ABSTRACT
In this study, the potential use of sweet whey as a base medium for lactic acid bacteria culture and its effect on the technological characteristics of lactobacilli was evaluated. Bacteria were grown on raw or deproteinized whey supplemented with different amounts of yeast extract and/or tomato juice. Results obtained showed that biomass production on whey supplemented with yeast extract was higher than those observed with tomato juice. Whilst, supplementation by either whey, tomato juice or yeast extract increased significantly the growth of both lactic acid bacteria, the performance was in the same range as that on a modified MRS medium. Additionally, acidification and proteolytic activities resulting from control and modified MRS medium were of comparable values. The present investigation demonstrates that sweet whey can be used as an alternative substrate for lactic acid bacteria cultural purposes and provide opportunity to make a new growth medium with low cost.
Key words: Acidification, bacterial growth, Lactobacillus, proteolytic activity, sweet whey, tomato juice, yeast extract.
Many sub-products of food industry that are released into the environment are a source of pollution. Among these, we are interested in whey from dairy industry. This sub-product is still poured in large quantities, simply in rivers and sewers. Because of its biochemical composition (lactose, proteins, vitamins), whey is an excellent culture medium for microorganisms and thus is a great source of pollution (Boudjema et al., 2009). In order to promote these high tonnages whey, we are interested in using sweet whey as a culture medium for lactic acid bacteria (LAB).
Indeed, LAB are demanding nutritionally (Desmazeaud, 1983), and culture media have to be supplemented with various peptide sources and growth factors (Pritchard and Coolbear, 1993; John et al., 2007). Rich and complex media, like MRS (De Man et al., 1960) or M17 (Terzaghi and Sandine, 1975) are available and especially suitable for the culture of LAB: they contain complex nitrogen supplementation, like peptones, yeast extract and meat extract, supplying LAB growth with peptidic nitrogen and growth factors. But, their use is limited to specific uses because of their high costs. This is why many researchers are interested in optimization of culture media for LAB by adding different sources of nitrogen like ram horn (Kurbanoglu, 2004), corn steep liquor (Cornelius et al., 1996; Marcela et al., 2016), whey protein hydrolysates (Mc Comas and Gilliland, 2003; Watanabe et al., 2004), malt sprout extract, casein hydrolysates (Vahvaselkä and Linko, 1987; Chiarini et al., 1992; HoráÄková, 2014), baker’s yeast cells (Altaf et al., 2007), vegetal substrates (Gardner et al., 2001; Nancib et al., 2001; Charalampopoulos et al., 2002; Djeghri-Hocine et al., 2006; Djeghri-Hocine et al., 2007a), sugarcane juice (Preeti et al., 2016), Palmyra palm jaggery (Reddy Tadi et al., 2017), and de-lipidated egg yolk (Djeghri-Hocine et al., 2007b). The most efficient nitrogen source seems to be yeast extract (Aeschlimann and Von Stokar, 1990; Arasaratnam et al., 1996; Göksungur and Gűvenç, 1997; Guha et al., 2013; Vethakanraj et al., 2013). But this is costly in industrial production. Therefore, attempts have been made to replace the yeast extract (Baralle and Borzani, 1988; Amrane and Prigent, 1993; Amrane, 2000; Gaudreau et al., 2005; Timbuntam et al., 2006).
Furthermore, other interesting constituents for the growth of bacteria and especially Lactobacillus genus that was studied, is the tomato juice, rich in simple sugars, minerals and vitamins. It stimulates growth, increases bacterial biomass, with shorter generation time and improves production of acid in medium (Gibson and Abdel-Malek, 1945; Miller and Puhan, 1980; Babu et al., 1992).
In our work, we investigated the possibility of valorization of sweet whey as culture medium for lactic acid bacteria belonging to Lactobacillus genus.
Bacterial strains
The following strains used in this work belong to the collection of the Laboratory of Biology of Microorganisms and Biotechnology, University of Oran 1 Ahmed Ben Bella, Algeria: Lactobacillus plantarum BH14; Lactobacillus casei CHTD27, Lactobacillus sp. CHM11 isolated from raw camel milk, Lactobacillus sp. V8 and V14 isolated from fresh ovin meat and Lactobacillus sp. LVK9, LVK11, LVK12 isolated from cow’s raw milk.
