Full Length Research Paper
ABSTRACT
The study was carried out to produce a microcapsule powder of total flavonoids and total total phenols of methanol extract of the cashew, using a complex coacervation encapsulation method. In the search for optimal conditions for encapsulation, a three level factorial design was set up, while taking into account factors like time and proportions in Arabic gum and gelatin. The kinetic of encapsulation follows a kinetic of 2nd order which gives polynomial equations of the second degree. The conditions found are respectively 45 min, 30% Arabic gum and 70% gelatin, for an encapsulation yield is 84.37%; the encapsulation rate is 77.9% for the total flavonoids and 76.5% for the total phenols. The powder obtained has a doubled concentration in total flavonoids and total phenols than the raw bark powder.
Key words: Encapsulation, complex coacervation, total flavonoids, total phenols, Anacardium occidentale.
INTRODUCTION
Humans have always used the products of their environment, especially plants for their medical needs. Plants have several therapeutic properties and their uses for the treatment of diseases in living beings are very old and have always been done empirically (Jeansheng and Cheng, 2018). These plants represent a primary source of medicines and have continued to provide humanity with new remedies to date. Now, research is showing more and more that the active ingredients in herbal medicines are often linked to secondary metabolites. Thus, the African and Malagasy Council for Higher Education (CAMES) program has empowered the Central Africa Network to carry out research activities on medicinal plants used in the treatment of complicated diseases, with a view to producing improved traditional medicines (Ngolo et al., 2018). In the perspective of researching molecules endowed with properties against these diseases, this study was oriented on one of the plants with proven pharmacological activities. It is Anacardium occidentale (Anacardiaceae).
A. occidentale is an 8-10 m tall tree, native to tropical America and introduced to all tropical countries. Phytochemical studies conducted on the plant reveal the presence of tannins, flavonoids (Annalisa et al., 2017), saponins (Dharamveer et al., 2013) and alkaloids (Alfa et al., 2017). Resorcinolic acid, ascorbic acid, carotenoids (α-carotene, β-carotene and β-cryptoxantin), vitamin C and phenols are also identified in cashew apples (Farid et al., 2014). The work of Madjitoloum et al. (2018b) isolated two flavonoids: quercetin-3-O--D-glucopyranoside and Kempferol-3-O--D-glucopyranoside. The decoction and infusion of the leaves and bark of the trunk are used in pharmacopoeia to treat several diseases namely: High blood pressure, gastrointestinal ailments, ulcers, and sore throat (Jeansheng and Cheng, 2018). In this practice, the effectiveness and quantity of the active ingredient in the solution is not controlled hence the need to extract it.
The performance of extracts in terms of biological activities is linked to extraction techniques. Speaking of extraction methods, apart from the so-called conventional extraction methods such as decoction, infusion, maceration, mechanical agitation and soxhlet, innovative methods of extraction, fractionation and identification of natural products of plant origin have been implemented in order to participate in technological advances faster, more efficient while reducing the quantities of solvent, energy and consumption of samples. Among these innovative extraction methods, microwave assisted extraction has also been implemented. This is also aiming to improve these traditional techniques that are long, tedious, and require large amounts of organic solvents harmful to our environment and health (Madjitoloum et al., 2018a). This technique can be influenced by the parameters such as power, time, solvent polarity, and liquid/solid ratio; its effects can be independent or interactive (Jing et al., 2016). Then, it is necessary to define the best extraction conditions in terms of biological activities and yield. Faced with these difficulties, the use of substances of plant origin is increasingly considered as an alternative or complementary means for poor populations (Raoufou and Kouami, 2013).
