presence of the odd numbered long alkanes identified in this study unlike in the other studies is due to the fact that the alkanes have poor volatility in steam (Radulovic et al., 2006) used in extraction methods. Some of the n-alkanes identified in this study such as n-docosane, hentriacontane, triacontane and tetracontane have also been identified in vanilla beans (Ramaroson-Raonizafinimanana et al., 1997). Pentanal, geranylacetone, heptanal, n-nonane, n-decane and n-docecane were also extracted and identified from dry beans (Phaseolus vulgaris), isolated by headspace solid phase microextraction (HS-SPME) (Oomah et al., 2007). Worth noting in this study is the accessions 11741 and 11719 that stood out distinctly in the dendrogram. These two accessions were most preferred by farmers, and ranked high in taste in a study carried out on sensory and cooking quality of lablab (Shivachi et al., 2012). The predominant volatile compounds in the two accessions are however different, indicating that their preference is attributable to different volatiles.
Apart from the volatile compounds assayed in this study, there are other major biochemical components of beans that would affect flavour but which were not evaluated here. These include lipids, carbohydrates, proteins, saponins, minerals and phenolic acids. Further, it should be noted that less than 5% of all the volatiles identified in foods have actually been found to contribute to the odour (Grosch, 2000). The compounds identified in this study are therefore not exhaustive and the actual volatiles that influence the characteristic odour of lablab may be identified by more sensitive quantitative measure-ments and more systematic sensory experiments of a large sample of lablab beans grown in different environments. The analysis carried out in this study is however important in the identification of the major volatiles that need to be assayed in more detailed studies. This is important because the flavour produced by the volatiles, which contribute to odour, may either be desirable or undesirable to consumers who are not familiar with the lablab. Volatiles associated with desirable flavour should be identified and positively selected for, while those associated with non-desirable flavour should be selected against.
The authors have not declared any conflict of interests.
REFERENCES
|
Adams RP (1995). Identification of essential oil components by gas-chromatography/mass spectroscopy. Carol Stream, USA: Allured Publishing Corp.
|
|
|
|
Babushok VI, Linstrom PJ, Reed JJ, Zenkevich IG, Brown RL, Mallard WG, Stein SE (2007). Development of a database of gas chromatographic retention properties of organic compounds. Journal of Chromatography A 1157:414-421.
Crossref
|
|
|
|
|
Bower JA (2000). Statistics for Food Science - Correlation and regression (part B). Journal of Nutrition and Food Sciences 2:80-85.
Crossref
|
|
|
|
|
El-Sayed AM (2005). The Pherobase: Database of Insect Pheromones and Semiochemicals. Lincoln, New Zealand.
|
|
|
|
|
Engle LM, Altoveris NC (eds) (2000). Collection, conservation and utilization of indigenous vegetables. In: Proceedings of a workshop, AVRDC, Shanhua, Tainan, Taiwan, 16-18 August 1999, Asian Vegetable Research and Dvlpt Centre (AVRDC), Shanhus, Taiwan, ROC, 142 p.
|
|
|
|
|
Grosch W (2000). Specificity of the human nose in perceiving food odorants. In: Schieberle P, Engel KH (eds), Frontiers of Flavour Science, Proceedings of the Ninth Weurman Flavour Research Symposium. Deutshe Forchungsanstalt für Lebensmittelchemie, Garching. pp. 213-219.
|
|
|
|
|
Hinterholzer A, Lemos T, Schieberle P (1998). Identification of the key odorants in raw French beans and changes during cooking. Z Lebensm Unters Forsch 207:219-222.
Crossref
|
|
|
|
|
Jaetzold R, Schmidt H, (1983). Farm Management Handbook of Kenya, Vol IIB, Central Kenya (Rift Valley and Central Provinces). Ministry of Agriculture in Cooperation with the German Agric. Team (GAT) of the German Agency for Technical Cooperation (GTZ). Typo-druck, Rossdorf, W. Germany. pp. 381-416.
|
|
|
|
|
Khah EM, Arvanitoyannis IS (2003). Yield, nutrient content and physico-chemical and organoleptic properties in green bean are affected by N:K ratios. Journal of Food, Agriculture and Environment 1(3&4):17-26.
|
|
|
|
|
Kim JS, Chung HY (2008). Characterization of volatile components in Field bean (Lablab lablab) obtained by simultaneous steam distillation and solvent extraction. Journal of Nutrition and Food Sciences 13:18-22.
Crossref
|
|
|
|
|
Kimani E, Wachira FN, Kinyua MG (2012). Molecular diversity of Kenyan Lablab bean (Lablab purpureus (L.) Sweet) accessions using Amplified Fragment Length Polymorphism markers. American Journal of Plant Sciences3:313-321.
Crossref
|
|
|
|
|
Maass BL, Jamnadass RH, Hanson J, Pengelly BC (2005). Determining sources of diversity in cultivated and wild lablab purpureus related to provenance of germplasm using amplified fragment length polymorphism (AFLP). Genetic Resources and Crop Evolution 52:683-695.
Crossref
|
|
|
|
|
Maass BL, Knox MR, Venkatesha SC, Angessa TT, Ramme S, Pengelly BC (2010). Lablab purpureus - A crop lost for Africa? Tropical Plant Biology 3(3):123-135.
Crossref
|
|
|
|
|
Mestres M, Busto O, Guasch J (2000). Analysis of organic sulfur compounds in wine odour. Journal of Chromatography A 881:569-581.
Crossref
|
|
|
|
|
Mortuza MG, Tzen JTC (2009). Physicochemical and functional properties of ten cultivars of seem (Lablab purpureus L.), an underexploited bean in Bangladesh. Journal of the Science of Food and Agriculture 89(8):1277-1283.