Media
Culture medium used was upon sweet whey, from local manufacturing plant soft cheeses (camembert) and tomato juice (TJ). Whey was deproteinized by heating in a water bath at 100°C for 30 min, then centrifuged at 5000 rpm for 15 min. The supernatant was filtered through a standard paper filter (DURIEUX, ref: 66301130). The same procedure, except the heat treatment was performed on the tomato after scraping (Boudjema et al., 2009).
Deproteinized whey was then supplemented with various concentrations of yeast extract (YE) and/or fresh tomato juice (pH is adjusted to 5.7 with 5N NaOH or 5N HCl) supplemented with 0.1 g of magnesium sulfate and 0.05 g of manganese sulphate. The whole was autoclaved at 120°C for 15 min.
Culture
After incubation for 24 h at 30°C, bacterial growth was measured by turbidimetry at a wavelength of 600 nm and a microbial count on MRS solid medium (results are expressed as U.F.C / ml).
Measuring the acidity produced
Measurement of the acidity produced by the bacterial strains was done by Dornic titration as described by Karam and Karam (1994).
Detection of cellular proteolytic activity
Proteolytic activity was sought in a solid medium on a Petri dish buffered to pH 7 with phosphate buffer (KH2PO4 / Na2HPO4 0.1 M) supplemented with 1% sterile reconstituted skim milk at 10%. According to the method described by Van den Berg et al. (1993), bacterial strains tested were seeded simultaneously on the surface of culture medium using multipoint inoculator. After incubation at 30°C for 48 h, proteolytic activity was revealed by the appearance of a clear halo around each bacterial colony. The dimension of this halo was later measured.
Optimization of culture medium
WY growth medium (Whey + yeast extract)
Results obtained clearly show that biomass production was improved by the non-supplemented whey (Figure 1). L. casei CHTD27 biomass was proportional to the amount of yeast extract added until concentrations above 8%, and then stabilized. For L. plantarum BH14, biomass continues to increase up to 10% of yeast extract. There are differences in biomass between the two strains for the same yeast extract concentration; this is probably due to the strain effect.

The stimulant effect of YE on bacterial growth was often reported (Baralle and Borzani, 1988; Amrane and Prigent, 1993; Champagne et al., 1999; Nancib et al., 2005; Djeghri-Hocine et al., 2007b). However, Boudjema et al. (2009) and Ghaly et al. (2003) reported that at high concentrations of YE, the cell concentration decreases, under the effect of a high toxicity of YE.
From the viewpoint of medium cost, the use of a culture medium with such concentrations of YE is not profitable. For this reason we have substituted yeast extract by the tomato juice.
WT growth medium (Whey + tomato juice)
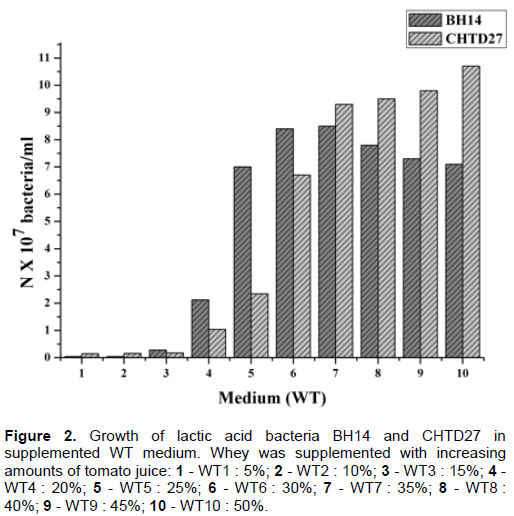
Figure 2 shows the results obtained. The addition of tomato juice to increase concentrations stimulates bacterial biomass proportionally, except for the strain L. plantarum BH14 where we recorded maximum growth in the presence of 35% (v / v) tomato juice (WT7 medium). A decrease in biomass was observed with higher quan-tities of tomato juice (WT 8, WT 9 and WT 10 medium).
This could be explained by the reduction of activity of β-galactosidase because of the high concentration of acids found in tomato juice.
Also, Inagawa et al. (1987) showed that phytic acid found in the corn syrup at a concentration of 4.4% reduces the activity of β-galactosidase in vivo, and therefore assimilation of lactose by bacteria.
Small biomass differences between the two strains were observed for the same concentration of tomato juice. Our observations agree well with the work of Babu et al. (1992) which showed that the addition of tomato juice in the skimmed milk stimulates the growth of lactobacilli.
