ABSTRACT
Wheat (Triticum aestivum L.) improvement via genetic transformation depends on an efficient regeneration system for recovery of transgenic events. This study reports a somatic embryogenesis-based regeneration system for two Kenyan wheat genotypes (Eagle 10 and Njoro bread wheat II) from mature embryos. The study investigated the efficiency of mercuric chloride, commercial bleach, and chlorine gas in surface sterilizing explants prior to in vitro culture. Callus induction and somatic embryogenesis were done by culturing wheat mature embryos in Murashige and Skoog (MS) medium supplemented with 2, 4-dichlorophenoxyacetic acid (2,4-D) used either singly or in combination with 1-naphthaleneacetic acid (NAA), 4-chlorophenoxyacetic acid (4-CPA) or 6-benzylaminopurine (BAP). Embryo germination and plantlet recovery were done by culturing embryogenic callus in MS medium without plant growth regulators (PGRs). Chlorine gas was significantly (p<0.001) the most effective in surface sterilization and maintenance of explant viability. All 2, 4-D concentrations tested (1, 2, 4 and 8 mg/l) induced embryogenic callus. A significantly higher callus induction rate and callus fresh weight were obtained when 2,4-D was used in combination with either NAA or 4-CPA than when it was used singly. Combining 2,4-D with BAP led to a significantly lower callus induction frequency. Somatic embryo germination was achieved in MS medium without plant growth regulators. These findings have the potential to inform future efforts in the application of modern biotechnology for accelerated wheat cultivar improvement.
Key words: Wheat, somatic embryogenesis, mature embryos.
Wheat (Triticum aestivum L.) is among the top five most important food crops in the world, in terms of production and consumption. In Kenya, apart from corn meal (‘Ugali’), food products from wheat flour are mostly preferred, making it the second most popular food crop after maize, in terms of consumption. With increasing population especially in Africa, the global demand for wheat is expected to increase by over 60% in 2050. However, due to diseases and climate change stresses, yield is on the decline with estimated projections of 30% over the same period (FAOSTAT, 2017). Notwithstanding that conventional breeding had been achieved, much success in wheat improvement, especially against rust and drought, other biotechnological approaches are needed to handle the challenges. This is especially so because of low frequency of meiotic recombination in the crop which often leads to colossal unwanted linkage drag (Choulet et al., 2014; Darrier et al., 2017). Genetic transformation, for example, is quicker and more precise for improving resistance against diseases in crops. Transgenes or cisgenes can be transferred to elite cultivars in relatively shorter time without compromising on agronomic traits. With emerging technology of genome editing such as CRISPR/Cas 9, it is now possible to up-regulate genes for disease resistance by mutating their specific promoter regions (Dale et al., 2017). Apart from genetic transformation, other alternative approaches include mutation breeding and in vitro selection. All these techniques depend on tissue culture as they involve in vitro manipulation of wheat axenic cultures. They also require an efficient regeneration system (Miroshnichenko et al., 2016).
Wheat, like many monocots, is recalcitrant to in vitro culture and its regeneration and transformation is highly dependent on genotype (Aydin et al., 2011); besides, somatic embryogenesis, which is mostly preferred in genetic transformation procedures, it is difficult to achieve in most cultivars. In fact, genetic transformation of wheat is almost restricted to a few cultivars including ‘Bob white’ and ‘Chinese spring’ because of their ease of regeneration. There is need to develop protocols for as many elite cultivars as possible in order to accelerate wheat improvement by modern biotechnology.
Monocots have a limited number of explants which can be regenerated into plants (Repellin et al., 2001). Explants studied so far in wheat include and not limited to immature embryos (Hafeez et al., 2012), mature embryos (Delporte et al., 2001; Filippov et al., 2006), spikes and anthers (Stober and Hessu (1997), and immature inflorescence (Redway et al., 1990; Sharma et al., 1995). However, only immature embryos have been efficient in regeneration (Bouiamrine et al., 2012). The challenge of seasonal availability of immature zygotic embryo explants requires setting up of greenhouses which are expensive to maintain (Zale et al., 2004). In view of this, there is need to optimise regeneration using mature embryos which are independent of season and can be easily conserved and stored for long inside dry seeds. Somatic embryogenesis of Kenyan wheat cultivars has so far not been explored. This is the first report of somatic embryogenesis of wheat genotypes bred in the country.