In addition to this concern, there is the procedure of formulating a powder by complex coacervation of the active extracts of A. occidentale. It should be noted that the quality and the rate of encapsulation of this powder depend on factors such as the time of mixing and the proportions of adjuvants such as gum arabic and gelatin. Encapsulation which is an inclusion technique to confine a substance in a polymeric matrix covered by one or more semi-permeable membranes, whereby the encapsulated compound becomes more stable than the one from which it was isolated. It becomes necessary to solve the shortcomings found in the adsorption technique (Deepak and Sheweta, 2020). In order to minimize the negative aspects, several techniques have been developed to implement different formulations to suit different applications. Nowadays, powder formulation has become one of the most attractive methods for immobilization and protection of active ingredients (Yuksel et al., 2018). To achieve this powder formulation, two main techniques have been developed following the example of adsorption and coacervation encapsulation (Yuksel et al., 2018). Coacervation can be simple or complex: Simple coacervation involves only a single polymer with the addition of strongly hydrophilic agents into the colloidal solution and complex coacervation, two or more polymers are used (Lv et al., 2012). The choice of complex coacervation is the easy release of the active ingredient into the solution.
Our objective is to protect the methanolic extract of A. occidentale trunk bark obtained under the optimal conditions of microwave-assisted extraction from adverse effects, minimizing the interactions between the active ingredient and the polymers of the formulation by complex coacervation (Mohammadinejad et al., 2016; Vázquez-González et al., 2021). To grasp this technique, a study on the kinetics and encapsulation parameters needed to be carried out followed by investigating the most influential levels of factors in complex coacervation.
MATERIALS AND METHODS
Plant material
Active ingredient
The active ingredient, called the active principe, in the formula is the methanol extract from the bark of the plant's trunk. This extract is obtained under optimal conditions of microwave assisted extraction at the time of 83 s, at the power of 620 W, at the solvent-matter ratio of 30 mL/g and at 63% water-methanol for which the values total phenols and total flavonoids, are respectively 655.90 mg EGA / 100 g DM and 82.94 mg EQ / 100 g DM of bark (Madjitoloum et al., 2018a). After filtration, the filtrate is concentrated using a rotary evaporator and dried in the jars, then crushed, pulverized and stored for further study.
Auxiliaries
The auxiliaries are adjuvants which have secondary and tertiary functions of the formula. These are gum Arabic and gelatin. Gum Arabic is a highly branched hydrocolloid and a polysaccharide polymer. Its solution has a density of negative charges compared to the acid function (Oumarou et al., 2010). Protein polymer gelatin is used as an encapsulation material thanks to its amphiphilic properties, its ability to interact with different types of molecules, its high molecular weight and the flexibility of its molecular chains. It has a density of positive charges in solution with respect to its amine functions to form ammonium ions (Marta et al., 2020).
Preparation of coacervates
The method used for the preparation of microcapsules is that of Sarunyoo et al. (2018) which has been adapted. The colloidalsolution is prepared by mixing the solutions of the two polymers with Moulinex to properly emulsify the solution: the gum arabic solution (2% m/v) with that of gelatin (8% m/v) at 40°C. The active principle (0.2 g) is introduced into the colloidal solution (1 mL) and the addition of CH3CO2H at 50% v/v is made to adjust the pH of the mixture to pH = 4.5 while stirring for 30 min. Then the addition of CH2O (37% w/v) 4 mL per 100 mL in the mixture allows crosslinking. Finally, the whole is incubated at 4°C for 30 min. Two phases were observed and after screening the coacervates are recovered and subjected to lyophilization to produce the powder of the microcapsules. The freeze-dryer (Scientz-10ND vacuum Freezer driyer) freezes the coacervate solution at -20°C for 4 h before producing the powder in 48 h.
Quantitative study of flavonoids and total phenols
For quantitative analysis, phytochemical quantification of total flavonoids and total phenols was carried out according to the protocol of Jothi et al. (2013) adapted by Madjitoloum et al. (2018a).
Study of the encapsulation parameters
Effect of time on encapsulation
Time is a parameter which influences the encapsulation of active extracts by complex coacervation. The mixing was carried out for a period of 0 to 120 min in steps of 15 min, the other parameters being constant. The models studied for modeling are as follows.
Influence of the proportion of gum arabic on the encapsulation
The proportion of the gum arabic solution varies from 0 to 100% in 25% steps and the time remains constant (45 min).
Influence of the proportion of gelatin on the encapsulation
We also varied the proportion of the gelatin solution from 0 to 100% in 25% steps, keeping the time constant (45 min).