However, the amount of biomass produced in a medium enriched with the tomato juice is significantly lower than that obtained with YE, which is probably due to a source of nitrogen deficiency in the tomato juice.
To overcome these limitations, and in order to obtain good productivity of biomass, we performed bacterial growth assays in a whey-based medium containing the two supplements (yeast extract and tomato juice).
WT growth medium (Whey + tomato juice+ yeast extract)
From results obtained previously, we chose the medium WT6 containing 30% tomato juice, because beyond this concentration the medium becomes cloudy, which influences the spectrophotometric reading.
In addition, we limited ourselves to a maximum concentration of 1% YE, because there was a good biomass production at this concentration. Amrane (2000) worked on the production of lactic acid by Lactobacillus helveticus cultured in the supplemented whey by YE and concluded that the concentrations of yeast extract greater than 20 g/L become toxic to the microorganism. Similarly, Ghaly et al. (2003) reported that at high concentrations of yeast extract, the cell concentration decreases as a result of the high toxicity of yeast extract. Bouguettoucha et al. (2007), Kulozik and Wilde (1999), Amrane and Prigent (1997), who worked on a milk free thermophilic bacterium L. helveticus and Norton et al. (1994), Schepers et al. (2006) and Sirisansaneeyakul et al. (2007), who have worked respectively on thermophilic lactic bacteria immobilized Lactobacillus lactis IO1, L. helveticus found that 10 g/L yeast extract was the optimal concentration for these strains, so they can give good growth and production of lactic acid.
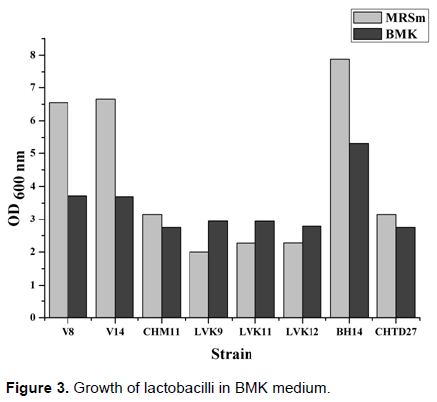
All previous results led us to retain WT6 medium at which we have added 1% yeast extract: the so-called BMK medium was chosen for remain study. Figure 3 shows the results obtained. We noticed that all tested strains grew in BMK medium to the extent that we achieved important cell densities indicating that it was favourable. The second observation was that growth was also a function of the strain. Indeed, we can distinguish three categories of strains according to their growth in that environment: those whose growth was much larger in the MRSm medium than BMK medium, those with almost identical growth in both media and those whose growth was higher in the BMK medium than MRSm medium.
All these observations indicate that BMK medium can be used to grow lactic acid bacteria and we wanted to examine whether the main technological characteristics of these bacteria were expressed in these trophic conditions.
Technological characteristics of lactobacilli grown in BMK medium
Production of lactic acid
On BMK medium, the strain CHTD27 gives an amount of lactic acid almost equivalent to that produced on MRSm medium (5 and 5.5 g/L respectively), but a very significant difference is observed for BH14 strain wherein the amount of lactic acid produced in MRSm is double that produced in BMK medium (Figure 4). Differences were also observed for the same medium depending on the strain.

These results are consistent with observations reported by several authors on the effect of yeast extract and differences in acidifying power of strains. Amrane and Prigent (1999), Kulozik and Wilde (1999) and Sherpers et al. (2006) showed that for a full use of the lactose, it is essential to supplement whey with nitrogen sources. According to Zhang et al. (2007), the addition of yeast extract to deproteinized whey has greatly increased not only the rate of acidification by Lactobacillus bulgaricus 11842 but also the activity of β-galactosidase. This activation leads to degradation of lactose and a good uptake of glucose which results in a good growth.
Proteolytic activity of the lactic acid bacterial strains
Results obtained (Figures 5, 6 and 7) show unequivocally that proteolytic activity observed is manifested in the same way in the two culture media tested. The result, (h/c) [h: diameter of the clear halo (mm); c: diameter of the colony (mm)] is substantially identical in the two culture media.
