ABSTRACT
The existence of Tomato yellow leaf curl virus (TYLCV) was figured out in different locations in Al-Ahsaa of Saudi Arabia. Polymerase chain reaction (PCR) results of samples collected showed that TYLCV existed in all locations. Using AVcore and ACcore primers, begomoviruses family were detected in symptomatic tomato plants and by using TYv2664 and TYc138 (specific primers for the detection of TYLCV), the results proved that the samples were infected with TYLCV. The lipid-soluble fraction of healthy and infected tomato leaves extract was compared using gas chromatography techniques. A total of 46 compounds were identified in both healthy and virus-infected leaf tissues; among which 37 metabolites were common between both samples and increased or decreased in concentration due to the virus attack. Nevertheless, eight compounds were exclusively detected in the infected samples with only one compound consumed and thus recognized only in the healthy samples. The classifications and roles of the identified metabolites were discussed from the point of view of plant defense mechanisms or virus resistance against plant defense.
Key words: Tomato yellow leaf curl virus (TYLCV), begomoviruses, Polymerase chain reaction (PCR), gas chromatography.
Tomato (Solanum lycopersicum, L.) is economically important in Saudi Arabia and is one of the most important vegetable crops in the world. It is considered as one of the most popular and widely grown vegetable crops worldwide with the area harvested in Saudi Arabia being 14,902 ha in 2016 (FAOSAT, 2016). The production of tomatoes in Saudi Arabia in 2016 was 503,217 tonnes with most of that production (60%) grown in greenhouses (FAOSTAT, 2016). Begomoviruses have one (monopartite) or two (bipartite) genomic components, denominated DNA-A and DNA-B, and are transmitted in a persistent manner by whiteflies of the species complex Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) to dicotyledonous plants (Fernandes et al., 2010). Tomato yellow leaf curl virus (TYLCV) is one of the most important harmful and invasive members of the genus begomovirus (family Geminiviridae), which is widespread over the world associated with tomato yellow leaf curl disease (Barboza et al., 2013). TYLCV is transmitted by whiteflies and can spread rapidly; it is also not transmitted through seed or by mechanical transmission.
Severe symptoms such as leaf curling, stunting, and yellowing showed on TYLCV-infected tomato plants, which cause serious production loss in tomato cultivation (Kil et al., 2016; Papayiannis et al., 2010). In addition to tomato, other cultivated plants including pepper (Capsicum species), common bean (Phaseolus vulgaris), cucurbit (Cucumis species) and eustoma (Eustoma grandiflora) have been reported to be TYLCV hosts (Anfoka et al., 2009; Kil et al., 2016). Polymerase chain reaction (PCR) was applied to detect and establish provisional identity of begomoviruses through amplification of 575 bp fragment of the begomoviral coat protein gene (CP), referred to as the 'core' region of the CP gene (core CP). The core CP fragment contains conserved and unique regions, and was hypothesized to constitute a sequence useful for begomovirus classification (Brown et al., 2001). Metabolomics is used nowadays as a high potential tool for understanding different metabolic changes in many biological systems and its applications have been recognized in the quality control validations and natural products research (Dai et al., 2010).
However, the use of metabolomics in investigating interactions between different organisms is until now infrequent. For example, a metabolic profile for Catharanthus roseus leaves infected with phytoplasma has been determined (Choi et al., 2004)and the aromatic metabolite profiles of Arabidopsis thaliana infected by Pythium sylvaticum has been investigated (Bednarek et al., 2005). To the knowledge of the authors, the metabolites that resulted from the interaction between the Tomato yellow leaf curl virus and its host the tomato plant (S. lycopersicum) has never been investigated. The main aim of this study was to explore the type of phytochemicals newly produced, increased or decreased in concentration during the attack of TYLCV on the tomato plant leaves. The study could ascribe to the better knowledge of the plant-virus chemical connection.
Plant material
Healthy tomato (S. lycopersicum, family Solanaceae) leaves samples (HTL) were identified and collected from the local fields and greenhouses in Al-Ahsaa, Eastern province, Saudi Arabia. Meanwhile, TYLCV naturally-infected tomato leaves samples (ITL) showing the typical symptoms of TYLCV infection (severe stunting, yellowing, curling of leaves and chlorosis on leaves) were collected from same field greenhouses (Figure 1). All plants were identified by experts and taxonomists in the College of Agriculture and Food Sciences, King Faisal University, Saudi Arabia with specimens deposited to the herbarium of the college.
