ABSTRACT
Investigation of secondary metabolites from the cryptic metabolic pathways in date palm fruit, garlic bulb and groundnuts from North-West Nigeria was conducted using liquid chromatography-mass spectrometry and mass spectrometry (LC-MS/MS). Results indicated the presence of several compounds which include Valinomycin, asperglaucide, asperphenamate, cyclo (L-Pro-L-Tyr). Cyclo (L-Pro-L-Val), emodin, physcion, integracin A and B, Monocerin, and fallacinol were detected at different concentrations in the different sampled materials. Asperglaucide, asperphenamate, cyclo (L-Pro-L-Tyr), cyclo (L-Pro-L-Val), and emodin were found in all the tested samples at different concentrations. Garlic bulb contains valinomycin, emodin and physcion which are compound that may have a potential inhibitory effect on severe acute respiratory syndrome coronavirus (SARS-CoV) with their maximum concentration of 55.15, 20.25 and 419.45 µg/kg respectively in the tested materials. Integracin A and B which are compound with potential inhibitory effect on the HIV-1 integrase enzyme found in Human immune deficiency virus (HIV) were detected in garlic bulb samples at the maximum concentration of (3.98 and 18.68 µg/kg) respectively. This study has identified the presence of a compound of immense pharmacological importance, and it may provide a veritable lead for natural product discovery.
Key words: Asperphenamate; cryptic pathway; date fruits; garlic bulb; groundnut seed; metabolites.
Secondary metabolites are compounds of natural origin that are often produced specifically in the cells of some animals, plant, fungi and bacteria. Secondary metabolites are involved in the homeostasis, modulation of organism health system such as excretion and overall health status of the body system (Baral et al., 2018). Production of these metabolites by some organism is regarded as an adaptive capacity of coping with stress caused by challenges of changing growth environment. This lead to overproduction of complex chemical types and it involves interaction in their structural and functional stabilization through cell signaling processes and pathway (Tanaka et al., 2013).
The biosynthetic capabilities of fungal and bacteria cryptic pathways in producing secondary metabolites which can be used as natural product is much more than it’s currently appreciated. Cryptic biosynthetic pathway involves manipulation and activation of biosynthetic genes. It has been previously enhanced using several methods which include, epigenetic modifier and genetic engineering (Jordan et al., 2016; Tanaka et al., 2013). Mixed fermentation using microbial cultivation of two or more microorganism has been reported to induce expression of cryptic pathway, leading to the production of different microbial natural products (Becerril and Susana, 2018). It can be deduced that competition between microbes encourages production of secondary metabolites via signaling molecules (auto-regulator/quorum sensing molecules and siderophore) in their environment (Jordan et al., 2016).
Alternatively, epigenetic modification in the producers strain may be related to the activation of metabolites precursors by producer active enzymes (Rutledge and Challis, 2015). However, it is worth noting that in many cases, direct contact is necessary between bacteria and fungi to elicit this effect. Recent studies have demonstrated that co-cultivation or co-contamination may be a remarkable successful approach for discovering new natural bioactive products (Becerril and Susana, 2018; Lal and Lal, 2011). Their biosynthesis can be greatly influenced by manipulating the type of host plant parts and concentration of the nutrient in the culture media (Jordan et al., 2016).
Some bacteria and fungi have cryptic gene clusters that produce secondary metabolites which is a leading source of drug discovery (Shen, 2015). Many of these microbial secondary metabolites are leading drug candidates as fungal bio-control agent of plants and animal pathogens, anti-metastatic agent, anti-inflammatory, antiviral and antibacterial agents (Abia et al., 2017). Cryptic products have been identified in tomato, maize meal, cassava fermented products, cashew nuts and animal feeds in different countries (Abia et al., 2017; Adetunji et al., 2019).
There is need to determine the differences in where these microbial organisms produce these cryptic pathway metabolites among the crop samples which are susceptible to fungal and bacteria co-contamination.