Our culture tests on deproteinized whey showed that it is too poor to the needs of lactobacilli. Its optimization through the addition of yeast extract and/or tomato juice led to the development of an ''inexpensive'' media for the growth of certain Lactobacilli in a similar way to that obtained in MRS medium. In addition, the results show that on BMK medium [whey supplemented with a mixture of tomato juice and yeast extract (at concentrations of 30 and 1%, respectively)], the biomass obtained is comparable to that observed in MRS medium.
The authors have not declared any conflict of interests.
REFERENCES
|
Aeschlimann A, von Stockar U (1990). The effect of yeast extract supplementation on the production of lactic acid from whey permeate by Lactobacillus helveticus. Appl. Microbiol. Biotechnol. 32:398-402.
Crossref
|
|
|
|
Altaf M, Naveena BJ, Reddy G (2007). Use of inexpensive nitrogen sources and starch for L (+) lactic acid production in anaerobic submerged fermentation. Bioresour. Technol. 98:498-503.
Crossref
|
|
|
|
|
Amrane A (2000). Effect of inorganic phosphate on lactate production by Lactobacillus helveticus grown on supplemented whey permeate. J. Chem. Technol. Biotechnol. 75:223-228.
Crossref
|
|
|
|
|
Amrane A, Prigent Y (1993). Influence of media composition on lactic acid production rate from whey by Lactobacillus helveticus. Biotechnol. Lett. 15:239-244.
Crossref
|
|
|
|
|
Amrane A, Prigent Y (1997). Growth and lactic acid production coupling for Lactobacillus helveticus cultivated on supplemented whey: influence of peptidic nitrogen deficiency. J. Biotechnol. 55:1-8.
Crossref
|
|
|
|
|
Amrane A, Prigent Y (1999). Analysis of growth and production coupling for batch cultures of Lactobacillus helveticus with the help of an unstructured model. Process Biochem. 34:1-10.
Crossref
|
|
|
|
|
Arasaratnam V, Senthuran A, Balasubramaniam K (1996). Supplementation of whey with glucose and different nitrogen sources for lactic acid production by Lactobacillus delbrueckii. Enzyme Microb. Technol. 19:482-486.
Crossref
|
|
|
|
|
Babu V, Mital BK, Garg SK (1992). Effect of tomato juice addition on the growth and activity of Lactobacillus acidophilus. Int. J. Food Microbiol. 17:67-70.
Crossref
|
|
|
|
|
Baralle SB, Borzani W (1988). Use of yeast autolysate to improve batch lactic fermentation of whey by Lactobacillus bulgaricus. Arq. Biol. Tecnol. 31:273-274.
|
|
|
|
|
Boudjema KA, Fazouane-Naimi F, Hellal A, Mechakra A (2009). Optimisation et modèle de production d'acide lactique par Streptococcus thermophilus sur lactosérum. Sci. Technol. C 29:80-90.
|
|
|
|
|
Bouguettoucha A, Balannec B, Nacef S, Amrane A (2007). A generalised unstructured model for batch cultures of Lactobacillus helveticus. Enzyme Microb. Technol. 41:377-382.
Crossref
|
|
|
|
|
Champagne CP, Gaudreau H, Conway J, Chartier N, Fonchy E (1999). Evaluation of yeast extracts as growth media supplements for lactococci and lactobacilli by using automated spectrophotometry. J. Gen. Appl. Microbiol. 45:17-21.
Crossref
|
|
|
|
|
Charalampopoulos D, Pandiella SS, Webb C (2002). Growth studies of potentially probiotic lactic acid bacteria in cereal based substrates. J. Appl. Microbiol. 92:851-859.
Crossref
|
|
|
|
|
Chiarini L, Mara L, Tabacchioni S (1992). Influence of growth supplements on lactic acid production in whey ultrafiltrate by Lactobacillus helveticus. Appl. Microbiol. Biotechnol. 36:461-464.
Crossref
|
|
|
|
|
Cornelius C, Erpicum T, Jacques P, Thonart P (1996). Comparison of fermentation industrial components such as corn steep and yeast extract for lactic acid bacteria production. Mededelingen van de Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen (Rijksuniversiteit te Gent). 61:1461-1463.
|
|
|
|
|
De Man JC, Rogosa M, Sharpe ME (1960). A medium for cultivation of lactobacilli. J. Appl. Microbiol. 23:130-135.