Primers for begomoviruses and TYLCV
Two sets of primers were used in this study to identify the TYLCV infection (Table 1). The first set of primers were AVcore and ACcore used for the detection of begomoviruses and the second set of primers were TYv2664 and TYc138 used for the detection of TYLCV (Table 1).
Extraction of total DNA from plant tissues
Total DNA was isolated from the infected tomato plants using DNeasy® Plant Mini Kit obtained from QIAGEN as manufacturer's instruction.
Polymerase chain reaction (PCR)
The extracted DNA was used as a template for PCR using set of primers as shown in Table 1. AVcore and ACcore primers were used as degenerate primers for begomoviruses group to amplify 575 bp while TYv2664 and TYc138 primers were used to amplify 316 bp of IR of TYLCV. PCR reactions were optimized for 25 µl and the final concentrations of reaction components were: 25 µM deoxynucleotide triphosphate (dNTPs), 2.5 µl of 10X PCR buffer, 2.5 mM MgCl2, 5 units Taq DNA polymerase, 1 µl of 10 µM of each complementary and viral-sense primers and 3 µl of DNA were used as target templates. PCR cycle parameters for AVcore and AC core primers were as follows: one cycle at 94°C for 2 min; 35 cycles at 94°C for 1 min, 55°C for 2 min, and 72°C for 2 min, followed by one cycle at 72°C for 10 min. PCR cycle parameters for TYv2664 and TYc138 primers were as follows: one cycle at 94°C for 5 min; 30 cycles at 94°C for 1 min, 62°C for 1 min, and 72°C for 1 min, followed by one cycle at 94°C for 1 min, 56°C for 1 min, and 72°C for 10 min. Five microliters aliquots of PCR products were analyzed on 1% agarose gels in 0.5X TBE buffer.
Plant sample preparation for analysis
Three hundred grams of the healthy and TYLCV-infected tomato leaves were isolated directly from the field to make the HTL and ITL samples, respectively. The samples were immediately placed into liquid nitrogen for preservation and enzyme deactivation and then pulverized into a powdered form and kept in -20°C until further analysis.
Metabolites extraction
The powdered samples were extracted by n-hexane (HPLC-grade, Fisher Chemicals) using a Soxhlet apparatus for 3 h (20 cycles, each) according to Shah and Alagawadi (2011). The n-hexane extracts were evaporated under reduced pressure to yield different residues. Twelve plants were used to give six HTL and ITL samples.
Metabolites isolation and identification
The n-Hexane extracts were investigated using gas-chromatography-mass spectrometry (GC-MS) for qualitative analysis and gas chromatography-flame ionization detection (GC/FID) quantitative analysis. The GC conditions involved the use of Shimadzu-QP-2010 machine equipped with a capillary column (DB-5 ms 30 m × 0.25 mm I.D., 0.25 μm). The chromatograph was programmed for an initial temperature of 50°C for 2 min followed by a 5°C/min temperature ramp to 280°C. The final temperature was maintained for 4 min. Injector and detector temperatures are maintained at 250 and 280°C, respectively. The initial head pressure of the carrier gas (He) was 90 kPa and a split injection system (ratio 1:20) was used. In GC/MS, the capillary column was directly coupled to a quadruple mass spectrometer (Shimadzu model QP2010S), the ionization mode was electron impact (EI) and ionization energy was 70 eV.
Components identification and percentage area calculation
Different separated compounds were identified using Kovat’s Retention indices (RI) calculated with respect to a set of co-injected homologous series of saturated hydrocarbon standards (C8 to C40, Sigma, UK). Compounds were identified by comparing their spectral data and RI with Wiley Registry of Mass Spectral Data 9th edition/NIST Mass Spectral Library (2011), and literature data (Adams, 2007). Some of the compounds were identified using authentic samples and those compounds are marked in Table 2. Calculations of peak percentage areas, based on FID response, are as follow:
Percent area of peak = (The FID peak area / the sum of all the FID peaks areas) × 100
Most of non-identified components are present as traces with relative abundances of less than 0.1%. The most important constituents identified in the n-hexane fractions analyzed are listed in Table 2. The percent area ratio was calculated for each component and displayed in Table 2. This ratio indicates that this component increased in concentration due to the virus attack (that is, the ratio will be more than 1) or decreased due to the attack (the ratio will be less than 1).