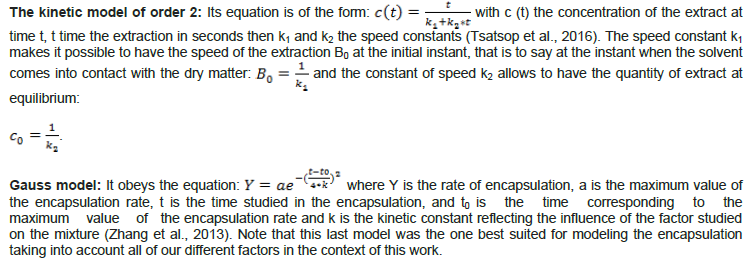
Encapsulation optimization
At the end of the tests, the experimental field for each of the three factors (time (min), proportion of the gum arabic solution (%) and proportion of the gelatin solution (%)) was chosen. A three-level factorial design is used to find the levels of the most influential factors in complex coacervation (Table 1).
Influence of the proportion of gum arabic on the encapsulation
The proportion of the gum arabic solution varies from 0 to 100% in 25% steps and the time remains constant (45 min).
Influence of the proportion of gelatin on the encapsulation
We also varied the proportion of the gelatin solution from 0 to 100% in 25% steps, keeping the time constant (45 min).
Encapsulation optimization
At the end of the tests, the experimental field for each of the three factors (time (min), proportion of the gum arabic solution (%) and proportion of the gelatin solution (%)) was chosen. A three-level factorial design is used to find the levels of the most influential factors in complex coacervation (Table 1).
Proposal of a model
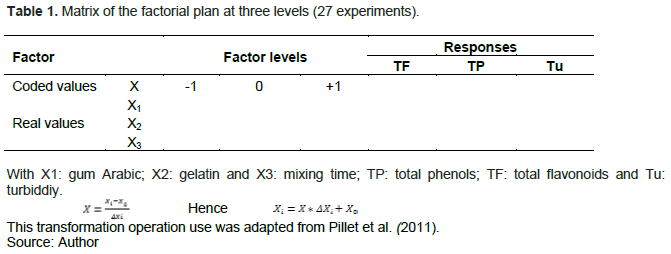
The proposed model has the advantage of properly representing the experimental responses studied in the experimental field of interest and making it possible to obtain an estimate of the value of the studied responses of acceptable quality. The model is as follows:
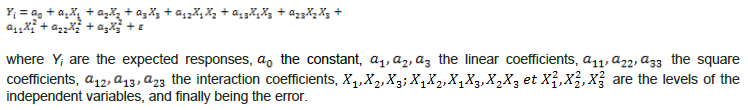
Validation of models
The performance of the model was measured by comparing the x values of the predicted responses with those observed. In addition to the linear regression coefficient (R²), other mathematical procedures and tools were used; the Absolute Analysis of Average Deviation (AADM), the bias factor (Bf) and the Accuracy factors Af1 (Catherine, 2015) and Af2 (Baranyi et al., 1999) were determined using the following expressions:
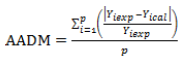

The graphical representations of the response surfaces of the postulated models were made using the STATGRAPHICS Centurion XV software.
Evaluation of the behaviour of the microcapsule powder
At the end of the encapsulation, a quantitative study of the powder obtained is made. The active ingredient present in microcapsules is characterized by several quantities.
Yield
The most common size of the powders obtained by coacervation is the encapsulation yield which is calculated according to the following formula:
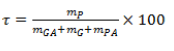
Where mP is the mass of the powder, mGA is the mass of the gum arabic, mG is the mass of the gelatin and mPA is the mass of the active ingredient. It is also the yield of the formulation.