Date palm (Phoenix dactylifera L.) is grown in many tropical countries and is the most popular fruit eaten in Northern Nigeria as high calorie appetizer (Arias et al., 2016). Date fruits have sweet flavour and high nutrition profile providing important essential nutrients like protein, fibre, carbohydrates, fat and minerals (Chandrasekaran and Bahkali, 2013). Groundnut (Arachis hypogea L seed has abundant protein, fat, vitamins, minerals and fibre. It is eaten locally by diabetic patient because it low glycaemic index (Nautiyal, 2002). Groundnut is very popular for its seeds which can be eaten raw, boiled, roasted or dried. Also, the seed oil is used for cooking food and has industrial application (Goswami et al., 2014). Garlic (Allium sativum L) bulb is commonly consumed for its medicinal and culinary use worldwide (Sharma et al., 2013). In Nigeria like other parts of the world, it is used in seasoning meat and meat products. Its medicinal properties include lowering of blood pressure, treat viral and bacterial diseases (Rastogi et al., 2016).
Several reports of fungal and bacterial contamination or co-contamination of date fruit, groundnut seeds and garlic bulb have been reported (Al-Meamar et al., 2017; Kachapulula et al., 2017; Moharam et al., 2013). The interaction between fungal and bacteria flora in these substrates may lead to the expression of the cryptic metabolic pathway and subsequent production of some useful metabolites. This study is focused on identifying products from the cryptic pathway which may be of pharmacological importance and relating it with substrate where they are found using high throughput screening with liquid chromatography-mass spectrometry/mass spectrometry.
Sampling and sample collection
Samples of garlic bulbs (16), groundnut seeds (30) and date palm fruits (45) were collected from three retail market points in each of the six zones of Zaria (Samaru, Gaskiya, Sabongari, Tudunwada, Kongilla and Tudunwada) located between Lat. 11° 06’ 40’’N and Long. 7°43’21’’E in Kaduna State, North West Nigeria. Thirty bulk samples (1 kg each) of randomly measured sample materials were obtained and kept in clean zip-lock bags. They were transported to the laboratory of Crop Protection Department, Ahmadu Bello University, Samaru-Zaria, Nigeria. A total of 91 samples (each representing a pack of the bulbs, fruits and the seeds, weighing approximately 500 g) were randomly collected. Each sample was quartered and about 100 g was taken and pulverized using a commercial Blender (Waring Commercial Blender 8010BU, Model HGBTWT, Connecticut, USA). The representative samples were stored at -4ºC to prevent further metabolite liberation by fungi and bacteria within samples prior to multi-mycotoxin analysis. Samples were kept in polypropylene bags and transported to the Center for Analytical Chemistry Laboratory in the Department of Agro-biotechnology, University of Natural Resources and Life Sciences, Vienna, Austria for analysis.
Analysis of mycotoxin
Reagents
Liquid chromatographic grade methanol (CH3OH) and acetonitrile were purchased from Merck (Germany) and VWR (Belgium) respectively. The Mass Spectrometry grade ammonium acetate and standards for fungi metabolite were brought from Sigma-Aldrich (Austria). Decontamination of water was carried out consecutively through reverse osmotic pressure and ultra-analytic system purchased from Veolia water (UK). A total of 34 working solutions were made and kept at -20°C in the fridge but were brought to 25°C before use. Fresh final working solution was mixed accordingly for the spiking experiment.
Garlic bulb, groundnut seeds and date fruits extraction
Each garlic bulb, groundnut seeds and date fruit were milled using a cyclone pulverizer which has one millimetre square sieve (Cyclotech, Sweden) before being homogenized. Five grams each were measured into the centrifuge tube (0.05 L polypropylene). Twenty millilitres of the separation solvent (acetic acid/water/acetonitrile 1:20:79, v/v/v) were added before being vortexed using a vortexed using laboratory rotary shaker (Model GFL 3017, Germany). Ratio of the dilution of the sample with the solvent was 1:1 and 5 ml of the dilution obtained from the extract were injected into the LC-MS/MS.