Statistical analysis
Six samples were used for both HTL and ITL (n=6), respectively and each sample was injected in triplicate. Quantitative values are expressed as mean ± standard error of mean of percentage areas and significance difference was determined using unpaired student-sample-t-test performed using SPSS statistical package version (SPSS for Windows, Version 11.5, SPSS Inc., Chicago, IL). P<0.05 was considered significant.
Detection of TYLCV
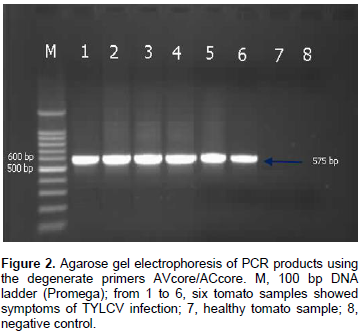
DNA of the expected sizes, 575 and 316 bp of begomoviruses and TYLCV, respectively, were amplified from symptomatic tomato plants using the primer pairs AVcore/ACcore (Figure 1) and TYv2664/TYc138 (Figure 2), respectively.
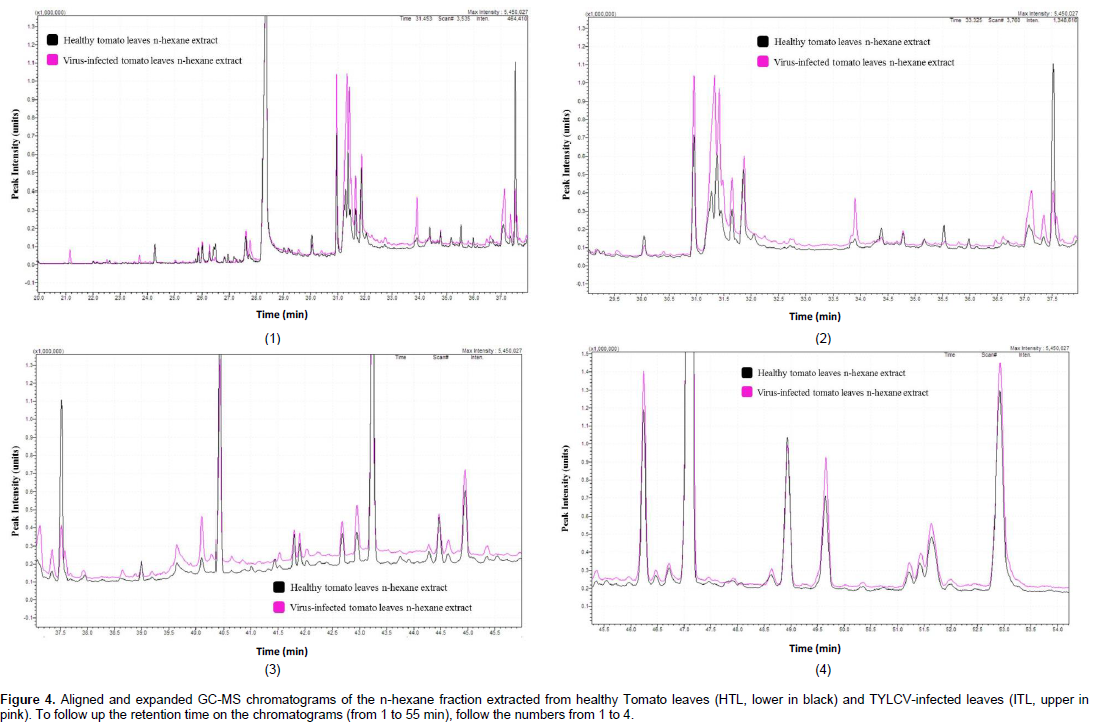
Analysis of n-hexane fraction
This study was performed to compare the lipid-soluble metabolic pool of compound in healthy tomato leaves to that of the TYLCV-infected leaves, which allows the identification of the newly synthesized metabolites, or those, which differ in concentration because of the virus attack. The gas chromatographic analysis of the n-hexane fraction (Figure 4 and Table 2) resulted in the separation of 84 components, 46 of which were identified, representing 80.40 and 82.64% of the total fraction contents of the HTL and ITL sample, respectively. Thirty-seven common compounds were identified between the two extracts; however eight compounds were produced uniquely in the infected tissue extracts and a compound was identified in the healthy samples only indicating its total consumption during the virus infection process. Similarly, six compounds were found in trace in the healthy tissues indicating that 14 compounds were more or less produced due to the virus infection (Table 2). The concentrations of the 12 compounds increased in the infected tissues in relation to the healthy ones. Nevertheless, ten common compounds decreased in concentration when the tissue was infected and both cases can be recognized from ITL/HTL ratio in Table 2. The change in concentration in ten common compounds was considered insignificant, and thus those compounds are considered to have no change in concentration due to the virus infection.