Crossref
|
|
|
|
|
Mureithi JG, Gachene CKK, Wamuongo JLW (2003). Legume cover crops research in Kenya: Experiences of the legume research network project. KARI Technical Note Series. No. 12.
|
|
|
|
|
Nursten HE (1977). The important volatile flavour components of foods. In: Birch GG, Brennan JG, Parker KJ (eds). Sensory Properties of
|
|
|
|
|
Foods Applied Science Publishers Ltd. London, UK pp 151-167.
|
|
|
|
|
Oomah BD, Liang LSY, Balasubramanian P, (2007). Volatile Compounds of Dry Beans (Phaseolus vulgaris L.). Plant Foods for Human Nutrition 62:177-183.
Crossref
|
|
|
|
|
Pengelly BC, Maass BL (2001). Lablab purpureus (L.) Sweet- diversity, potential use and determination of a core collection of this multi-purpose tropical legume. Genetic Resources and Crop Evolution 48:261-272.
Crossref
|
|
|
|
|
Pengelly BC, Lisson SN, (2003). Strategies for using improved forages to enhance production in Bali cattle. In: Entwistle K & Lindsay DR (eds). Strategies to improve Bali cattle in eastern Indonesia. Proceedings of a workshop 4-7 February 2002, Bali, Indonesia. ACIAR Proceedings no. 110. Australian Centre for International Agricultural Research (ACIAR), Canberra, Australia. pp. 29-33.
|
|
|
|
|
Quirien van O, Keith T (2005). Sensory evaluation techniques In: Methods in Development Research: Combining Qualitative and Quantitative Approaches (eds) Campbell J. R., Holland J. Kent, Great Britain. pp. 107-118
|
|
|
|
|
Radulovic N, Stojanovic G, Palic R, Alagic S (2006). Chemical composition of the ether and ethyl acetate extracts of serbian selected tobacco types: Yaka, Prilep and Otlja. Journal of Essential Oil Research 18(5):562-565.
Crossref
|
|
|
|
|
Ramaroson-Raonizafinimanana B, Gaydou ÉM, Bombarda I (1997). Hydrocarbons from three vanilla bean species: V. fragrans, V.madagascariensis, and V. tahitensis. Journal of Agricultural and Food Chemistry 45:2542-2545.
Crossref
|
|
|
|
|
Ravinaik K, Hanchnamani CN, Patil MG, Imamsaheb SJ (2015). Evaluation of dolichos genotypes (Dolichos lablab L.) under North eastern dry zone of Karnataka. The Asian Journal of Horticulture 10:49-52.
Crossref
|
|
|
|
|
SAS version 8.1 (2000) SAS Institute Inc., Cary, NC, USA.
|
|
|
|
|
Scott J, Maiden M (1998). Socio-economic survey of three bean growing areas in Malawi. Occasional paper series No. 24, CIAT, Kampala, Uganda.
|
|
|
|
|
Seo J, Chang H, Ji W, Lee E, Choi M, Kim H, Kim J (1996). Aroma components of traditional Korean soy sauce and soybean paste fermented with the Same Meju. Journal of Microbiology and Biotechnology 6: 278-285.
|
|
|
|
|
Shivachi A, Kinyua MG, Kiplagat KO, Kimurto PK, Towett BK (2012). Cooking time and sensory evaluation of selected Dolichos (Lablab purpureus) genotypes. African Journal of Food Science and Technology 3(7): 155-159.
|
|
|
|
|
Spence C, Levitan C, Shankar MU, Zampini M (2010). Does food color influence taste and flavor perception in humans? Chemosensory Perception 3:68-84.
Crossref
|
|
|
|
|
Tefera A (2006). Towards improved vegetable use and conservation of cowpea (Vigna unguiculata) and lablab (Lablab purpureus): Agronomic and participatory evaluation in northeastern Tanzania and genetic diversity study. Goettingen: Cuvillier Velag 2006.
|
|
|
|
|
van Ruth SM, Roozen JP (2000). Influence of mastication and artificial saliva on odour release in a model mouth system. Food Chemistry 71:339-345.
Crossref
|
|
|
|
|
van Ruth SM, Dings L, Buhr K, Posthumus MA (2004). In vitro and in vivo volatile flavour analysis of red kidney beans by proton transfer reaction-mass spectrometry. Food Research International 37:785-791.
Crossref
|
|
|
|
|
van Ruth SM, Dings L, Aprea E, Odake S (2005).Comparison of volatile flavour profiles of Kidney beans and soybeans by GC-MS and PTR-MS. Food Science and Technology Research 11(1):63-70.
Crossref
|
|
|
|
|
Venkatachalam M, Sathe SK (2007). Val bean (Lablab purpureus L.) proteins: Composition and biochemical properties. Journal of the Science of Food and Agriculture 87: 1539-1549.
Crossref
|
|
|
|
|
Verdcourt B (1979). Lablab. In: A manual of new Guinea legumes. Botanical Bulletin 11:537.
|
|
|
|
|
Waldmueller LF (1992). Lablab purpureus In: Agricultural Extension Handbook for Lamu District, Kenya. Lamu pp. 145-146.
|
|
|
|
|
Wanjekeche E, Mwangi J, Kamidi M, Powon P, Khaemba J (2000). Farmer participation in the evaluation of grain legumes in North-Western Kenya. In: KARI. Participatory technology development for soil management by small holders in Kenya. Proceedings of the 2nd Scientific Conference of the Soil Management and Legume Research Network Projects, Mombasa, Kenya. p39.
|
|
|
|
|
Zhang Y, Li X, Lo CK, Guo ST (2010). Characterization of the volatile substances and aroma components from traditional soypaste. Molecules 15:3421-3427.
Crossref
|
|