Encapsulation rate
The optimized encapsulation rate of the extract is the content of total phenols or total flavonoids encapsulated on the content of total phenols or total flavonoids introduced expressed as a percentage (%) according to the formula:
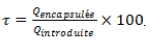
RESULTS AND DISCUSSION
Study of the influence of factors on coacervation encapsulation
Effect of time on coacervation encapsulation
The influence of time on complex coacervation is shown in Figure 1. The first part of the curves corresponds to an increasing curve from time t = 0 min to time t = 45 min where the optimum is reached at 44.28 NTU of the turbidity of the mixture and the degree of encapsulation of the total phenols and flavonoids respectively at 503.88 mg EGA/100 g P and 62.32 mg EQ/100 g P with a concavity turned upwards at t = 30 min. In this phase, the methanol extract of the bark from the trunk of the cashew is being encapsulated by polymers (gum arabic and gelatin). The second part corresponds to a decreasing curve at the end of t = 45 min to t = 120 min with an upward concavity at t = 75 min with a reduction in turbidity up to 20.62 NTU and the rate of encapsulation total phenols and flavonoids at 395.82 mg EGA/100 g P and 49.56 mg EQ/100 g P respectively. This phase corresponds to destruction of the microcapsules. The explanation that one could bring to these results is that at t = 45 min, the equilibrium of encapsulation of the active substance of the matrix and the polymers is reached, this is the isoelectric point. Beyond the point, the mixture mixing apparatus breaks the bonds between the polymers and there is degradation of the microcapsules which dissolve in the mixture. The study terminals chosen are [30; 60 min].
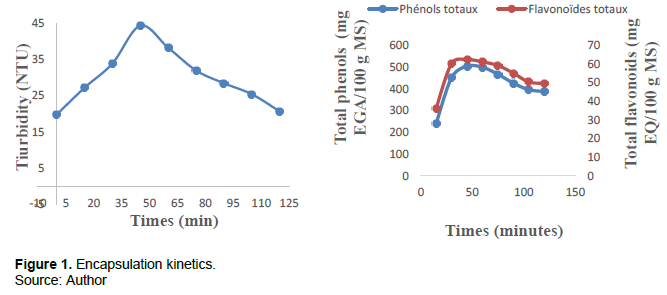
Kinetic modeling of encapsulation by coacervation
Two kinetic models allowed us to describe the rate of encapsulation:
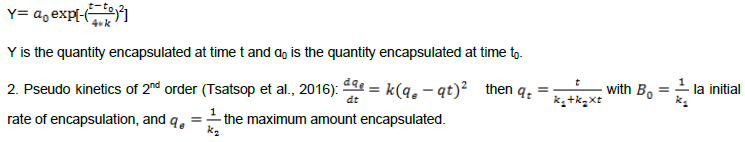
We notice that the pseudo kinetics of 2nd order adjust better because the curves contain an increasing part up to a maximum and an almost constant part for flavonoids and total phenols. As for the Gauss model the curve decreases sharply after their maximum for turbidity. This was justified by the values of the constants in Table 2.
Pseudo kinetics of 2nd order has well described the encapsulation with respect to its R2 which are superior to those of the Gauss model. However, these two models are valid. So in kinetics of 2nd order, the first phase corresponds to the rapid approximation of molecules (k1) to coacervation and the second corresponds to the slow approximation (k2) to crosslinking. The adjustment curves of the Gauss models and kinetics order 2 (Figures 2 and 3) show that the rate of encapsulation and the turbidity of the mixture depend on the mixing time.