Plant material
Two Kenyan wheat varieties, Eagle 10 and Njoro bread wheat II, obtained from the Kenya Agricultural and Livestock Research Organization (KALRO), Njoro, Kenya, were used in the current study.
Explant preparation
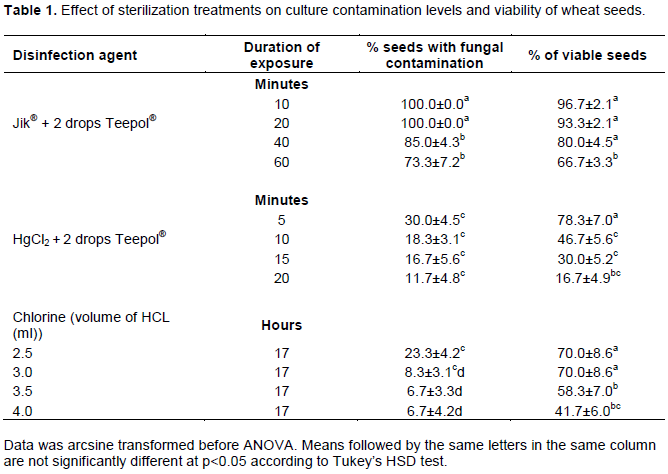
Intact mature seeds were used as initial explants for callus induction. Three disinfection methods were investigated for their effectiveness in establishing axenic cultures as well as maintaining explant viability. The first surface disinfection method involved the use of commercial bleach, Jik® (3.85% NaClO). Seeds were first vortexed in 70% ethanol for 3 min followed by three rinses with sterile distilled water. They were then soaked in 100% Jik® plus 2 drops of Teepol detergent, with varying exposure times of 10, 20, 40 and 60 min. The second method involved the use of mercuric chloride. Seeds were first vortexed in 70% ethanol for 3 min then rinsed thrice before being soaked in 0.1% mercuric chloride plus 2 drops Teepol for varying exposure times of 5, 10, 15 and 20 min. The third method involved chlorine gas disinfection in which dry seeds were fumigated with chlorine gas in a closed 8-litre container for 17 h. To generate chlorine gas, varying volumes of concentrated hydrochloric acid (2.5, 3.0, 3.5 and 4.0 ml) were added to 150 ml of Jik®.
Media composition and preparation
The culture media used for callus induction and regeneration were full strength MS (Murashige and Skoog, 1962) basal salts and half strength MS for root induction and proliferation. The medium was obtained from Duchefa Biochemie B.V., Netherlands. After addition of the supplements, and 8 g l-1 of agar powder (Thomas Baker), the pH of medium was adjusted to 5.7 ± 0.1 using either 1 N HCl or 1 N NaOH solutions. The medium in volumes of 50 ml was then autoclaved at 1.06 kg cm-2 steam pressure (≈ 121 o C) for 15 min.
Callus induction and maintenance media were supplemented with 2, 4-D in the range of 0.5 to 8 mg/l used either alone or in combination with either 0.2 mg/l of NAA or 4-CPA. These media were also augmented by 300 mg/l casein hydrolysate, 200 mg/l proline and 200 mg/l glutamine. After 4 to 7 days depending on the efficiency of callus induction and growth in different auxin concentrations, endosperm and germinating shoots were cut out and callus fragments were sub-cultured to medium of the same composition but with addition of 10 mg/l silver nitrate for callus maintenance.
Regeneration medium was the same as callus maintenance medium but devoid of plant growth regulators (PGRs). Regenerated plants were transferred to PGR-free ½ MS for root proliferation.
Inoculation and culture conditions
Surface disinfected seeds were aseptically inoculated onto sterile agar medium under a laminar flow cabinet. The cultures were incubated for 30 days in the dark at a temperature of 28±2°C for callus induction and maintenance. Callus initiated was then sub-cultured to regeneration medium and incubated at the same temperature in light of approximately 60 µmole photons m-2 s-1 and a 16-hour photoperiod.
Experimental design and data analysis
Effectiveness of disinfection and severity of the treatment on the seeds were assessed through percentage contamination scores and percentage viability as measured by actual germination scores. For seed disinfection and callus induction experiments, each treatment had 6 replicates with 5 explants per replicate. Each experiment was repeated thrice. A completely randomised design was used in the arrangement of the culture vessels.