LC-MS/MS parameters
Detection and quantification of extracts was achieved through a described procedure (Malachova et al., 2014). Briefly, a QTrap 5500 multimycotoxin LC-MS/MS system (Applied Biosystem, California, United State of America) furnished with TurboV spray ESI source and Ultra High Performance Liquid Chromatography system (UHPLC) (Agilent, Germany). The Chromatographic separation of extracts was performed at 25°C on a 150 × 4.6 mm, 5-μm Gemini C18-column equipped with a C18 security guard cartridge, 4 × 3 mm i.d. (Phenomenex). Elution was carried out in binary gradient mode. Both mobile phases contained 5 mM ammonium acetate and were composed of methanol/water/acetic acid 10:89:1 (v/v/v; eluent A) and 97:2:1 (v/v/v; eluent B), respectively. After an initial time of 2 min at 100% A, the proportion of B was increased linearly to 50% within 3 min. Further linear increase of B to 100% within 9 min was followed by a hold-time of 4 min at 100% B and 2.5 min column re-equilibration at 100% A. The flow rate was 1000 μL/min. ESI–MS–MS was performed in the scheduled multiple reaction monitoring (sMRM) mode both in positive and negative polarities in two separate chromatographic runs. The sMRM detection window of each analyte was set to the respective retention time ±27 s and ±42 s in positive and in negative mode, respectively. The target scan time was set to 1 s. The settings of the ESI source were as follows: source temperature: 550 °C; curtain gas: 30 psi; ion source gas 1 (sheath gas): 80 psi; ion source gas 2 (drying gas): 80 psi; ion-spray voltage: −4500 V and +5500 V, respectively; collision gas: (nitrogen) medium. Confirmatory identification was obtained through the acquisition of two sMRMs per analyte, which yields 4.0 identification points according to commission decision 2002/657/EC.
The method precision was tested through proficiency testing organized by Bureau Interprofessionel des Etudes Analytique (BIPEA) (Gennevilliers, France) in accordance with ISO 13525:2015. All the results of the extracts were between -2< x < 2 which was a satisfactory range. The percentage of contaminated samples, maximum and median concentration (μgkg-1) of toxins and metabolites were determined from the data collected for each of the samples analysed.
Metabolites were quantified by external calibration (1/x weighted) using a multi-component standard prepared from authentic standards.
Secondary metabolites produced through the expression of the cryptic pathway may be of great pharmacological importance. The tested samples are: defective date fruit (DDF) (21), healthy date fruits (HDF) (24), garlic bulb (16) and groundnut seed (30) samples and result presented in Table 1.
Valinomycin was detected only in 9/16 (56%) of the garlic sample with a median and maximum values of (44.99 and 55.15) µg/ kg (Table 1 and Figure 1a). Valinomycin is a product of bacterial contamination. It is found in garlic from different markets in the sampled area. It is a cyclic peptide which readily dissolves in the membrane lipid bilayer and thus enhanced its bioavailability in target cells. It is an antibiotic which greatly affects the metabolic pathways of several bacteria (Shen, 2015).
Asperglaucide was found naturally occurring in all the samples tested. The lowest value found in the food samples tested was 2.42 µg/ kg in groundnut seeds while the maximum concentration of 2886.40 µg/ kg was found in garlic bulb. Also, the maximum values of 13.52 µg/ kg of asperglaucide was obtained in defective date fruits.
Asperphenamate was also detected in all tested crop samples. Its median values are (2.41, 2.56, 267.24, and 3.41) µg/ kg in DDF, HDF, garlic bulbs and groundnut seeds (Table 1 and Figure 1b). Presence of Asperglaucide in all tested samples with its highest concentration of 2886.40 µg/kg in garlic showed that the metabolites can be produced in different substrates. It was also observed that it was detected in 100% of all samples which indicated that the growth environment for this microbial contaminant is conducive for the biosynthesis of Asperglaucide. Natural occurrence of Asperglaucide has been previously reported at different concentration in different substrates in several countries (Abass et al., 2017; Andersen and Frisvad, 2004; Humer et al., 2016).