Crossref
|
|
|
|
|
Desmazeaud M (1983). Nutrition of lactic acid bacteria: state of the art. Le Lait 63:267-316.
Crossref
|
|
|
|
|
Djeghri-Hocine B, Boukhemis M, Zidoune N, Amrane A (2006). Horse bean extract for the supplementation of lactic acid bacteria culture media. J. Food Technol. 4:299-302.
|
|
|
|
|
Djeghri-Hocine B, Boukhemis M, Zidoune N, Amrane A (2007a). Growth of lactic acid bacteria on oilseed crop pea and chickpea based media. World J. Microbiol. Biotechnol. 23:765-769.
Crossref
|
|
|
|
|
Djeghri-Hocine B, Boukhemis M, Zidoune N, Amrane A (2007b). Evaluation of de-lipidated egg yolk and yeast autolysate as growth supplements for lactic acid bacteria culture. Int. J. Dairy Technol. 60:292-296.
Crossref
|
|
|
|
|
Gardner NJ, Savard T, Obermeier P, Caldwell G, Champagne CP (2001). Selection and characterization of mixed starter cultures for lactic acid fermentation of carrot, cabbage, beet and onion vegetable mixtures. Int. J. Food Microbiol. 64:261-275.
Crossref
|
|
|
|
|
Gaudreau H, Champagne CP, Jelen P (2005). The use of crude cellular extracts of Lactobacillus delbrueckii ssp. bulgaricus 11842 to stimulate growth of a probiotic Lactobacillus rhamnosus culture in milk. Enzyme Microb. Technol. 36:83-90.
Crossref
|
|
|
|
|
Ghaly AE, Tango MSA, Dams MAA (2003). Enhanced lactic acid production from cheese whey with nutrient supplement addition. Agricultural Engineering international: the CIGR J. Sci. Res. Dev. 5:364-369.
|
|
|
|
|
Gibson T, Abdel-Malek Y (1945). The formation of carbon dioxide by lactic acid bacteria and Bacillus licheniformis and a cultural method of detecting the process. J. Dairy Res. 14:35-44.
Crossref
|
|
|
|
|
Göksungur Y, Güvenç U (1997). Batch and continuous production of lactic acid from beet molasses by Lactobacillus delbrueckii IFO 3202. J. Chem. Technol. Biotechnol. 69:399-404.
Crossref
|
|
|
|
|
Guha A, Banerjee S, Bera D (2013). Production of lactic acid from sweet meat industry waste by Lactobacillus delbruki. Int. J. Res. Eng. Technol. 2:630-634.
Crossref
|
|
|
|
|
HoráÄková S, SedláÄková P, Sluková M, Plocková M (2014). Influence of Whey, Whey Component and Malt on the Growth and Acids Production of Lactobacilli in Milk. Czech J. Food Sci. 32:526-531.
|
|
|
|
|
Inagawa J, Kiyosawa I, Nagasawa T (1987). Effect of phytic acid on the hydrolysis of lactose with β-galactosidase. Agr. Biol. Chem. 51(11):3027-3032.
Crossref
|
|
|
|
|
John RP, Nampoothiri KM, Pandey A (2007). Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 74:524-534.
Crossref
|
|
|
|
|
Karam NE, Karam H (1994). Alimentation, génétique et santé de l'enfant, Eds J.F. Desjeux et M.Touhami, L'harmattan, Paris. pp. 257-264.
|
|
|
|
|
Kulozik U, Wilde J (1999). Rapid lactic acid production at high cell concentrations in whey ultrafiltrate by Lactobacillus helveticus. Enzyme Microb. Technol. 24:297-302.
Crossref
|
|
|
|
|
Kurbanoglu EB (2004). Enhancement of lactic acid production with ram horn peptone by Lactobacillus casei. World J. Microbiol. Biotechnol. 20:37-42.
Crossref
|
|
|
|
|
Marcela PB, Luciana FC, Daiane CS, Jonas C (2016). L-(+)-Lactic acid production by Lactobacillus rhamnosus B103 from dairy industry waste. Braz. J. Microbiol. 47:640-646.
Crossref
|
|
|
|
|
Mc Comas KA, Gilliland SE (2003). Growth of probiotic and traditional yogurt cultures in milk supplemented with whey protein hydrolysate. J. Food Sci. 68:2090-2095.