Data for percentage callus induction, callus fresh weight, percentage embryogenic callus induction and regeneration were subjected to one-way analysis of variance (ANOVA) and means separated by Tukey’s HSD test at p ≤ 0.05. These analyses were completed using GenStat® computer software 15th edition. Prior to ANOVA, arcsine transformation of data (percentages) was done where necessary based on the relation Y = arcsine √p, where p = the proportion obtained by dividing the respective percentage value by 100 as described by Rangaswamy (2010).
Explant sterilization
Disinfection of explants is a crucial step in explant preparation prior to in vitro culture. Studies have shown that the method applied may have a significant effect on morphogenic responses. This may include variations in callus induction, somatic embryogenesis and regeneration frequencies depending on the disinfectant used (Teng et al., 2002; Çölgeçen et al., 2011). In the present study, preliminary results showed that 50% Jik® produced 100% contamination even when exposure time was extended to one hour. Consequently, full strength Jik® (3.85% NaOCl) was used in subsequent experiments. However, this was still not effective because contamination rates remained at > 70% (Table 1). Mercuric chloride was more effective than Jik® but its phyto-toxicity levels were higher than those of Jik® and this later affected callus induction frequency and vigour. Vapour disinfection by overnight fumigation of seeds with chlorine gas was the most effective surface-disinfection treatment, producing up to 93.3% disinfection (Table 1). The optimal balance between the need for high disinfection and low toxicity was achieved using 3.0 ml HCL in 150 ml Jik, as the source of chlorine gas (Table 1). The results obtained in the present study with respect to commercial bleach and mercuric chloride contrast with those reported by Bi and Wang (2008) and Parmar et al., (2012), who achieved success in using the two disinfectants for disinfection of wheat seeds. This could be due to different environments from which the seeds were obtained. The chlorine vapour disinfection protocol used in this study is adopted from the protocol used by Clough and Bent (1998) in sterilization of arabidopsis seeds. There is no report to date of previous use of this method in wheat seeds.
Callus induction and regeneration
In the present study, callus formation in mature zygotic embryos started on day 2 or 3 depending on the concentration or combination of auxins used. When 2 mg/l 2,4-D was used alone, callus induction started on day three. Augmentation of 2, 4-D with either 0.2 mg/l NAA or 4-CPA reduced the time for initiation of callusing by a day and triggered faster proliferation of callus. Callus fresh masses in augmented media were significantly larger (p<0.05) than those in media containing 2,4-D alone after 30 days in culture (Table 2). Embryos which had not formed callus by day seven remained non-responsive throughout the culture period. Callusing also failed to occur in both cultivars in medium containing 0.5 mg/l and lesser 2, 4-D.
Early separation of callusing embryos from the rest of the seed was crucial for further growth and maintenance of the callus probably because it removed the interfering influence of plant growth regulators emanating from the germinating seed. Except for calli from 1 and 2 mg/l 2,4-D alone, which had lots of roots on them by day seven (Day of first subculture), calli from all other treatments were non-morphogenic at this stage, with smooth, less nodular and relatively dry surfaces. Occasionally, watery and spongy calli was observed. Combinations of auxins (2,4-D and 4-CPA) and higher concentrations of 2, 4-D inhibited normal germination and produced callus with no roots. This is consistent with known effects of auxins (Wilkins, 1984). In single-auxin media, 2, 4-D was generally more superior to 4-CPA or NAA in callus induction (data not shown). Similar results have been reported by Hakan and Ismet (2004) and Miroshnichenko et al., (2013). In the study herein, combinations of NAA or 4-CPA with 2, 4-D improved somatic embryogenesis suggesting the importance of combining a superior auxin with less effective ones for synergy. The combination of 2 mg/l 2, 4-D with 0.2 mg/l of either 4-CPA or NAA proved beneficial for callus induction frequency, callus fresh mass, somatic embryogenesis and plant regeneration in both cultivars (Tables 2 and 3). Hakan and Ismet (2004) reported similar synergistic effect with a combination of 2, 4-D and NAA. Filippov et al. (2006) also reported improved somatic embryogenesis when either IAA, IBA or NAA were used in combination with dicamba.
Augmentation of 2 mg/l 2, 4-D with 0.2 mg/l TDZ resulted in browning and necrosis of calli from as early as seven days after subculture. However, the combination of 2 mg/l 2, 4-D with 0.2 mg/l BAP caused a significant reduction in callus induction frequency and fresh mass (Table 2). The inhibitory effects of these cytokinins on callus induction in the present study are in agreement with the findings of Rashid et al., (2002). This combination of BAP and 2, 4-D supported normal germination of seeds which inhibited callus induction.