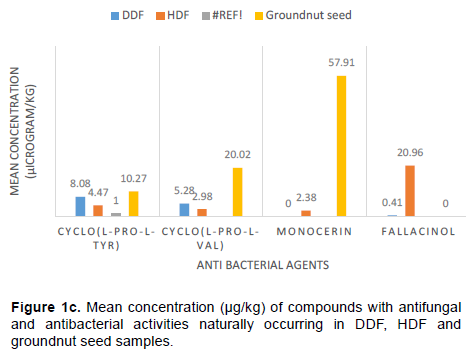
Cyclic dipeptide identified in the samples was cyclo (L-Pro-L-Tyr) and cyclo (L-Pro-L-Val). Garlic bulb samples were positive for these two cyclo dipeptides. The maximum values recorded were (1458.00 and 1481.00) µg/ kg for cyclo (L-Pro-L-Tyr) and cyclo (L-Pro-L-Val) respectively (Table 1 and Figure 1c). A total of 18/21 (86%) of DDF and 9/24 (38%) HDF were positive for cyclo (L-Pro-L-Val). Their respective median values were 8.42 and 3.71 µg/kg. The result of this study showed that the two cyclo dipeptides may be a natural contaminant of garlic bulb. These cyclo-peptides have been isolated from Streptomyces species, they possess antibacterial activities and has inhibitory effect on some plant pathogens (Nishanth et al., 2012).

Emodin was found in 100% of garlic samples, 7/21 (33.33%) samples of DDF, 1/24 (4.1%) in HDF and 8/30 (27%) in groundnut seeds. Their respective maximum values were 99.46, 2.38, 20.25, 131.38 and 1.48 µg/ kg (Table 1 and Figure 1a). Natural occurrence of emodin in unripe fruits, vegetables and different herbs has been previously reported (Dong et al., 2016); to the best of our knowledge this is the first report of emodin in garlic bulbs, groundnut seeds and date fruits in Nigeria. Unlike emodin, physcion is found in 50% of the garlic bulb samples and only one sample each of DDF and HDF (Table 1 and Figure 1a). The presence of physcion in half of the garlic samples tested indicated its appreciable presence in the study area. These metabolites can be exploited for its documented pharmacologically activities. Emodin and physcion have been documented to possess anti-SARS-CoV activities (Ho et al., 2007). They have also been used in Traditional Chinese Medicine in the treatment of microbial infections, liver diseases and are good anti-inflammatory agent (Ahirwar and Jain, 2015).
Integracin A and integracin B are found only in garlic samples at maximum values of (3.98 and 18.68) µg/ kg respectively (Table 1 and Figure 1d) while Monocerin was detected in garlic 11/16 (69%) and groundnut seeds 2/30 (7%) (Table 1 and Figure 1e). Their respective median values are 1.66 and 55.41 µg/ kg respectively. These fungi metabolites are products of the cryptic metabolic pathway with beneficial medicinal properties. They have been reported to have activities against HIV-1 integrase enzymes (Sivro et al., 2019). In addition, Monocerin has shown appreciable pharmacological activities against plant pathogens like powdery mildew of wheat (Robeson and Strobel, 1982).
Fallacinol (Table 1 and Figure 1e) is found in two samples of garlic bulb and DDF with their respective maximum values of 0.41 and 35.54 µg/kg. Fallacinol is an antimicrobial metabolites found in some medicinal plants (Bitchagno et al., 2015).
The microbial secondary metabolites identified in this study are of significant pharmacological importance. This study will serve as a template for further work on isolation and subsequent development of natural biologically active product that can be effective in managing several diseases of plants and animals.
Several products of cryptic metabolic pathways of pharmacological importance were identified using LC-MS/MS chromatographic techniques. Valinomycin, integrin A and C were found naturally occurring in only
garlic bulb samples while Asperglaucide, Asperphenamate, the Cyclo dipeptides and Emodin are found as natural contaminants of all the sample tested. This study detected Asperglaucide and Asperphenamate in 100% of DDF, HDF and groundnut seed tested. These secondary metabolites detected in this study may be an important lead in medicinal plant drug discovery.