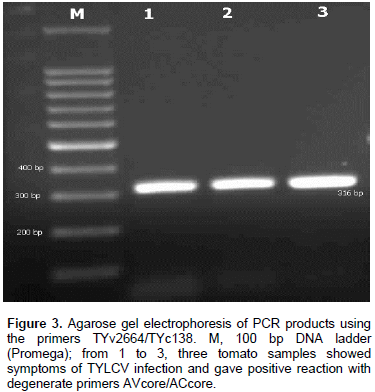
In the present study, the detection of begomoviruses in tomato was done and the results agreed with that of Alhudaib et al. (2014)and Rezk (2016)who used the degenerate primers of AVcore and ACcore to detect the begomoviruses in infected tomato samples in Saudi Arabia. Also, a leaf curl disease with symptoms typical of begomoviruses was observed in bean (P. vulgaris) at the Main Research Farm of the Indian Institute of Pulses Research, Kanpur, India (Kamaal et al., 2015) whereas Herrera-Vásquez et al. (2016)detected begomoviruses in commercial tomato plots using both production systems (open ï¬eld and greenhouse) in Panama with different degenerate primers. Just et al. (2014) stated that, imported tomato fruits infected with Tomato yellow leaf curl virus (TYLCV) were identified on the market in northern Europe using paper-based FTA Classic Cards (Whatman), PCR and partial DNA sequence analysis.
Impact of the metabolites pool changes due to the virus attack
Analysis of Table 2 discloses many classes of lipid-soluble components in the healthy and infected tissues of tomato leaves with different ratios. Sterols and triterpenes are the main class of compounds, which can be identified in the metabolites of both healthy and virus-infected leaves (Table 2). Although there was total decline in sterols and triterpenes concentration, 3-β-Stigmast-5-en-3-ol increased by 1.85 folds due to the virus infection. The concentration of β-Sitosterol represents nearly 25% of the total lipid-soluble fraction and it declined in the virus-infected leaves to reach 0.7 of its amount in healthy tissues. Accumulation of stigmasterol is a characteristic for plants during pathogens infection (Griebel and Zeier, 2010). Stigmasterol is chemically produced from β-sitosterol through C22 desaturation and this can explain the consumption of β-sitosterol and the increase in stigmasterol concentration due to the virus attack. Campesterol is produced uniquely in the virus-infected tomato leaves tissues. Campesterol are the precursor of steroidal phytohormones called brassinosteroids (Schaller, 2003), which are of vital role in plant defense mechanism against any pathogen attack (Choudhary et al., 2012).
The increase in Campesterol concentration due to the virus attack might indicate an effect applied by the virus to weaken the plant defense through prevention of the production of brassinosteroids. On the other hand, a low ratio of Campesterol to sitosterol is needed for high plant cell membrane integrity and functionality (Schaeffer et al., 2001), though this ratio was affected by the production of Campesterol in the virus infected tissues which could lead to interruption and weakness of cell member, that is, the curling effect. Sesquiterpenes are another class of compounds which are found in high ratio in the lipid soluble metabolic pool of tomato leaves. Although the total sesquiterpene compounds concentration insignificantly changed due to the virus infection, many individual components were produced exclusively or showed meaningful increase in concentration due to infection. Sesquiterpenes in tomato leaves metabolic pool can be divided into three main classes; Fernesols, bisabolines and abietic acid derivatives. Fernesols represent around 11.6% of the total lipid-soluble fraction of tomato leaves and although this whole ratio did not change due to the virus infection, many compounds were individually increased or decreased.