By day 21, embryogenic callus fragments could easily be distinguished from those that were non-embryogenic. Embryogenic callus had relatively dry and nodular surfaces with visible white embryoids which quickly turned into green spots when the callus was sub-cultured to medium without PGRs (Figure 1a). Non-embryogic callus had watery surfaces without any organized structures (Figure 1b). This type of callus did not give shoots when sub-cultured to regeneration medium, although some turned green. Development of chlorophyll in non-embryonic calli is not unique to this study as similar results had been reported by Parmar et al., (2012). In some cases, a single callus had both embryogenic and non-embryogenic regions.
Frequency of somatic embryogenesis differed depending on the auxin concentration or combination and genotype (Table 3). Within a week of sub-culture of embryogenic calli to medium without PGRs in light, leaf-like structures started to develop from globular protrusions of the callus (Figure 1a). Most of these leaf-like structures did not however develop into plantlets. In fact, a higher percentage of calli which had been identified as embryogenic failed to regenerate any shoot resulting in generally low percentages of plantlet regeneration (Table 3). Regenerated shoot buds (Figure 1c) quickly elongated and developed roots when transferred to ½ MS medium without PGRs (Figure 1d).
Even though both wheat varieties showed a similar pattern of response in all stages of the culture process, genotypic differences were observed in the response to the different PGR concentrations and combinations. When 2,4-D was used alone, callus induction frequency for cv. Eagle 10 was optimum at 4 mg/l 2,4-D while for cv. Njoro BW II the optimum frequency was at 2 mg/l 2,4-D (Table 2). Optimal callus fresh mass for both cultivars was in medium with 2 mg/l 2,4-D. When combinations of auxins were used, cv. Eagle 10 showed the best response in terms of callus induction frequency in the combination of 2 mg/l 2, 4-D + 0.2 mg/l 4-CPA while cv. Njoro responded best in the combination of 2 mg/l 2, 4-D and 0.2 mg/l NAA.
There are some previous reports on the unique response of different wheat genotypes to different media (Bi et al., 2007; Aydin et al., 2011; Parmar et al., 2012). The findings of the present study agree with these previous studies.
Combination of auxins was more superior in stimulating callus induction and somatic embryogenesis compared to single auxin treatments. In vitro regeneration of wheat is cultivar dependent. There is need for further work to establish the optimal media and PGRs for other elite wheat cultivars bred in Kenya.
The authors have not declared any conflict of interests.
REFERENCES
|
Aydin M, Tosun M, Haliloglu K (2011). Plant regeneration in wheat mature embryo culture. African Journal of Biotechnology 10(70):15749-15755.
Crossref
|
|
|
|
Bi RM, Kou M, Chen LG, Mao SR, Wang H G (2007). Plant regeneration through callus initiation from mature embryo of Triticum. Plant breeding 126(1):9-12.
Crossref
|
|
|
|
|
Bi R, Wang H (2008). Primary studies on tissue culture from mature embryos in diploid and tetraploid wheat. Frontiers of Agriculture in China 2(3):262-265.
Crossref
|
|
|
|
|
Bouiamrine E, Diouri M, El-Halimi R (2012). Somatic embryogenesis and plant regeneration capacity from mature and immature durum wheat embryos. International Journal of Biosciences 2(9):29-39.
|
|
|
|
|
Choulet F, Alberti A, Theil S, Glover N, Barbe V, Daron J, Leroy P (2014). Structural and functional partitioning of bread wheat chromosome 3B. Science 345(6194):1249721. https://doi.org/10.1126/science.1249721
Crossref
|
|
|
|
|
Clough SJ, Bent AF (1998). Floral dip: a simplified method for Agrobacteriumâ€mediated transformation of Arabidopsis thaliana. The Plant Journal 16(6):735-743.
Crossref
|
|
|
|
|
Çölgeçen H, Caliskan UK, Toker G (2011). Influence of different sterilization methods on callus initiation and production of pigmented callus in Arnebia densiflora Ledeb. Turkish Journal of Biology 35(4):513-520.
|
|
|
|
|
Dale J, Paul JY, Dugdale B, Harding R (2017). Modifying bananas: From transgenics to organics? Sustainability 9(3):333.