Crossref
|
|
|
|
|
Miller B, Puhan Z (1980). Mögllihkeiten zur Verkürzung deer. Fermentation bei Acidophilus-Milch. Schweig. Milchwissenchaft. 9:49-56.
|
|
|
|
|
Nancib A, Nancib N, Meziane-Cherif D, Boubendir A, Fick M, Boudrant J (2005). Join effect of nitrogen sources and B vitamin supplementation of date juice on lactic acid production by Lactobacillus casei subsp. rhamnosus. Bioresour. Technol. 96:63-67.
Crossref
|
|
|
|
|
Nancib N, Nancib A, Boudjelal A, Benslimane C, Blanchard F, Boudrant J (2001). The effect of supplementation by different nitrogen sources on the production of lactic acid from date juice by Lactobacillus casei subsp. rhamnosus. Bioresour. Technol. 78:149-153.
Crossref
|
|
|
|
|
Norton S, Lacroix C, Vuillemard JC (1994). Reduction of yeast extracts supplementation in lactic acid fermentation of whey permeate by immobilized cell technology. J. Dairy Sci. 77:2494-2508.
Crossref
|
|
|
|
|
Preeti C, Dimpi G, Raman K, Pooja A, Suman D (2016). Lactic Acid Production Vis-à-Vis Biowaste Management Using Lactic Acid Bacteria. World Appl. Sci. J. 34:1542-1552.
|
|
|
|
|
Pritchard GG, Coolbear T (1993). The physiology and biochemistry of the proteolytic system in lactic acid bacteria. FEMS Microbiol. Rev. 12:179-206.
Crossref
|
|
|
|
|
Reddy Tadi SR, EVR A, Limaye AM, Sivaprakasam S (2017). Enhanced production of optically pure d (-) lactic acid from nutritionally rich Borassus flabellifer sugar and whey protein hydrolysate based-fermentation medium. Biotechnol. Appl. Biochem. 64:279-289.
Crossref
|
|
|
|
|
Schepers AW, Thibault J, Lacroix C (2006). Continuous lactic acid productions in whey permeate/yeast extract medium with immobilized Lactobacillus helveticus in a two stage process: model and experiments. Enzyme Microb. Technol. 38:324-337.
Crossref
|
|
|
|
|
Sirisansaneeyakul S, Luangpipat T, Vanichsriratana W, Srinophakun T, Chen HH, Chisti Y (2007). Optimization of lactic acid production by immobilized Lactococcus lactis IO-1. J. Ind. Microbiol. Biotechnol. 34:381-391.
Crossref
|
|
|
|
|
Terzaghi BE, Sandine WE (1975). Improved medium for lactic streptococci and their bacteriophage. Appl. Microbiol. 29:807-813.
|
|
|
|
|
Timbuntam W, Sriroth K, Tokiwa Y (2006). Lactic acid production from sugar-cane juice by a newly isolated Lactobacillus sp. Biotechnol. Lett. 28:811-814.
Crossref
|
|
|
|
|
Vahvaselkä MI, Linko P (1987). Lactic acid fermentation in milk ultrafiltrate by Lactobacillus helveticus. In: Neyssel OM, van der Meer RR, Huyben KCAM (eds) Proceedings of the 4th European Congress on Biotechnology, vol 3, Elsevier, Amsterdam, pp. 317-320.
|
|
|
|
|
Van den Berg DJC, Smits A, Pot B, Ledeboer AM, Kersters K, Verbakel JMA, Verrips CT (1993). Isolation, screening and identification of lactic acid bacteria from traditional food fermentation process and culture collections. Food Biotechnol. 7:189-205.
Crossref
|
|
|
|
|
Vethakanraj HS, Sayanti S, Ashoke RT, Shaon RC (2013). Screening of bacteria for lactic acid production from whey water. Am. J. Biochem. Biotechnol. 9:118-123.
Crossref
|
|
|
|
|
Watanabe M, Kaburagi T, Seto Y, Morita F, Suzuki Y, Nakajima H (2004). Whey protein hydrolyzate for growth promotion of lactic acid bacteria. Jpn Kokai Tokkyo Koho, pp 11. Coden: JKXXAF JP 2004057047.
|
|
|
|
|
Zhang ZY, Jin B, Kelly JM (2007). Production of lactic acid from renewable materials by Rhizopus fungi. Biochem. Eng. J. 35:251-263.
Crossref
|
|