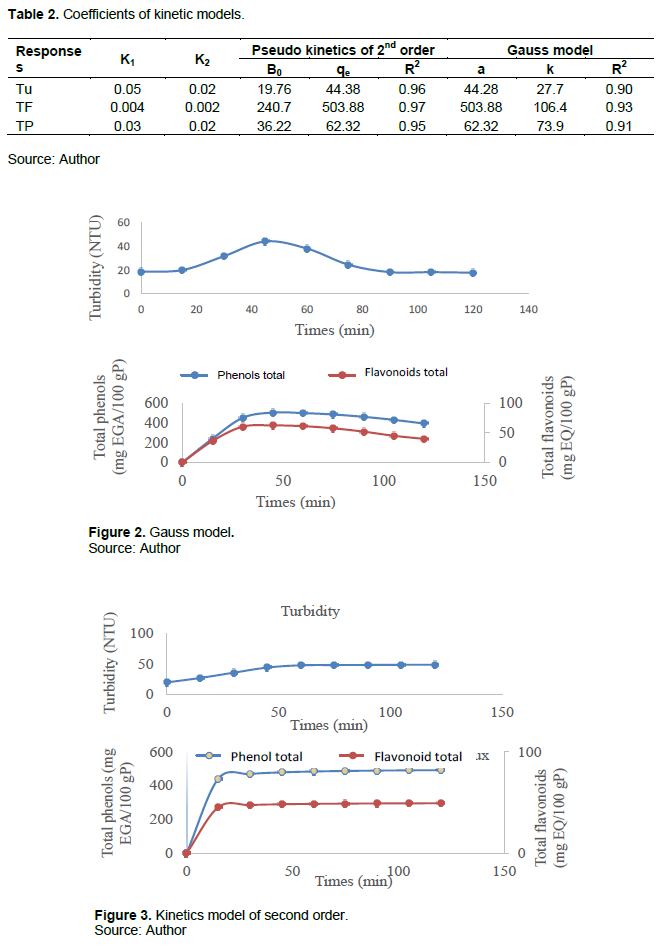
Effect of gum arabic solution on coacervation encapsulation
Figure 4 shows the turbidity of the mixture, total phenols and flavonoids as a function of the proportion of the gum arabic solution. The mixture is made with a variation of the proportions of the gum arabic solution and the time, are constants.
For the effect of gum arabic, we find that the curves reach their maximum at around 30% which is the equilibrium point then they decrease. The phenomenon of decrease is that the more the gum arabic is increased, the more the density of negative charges increases and the bonds between the polymers break from where there is degradation of the microcapsules: this is dilution. The limits set for the experiment are [20; 40%].
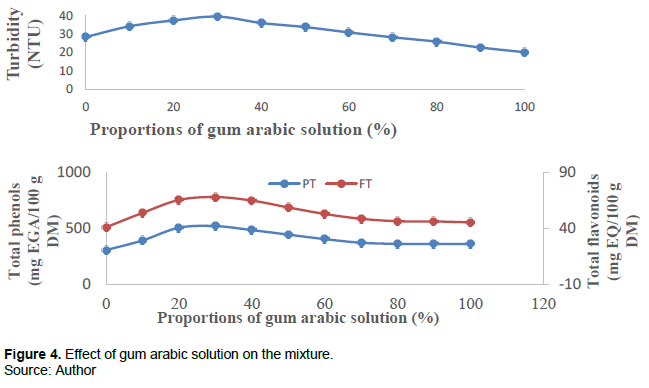
Effect of gelatin solution on coacervation encapsulation
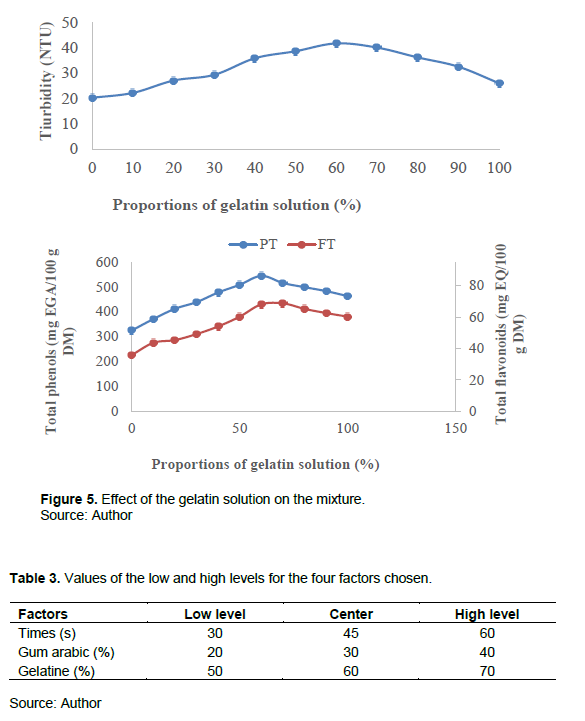
One of the parameters studied during encapsulation is the effect of the proportion of the gelatin solution. This is done in different proportions and the time is kept constant. Figure 5 illustrates the evolution of the turbidity of the mixture and that of the encapsulated total phenols and flavonoids.