The authors have not declared any conflict of interests.
REFERENCES
|
Abass AB, Awoyale W, Sulyok M, Alamu EO (2017). Occurrence of Regulated Mycotoxins and Other Microbial Metabolites in Dried Cassava Products from Nigeria. Toxins 9(207):1-13.
Crossref
|
|
|
|
Abia WA, Warth B, Ezekiel CN, Sarkanj B, Turner PC, Marko D, Krska R, Sulyok M (2017). Uncommon toxic microbial metabolite patterns in traditionally home-processed maize dish (fufu) consumed in rural Cameroon. Food and Chemical Toxicology 107:10-19.
Crossref
|
|
|
|
|
Adetunji MC, Aroyeun SO, Michael B, Sulyok M, Krska R, Mwanza M (2019). Food Additives and Contaminants: Part A Fungal metabolite and mycotoxins profile of cashew nut from selected locations in two African countries. Food Additives and Contaminants: Part A 00(00):1-13.
|
|
|
|
|
Ahirwar K, Jain SK (2015). Aloe-emodin novel anticancer Herbal Drug. International Journal of Phytomedicine 3(2011):27-31.
|
|
|
|
|
Al-Meamar TS, Al-Jassani MJ, Hamed NS (2017). Contamination of date fruit by a flatoxigenic fungi and a flatoxins in Hilla City, Iraq. Journal of Global Pharma Technology 9(12):438-446.
|
|
|
|
|
Andersen B, Frisvad JC (2004). Natural occurrence of fungi and fungal metabolites in moldy tomatoes. Journal of Agricultural and Food Chemistry 52(25):7507-7513.
Crossref
|
|
|
|
|
Arias E, Hodder AJ, Oihabi A (2016). FAO support to date palm development around the world: 70 years of activity. Emirates Journal of Food and Agriculture 28(1):1-11.
Crossref
|
|
|
|
|
Baral B, Akhgari A, Metsä-ketelä M (2018). Activation of microbial secondary metabolic pathways : Avenues and challenges. Synthetic and Systems Biotechnology 3(3):163-178.
Crossref
|
|
|
|
|
Becerril A, Susana A (2018). Uncovering production of specialized metabolites by Streptomyces argillaceus : Activation of cryptic biosynthesis gene clusters using nutritional and genetic approaches. PLoS ONE (May 24), 1-14.
Crossref
|
|
|
|
|
Bitchagno GTM, Fonkeng LS, Kopa TK, Tala MF, Wabo HK, Tume CB, Tane P, Kuiate J (2015). Antibacterial activity of ethanolic extract and compounds from fruits of Tectona grandis (Verbenaceae). BMC Complementary and Alternative Medicine 15(265):1-6.
Crossref
|
|
|
|
|
Chandrasekaran M, Bahkali AH (2013). Valorization of date palm (Phoenix dactylifera) fruit processing by-products and wastes using bioprocess technology - Review. Saudi Journal of Biological Sciences 20(2):105-120.
Crossref
|
|
|
|
|
Dong X, Fu J, Yin X, Cao S, Li X, Lin L (2016). Emodin : A Review of its Pharmacology. Toxicity and Pharmacokinetics 1218:1207-1218.
Crossref
|
|
|
|
|
Goswami D, Dhandhukia P, Patel P, Thakker JN (2014). Screening of PGPR from saline desert of Kutch: Growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiological Research 169(1):66-75.
Crossref
|
|
|
|
|
Ho T, Wu S, Chen J, Li C, Hsiang C (2007). Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antiviral Research 74:92-101.
Crossref
|
|
|
|
|
Humer E, Lucke A, Harder H, Metzler-Zebeli BU, Böhm J, Zebeli Q (2016). Effects of citric and lactic acid on the reduction of deoxynivalenol and its derivatives in feeds. Toxins 8(10):1-10.