Crossref
|
|
|
|
|
Darrier B, Rimbert H, Balfourier F, Pingault L, Josselin AA, Servin B, Sourdille P (2017). High-resolution mapping of crossover events in the hexaploid wheat genome suggests a universal recombination mechanism. Genetics 206(3):1373-1388.
Crossref
|
|
|
|
|
Delporte F, Mostade O, Jacquemin JM (2001). Plant regeneration through callus initiation from thin mature embryo fragments of wheat. Plant Cell, Tissue and Organ Culture 67(1):73-80.
Crossref
|
|
|
|
|
FAOSTAT (2017). Agriculture data. Available at http://faostat.Fao.org. Accessed on 10th June 2019 http://faostat
|
|
|
|
|
Filippov M, Miroshnichenko D, Vernikovskaya D, Dolgov S (2006). The effect of auxins, time exposure to auxin and genotypes on somatic embryogenesis from mature embryos of wheat. Plant Cell, Tissue and Organ Culture 84(2):213-222.
Crossref
|
|
|
|
|
Hafeez I, Sadia B, Sadaqat NA, Kainth RA, Iqbal MZ, Khan IA (2012). Establishment of efficient in vitro culture protocol for wheat land races of Pakistan. African Journal of Biotechnology 11(11):2782-2790.
Crossref
|
|
|
|
|
Miroshnichenko DN, Filippov MV, Dolgov SV (2013). Medium optimization for efficient somatic embryogenesis and in vitro plant regeneration of spring common wheat varieties. Russian Agricultural Sciences 39(1):24-28.
Crossref
|
|
|
|
|
Miroshnichenko D, Chernobrovkina M, Dolgov S (2016). Somatic embryogenesis and plant regeneration from immature embryos of Triticum timopheevii Zhuk. and Triticumkiharae Dorof. et Migusch, wheat species with G genome. Plant Cell, Tissue and Organ Culture 125(3):495-508.
Crossref
|
|
|
|
|
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15(3):473-497.
Crossref
|
|
|
|
|
Parmar SS, Sainger M, Chaudhary D, Jaiwal PK (2012). Plant regeneration from mature embryo of commercial Indian bread wheat (Triticum aestivum L.) cultivars. Physiology and Molecular Biology of Plants 18(2):177-183.
Crossref
|
|
|
|
|
Rangaswamy R (2010). A textbook of agricultural statistics. New Age International Publishers, New Delhi 531 p.
|
|
|
|
|
Rashid H, Ghani RA, Chaudhry Z, Naqvi SMS, Quraishi A (2002). Effect of media, growth regulators and genotypes on callus induction and regeneration in wheat (Triticum aestivum). Biotechnology 1(1):49-54.
Crossref
|
|
|
|
|
Redway FA, Vasil V, Lu D, Vasil IK (1990). Identification of callus types for long-term maintenance and regeneration from commercial cultivars of wheat (Triticum aestivum L.). Theoretical and Applied Genetics 79(5):609-617.
Crossref
|
|
|
|
|
Repellin A, Båga M, Jauhar PP, Chibbar RN (2001). Genetic enrichment of cereal crops via alien gene transfer: new challenges. Plant Cell, Tissue and Organ Culture 64(2-3):159-183.
Crossref
|
|
|
|
|
Sharma VK, Rao A, Varshney A, Kothari SL (1995). Comparison of developmental stages of inflorescence for high frequency plant regeneration in Triticum aestivum L. and T. durum Desf. Plant Cell Reports 15(3-4):227-231.
Crossref
|
|
|
|
|
Stober A, Hessu D (1997). Spike pre-treatments, anther culture conditions and anther culture response of 17 German varieties of spring wheat (Triticum aestivum L.). Plant Breeding 116(5):443-447.
Crossref
|
|
|
|
|
Teng WL, Sin T, Teng MC (2002). Explant preparation affects culture initiation and regeneration of Panax ginseng and Panax quinquefolius. Plant Cell, Tissue and Organ Culture 68(3):233-239.
Crossref
|
|
|
|
|
Wilkins BM (1984). Advanced plant physiology. English Language Book Society/Longman. Avon.
|
|
|
|
|
Zale JM, Borchardt-Wier H, Kidwell KK, Steber CM (2004). Callus induction and plant regeneration from mature embryos of a diverse set of wheat genotypes. Plant Cell, Tissue and Organ Culture 76(3):277-281.
Crossref
|
|