The most abundant fernesol-type sequitepene in tomato leaf is methyl farnesoate, which represents 9.82% in healthy tissue, and this percentage decreased to 8.37 in the infected tissues. Fernesols are insect hormones (Nagaraju, 2007)and prevent fungal mycelia development with slight anti-fungal properties (Hornby et al., 2001). Bisabolene-type compound are another type of component that belong to the sesquiterpenes pool. Bisabolenes are recognized as sexual pheromones (Brézot et al., 1994; Lu and Teal, 2001)and thus they, together with Fernesols could be emitted by the plant to attract insects (War et al., 2012)as a way to resist the virus attack. Abietic acid derivatives is another class of sesquiterpenes, which is represented in the total lipid-soluble fraction tomato leaves extract by nearly 11%. This ratio did not change significantly between the infected and non-infected leave tissues. Abietic acid and its derivatives are diterpenes which are known for their role in plant defense mechanisms and are recognized for their tissue healing properties and pathogen trapping capabilities (Costa et al., 2016).
Phytols are acyclic diterpene alcohols, which decreased due to the virus attack on the plant. Although phytol and phytol acetate concentration decreased, isophytol was produced uniquely in the attacked tissues. The production of isophytol can explain the decrease in concentrations in phytol and its acetate. The role of isophytol as a production in the virus infected tissues is not clear and needs further investigation. Fatty acids have been identified in both the HTL and ITL samples with 9.11 and 11.6%, respectively. The main fatty acids found in both extracts was stearic acids and its derivative; stearylaldehyde and its methyl ester. The concentration of stearylaldehyde has dramatically decreased in ITL tissues to reach 0.2% of its original concentration in HTL. However, the concentration of stearic acid and its methyl ester has increased by 1.4% for both compounds due to the virus attack and this could explain the decline in stearylaldehyde concentration.
TYLCV has been identified in local area of Al-Ahsaa region, Eastern province of Saudi Arabia using means of specific PCR primers. The fat-soluble metabolites resulting from the virus attack on the tomato plants has been revealed using means of GC/MS and quantified using GC/FID. Forty-six compounds were separated in both healthy and virus-infected leaf tissues, among which eight compounds were exclusively detected in the infected samples and only one compound was consumed and thus recognized only in the healthy samples.
The authors have not declared any conflict of interests
The authors are thankful to Dr. Mohamed S. Al-Saikhan, the Supervisor of Central Labs, College of Agricultural and Food Sciences, King Faisal University for assistance during scientific experiments.
REFERENCES
|
Adams, RP (2007). Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th Edition ed. Allured Publishing corporation, Chicago, Illinois, USA.
|
|
|
|
Alhudaib K, Alaraby W, Rezk AA (2014). Molecular characterization of tomato yellow leaf curl disease associated viruses in Saudi Arabia. Int. J. Virol. 10, 180-191.
Crossref
|
|
|
|
|
Anfoka G, Haj Ahmad F, Abhary M, Hussein A (2009). Detection and molecular characterization of viruses associated with tomato yellow leaf curl disease in cucurbit crops in Jordan. Plant Pathol. 58:754-762.
Crossref
|
|
|
|
|
Barboza N, Blanco-Meneses M, Hallwass M, Moriones E, Inoue-Nagata AK (2013). First Report of Tomato yellow leaf curl virus in Tomato in Costa Rica. Plant Dis. 98:699-699.
Crossref
|
|
|
|
|
Bednarek P, Schneider B, Svatos A, Oldham NJ, Hahlbrock K (2005). Structural complexity, differential response to infection, and tissue specificity of indolic and phenylpropanoid secondary metabolism in Arabidopsis roots. Plant Physiol. 138:1058-1070.
Crossref
|
|
|
|
|
Brézot P, Malosse C, Mori K., Renou M (1994). Bisabolene epoxides in sex pheromone innezara viridula (L.) (Heteroptera: Pentatomidae): Role ofcis isomer and relation to specificity of pheromone. J. Chem. Ecol. 20:3133.
Crossref
|
|
|
|
|
Brown JK, Idris AM, Torres-Jerez I, Banks GK, Wyatt SD (2001). The core region of the coat protein gene is highly useful for establishing the provisional identification and classification of begomoviruses. Arch. Virol. 146:1581-1598.
Crossref
|
|
|
|
|
Choi YH, Tapias EC, Kim HK, Lefeber AWM, Erkelens C, Verhoeven JTJ, Brzin J, Zel J, Verpoorte R, (2004). Metabolic Discrimination of Catharanthus roseus Leaves Infected by Phytoplasma Using 1H-NMR Spectroscopy and Multivariate Data Analysis. Plant Physiol. 135:2398-2410.