Crossref
|
|
|
|
|
Jordan PA, Moore BS, Jordan PA, Moore BS (2016). Biosynthetic Pathway Connects Cryptic Ribosomally Synthesized Posttranslationally Modified Peptide Genes with Pyrroloquinoline Alkaloids. Cell Chemical Biology 23(12):1504-1514.
Crossref
|
|
|
|
|
Kachapulula PW, Akello J, Bandyopadhyay R, Cotty PJ (2017). Aflatoxin contamination of groundnut and maize in Zambia: Observed and potential concentrations. Journal of Applied Microbiology 122(6):1471-1482.
Crossref
|
|
|
|
|
Lal D, Lal R (2011). Discovering Metabolic Products of Cryptic Biosynthetic Pathways. Indian Journal of Microbiology 51(3):414-419.
Crossref
|
|
|
|
|
Malachová A, Sulyok M, Beltrán E, Berthiller F, Krska R (2014). Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. Journal of Chromatography A.
Crossref
|
|
|
|
|
Moharam MHA, Farrag ESH, Mohamed MDA (2013). Pathogenic fungi in garlic seed cloves and first report of Fusarium proliferatum causing cloves rot of stored bulbs in upper Egypt. Archives of Phytopathology and Plant Protection 46(17):2096-2103.
Crossref
|
|
|
|
|
Nautiyal PC (2002). Groundnut Post-harvest Operations. In INPhO - Post-harvest Compendium. P. 127.
|
|
|
|
|
Nishanth Kumar S, Mohandas C, Siji JV, Rajasekharan KN, Nambisan B (2012). Identification of antimicrobial compound, diketopiperazines, from a Bacillus sp. N strain associated with a rhabditid entomopathogenic nematode against major plant pathogenic fungi. Journal of Applied Microbiology 113(4):914-924.
Crossref
|
|
|
|
|
Rastogi S, Pandey MM, Rawat AKS (2016). Traditional herbs: A remedy for cardiovascular disorders. Phytomedicine 23(11):1082-1089.
Crossref
|
|
|
|
|
Robeson DJ, Strobel GA (1982). Monocerin, a phytotoxin from Exserohilum turcicum (≡ Drechslera turcica). Agricultural and Biological Chemistry 46(11):2681-2683.
Crossref
|
|
|
|
|
Rutledge PJ, Challis GL (2015). Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nature Reviews Microbiology 13(August 2015):1-15.
Crossref
|
|
|
|
|
Sharma J, Gairola S, Gaur RD, Painuli RM, Siddiqi TO (2013). Ethnomedicinal plants used for treating epilepsy by indigenous communities of sub-Himalayan region of Uttarakhand, India. Journal of Ethnopharmacology 150(1):353-370.
Crossref
|
|
|
|
|
Shen B (2015). A New Golden Age of Natural Products Drug Discovery. Cell 163(6):1297-1300.
Crossref
|
|
|
|
|
Sivro A, Schuetz A, Sheward D, Joag V, Yegorov S, Liebenberg LJ, Yende-Zuma N, Stalker A, Mwatelah RS, Selhorst P, Garrett N, Samsunder N, Balgobin A, Nawaz F, Cicala C, Arthos J, Fauci AS, Anzala AO, Kimani J, Bagaya BS, Kiwanuka N, Williamson C, Kaul R, Passmore J-AS, Phanuphak N, Ananworanich J, Ansari A, Karim QA, Abdool Karim SS, McKinnon LR, on behalf of the CAPRISA004 and RV254 study groups (2019). Integrin α4β7 expression on peripheral blood CD4+ T cells predicts HIV acquisition and disease progression outcomes. Science Translational Medicine 10(425):1-23.
Crossref
|
|
|
|
|
Tanaka Y, Kasahara K, Hirose Y, Murakami K, Kugimiya R, Nbrc A, Nrrl S (2013). Activation and Products of the Cryptic Secondary Metabolite Biosynthetic Gene Clusters by Rifampin Resistance (rpoB) Mutations in Actinomycetes. Journal of Bacteriology 195(13):2959-2970.
Crossref
|
|