Crossref
|
|
|
|
|
Choudhary SP, Yu JQ, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS (2012). Benefits of brassinosteroid crosstalk. Trends Plant Sci. 17:594-605.
Crossref
|
|
|
|
|
Costa MS, Rego A, Ramos, V, Afonso TB, Freitas S, Preto M, Lopes V, Vasconcelos V, Magalhães C, Leão PN (2016). The conifer biomarkers dehydroabietic and abietic acids are widespread in Cyanobacteria. Sci. Rep. 6:23436.
Crossref
|
|
|
|
|
Dai H, Xiao C, Liu H, Tang H (2010). Combined NMR and LC-MS Analysis Reveals the Metabonomic Changes in Salvia miltiorrhiza Bunge Induced by Water Depletion. J. Proteome Res. 9:1460-1475.
Crossref
|
|
|
|
|
Food and Agriculture Organization of the United Nations (FAOSTAT) (2016). Food and Agricultural Organization of United Nations, Crop (Tomatoes).
|
|
|
|
|
Fernandes FR, Albuquerque LCd, Inoue-Nagata AK (2010). Development of a species-specific detection method for three Brazilian tomato begomoviruses. Trop. Plant Pathol. 35, 043-047.
Crossref
|
|
|
|
|
Griebel T, Zeier J (2010). A role for beta-sitosterol to stigmasterol conversion in plant-pathogen interactions. The Plant J. Cell Mol. Biol. 63:254-268.
|
|
|
|
|
Herrera-Vásquez JA, Ortega D, Romero AB, Davino S, Mejía LC, Panno S, Davino M (2016). Begomoviruses Infecting Tomato Crops in Panama. J. Phytopathol. 164:102-113.
Crossref
|
|
|
|
|
Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussault P, Nickerson KW (2001). Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992.
Crossref
|
|
|
|
|
Just K, Leke WN, Sattar MN, Luik A, Kvarnheden A (2014). Detection of Tomato yellow leaf curl virus in imported tomato fruit in northern Europe. Plant Pathol. 63(6):1544-1460.
Crossref
|
|
|
|
|
Kamaal N, Akram M, Agnihotri A K (2015). Molecular evidence for the association of Tomato leaf curl Gujarat virus with curl disease of Phaseolus vulgaris L. J. Phytopathol. 163(1):58-62.
Crossref
|
|
|
|
|
Kil EJ, Kim S, Lee YJ, Byun HS, Park J, Seo H, Kim CS, Shim JK, Lee JH, Kim JK, Lee KY (2016). Tomato yellow leaf curl virus (TYLCV-IL): a seed-transmissible geminivirus in tomatoes. Scientific reports. 6:19013.
Crossref
|
|
|
|
|
Lu F, Teal PEA (2001). Sex pheromone components in oral secretions and crop of male caribbean fruit flies, Anastrepha suspensa (Loew). Arch. Insect Biochem. Physiol. 48:144-154.
Crossref
|
|
|
|
|
Nagaraju GPC (2007). Is methyl farnesoate a crustacean hormone? Aquaculture 272:39-54.
Crossref
|
|
|
|
|
Papayiannis LC, Katis NI, Idris AM, Brown JK (2010). Identification of weed hosts of tomato yellow leaf curl virus in cyprus. Plant Dis. 95:120-125.
Crossref
|
|
|
|
|
Rezk AA (2016). Molecular characterization of tomato yellow leaf curl virus (TYLCV) infecting pepper and common bean. Int. J. Virol. 12(1-3):1-9.
Crossref
|
|
|
|
|
Schaeffer A, Bronner R, Benveniste P, Schaller H (2001). The ratio of campesterol to sitosterol that modulates growth in Arabidopsis is controlled by sterol methyltransferase 2;1. Plant J. Cell Mol. Biol. 25:605-615.
|
|
|
|
|
Schaller H (2003). The role of sterols in plant growth and development. Prog. Lipid Res. 42, 163-175.
Crossref
|
|
|
|
|
Shah AS, Alagawadi KR (2011). Anti-inflammatory, analgesic and antipyretic properties of Thespesia populnea Soland ex. Correa seed extracts and its fractions in animal models. J. Ethnopharmacol. 137:1504-1509.
Crossref
|
|
|
|
|
War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012). Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 7:1306-1320.
Crossref
|
|