Increasing the amount of gelatin increases the turbidity of the mixture and the content of total phenols and total flavonoids. This could be explained by the gradient of encapsulation of the microcapsules between the polymer and the gelatin matrix which is high when the percentage used is large. The application bounds for the experiment plan adopted are [50; 70%]. Thus, the summary of the preliminary tests is presented in Table 3.
The specifications which will allow us to define a compromise zone are defined with turbidity greater than or equal to the value of 44.28 NTU; the content of total phenols greater than or equal to 503.88 mg EGA/100 g P and the total flavonoid content greater than or equal to 62.32 mg EQ/100 g P.
The experiment plan
At the end of the experiment plan, the statistical analysis allowed us to have the validation indicators of the models
designed in Table 4.
For a model to be validated, R2 must be adjusted ≥ 80%; Bias factor and accuracy factor ? [0.75 and 1.25]; and 0 ≤ AADM ≤ 0.3. We have three models which are turbidity model (YT), total phenol model (YTP) and total flavonoid model (YTF). All three models are valid because their validation indicators are in the standards. The equations of these models are as follows:
YT (NTU) = -74.4 + 0.9X1 + 0.4X2 + 4.2X3 - 0.004X1X2 + 0.004X1X3 - 0.01X2X3 - 0.01X12 + 0.003X22 - 0.04X32
YTP (mg EAG/100 gP) = -763.5 + 11.7X1 + 2.4X2 + 46.6X3 - 0.03X1X2 - 0.004X1X3 - 0.1X2X3 - 0.2X12 + 0.04X22 - 0.46X32
YTF (mg EQ/100 gP) = -166.6 + 0.5X1 + 2.4X2 + 6.9X3 - 0.002X1X2 + 0.01X1X3 - 0.03X2X3 - 0.02X12 - 0.01X22 - 0.1X32
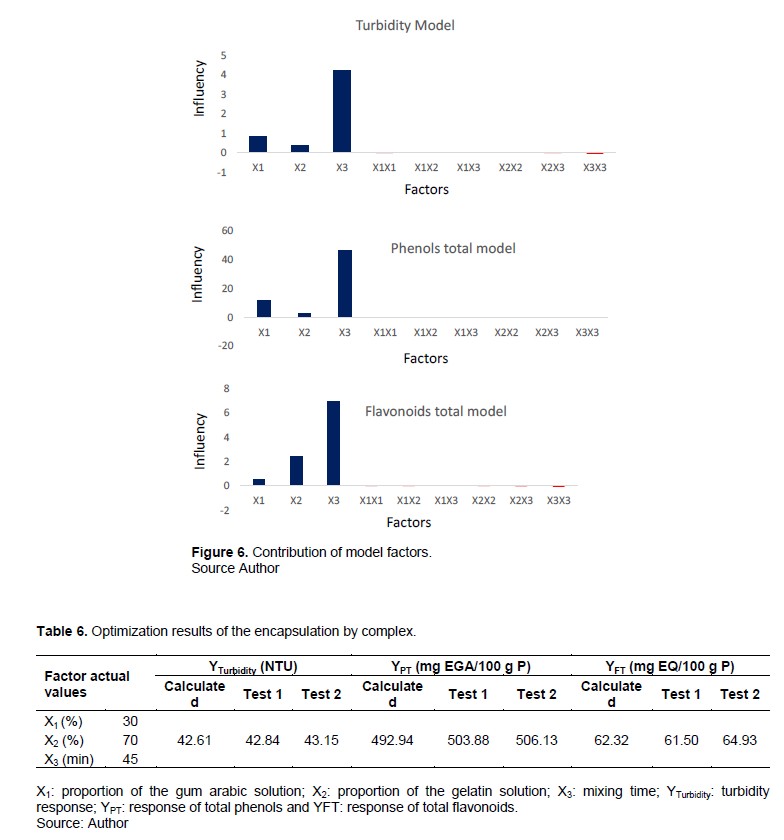
The factor coefficients are shown in Table 5. All the factors X1, X2 and X3 have a positive and significant influence on the encapsulation. While all interactions and quadratic effects have insignificant influence. Figure 6 presents the contribution of the factors of the models. It confirms that the direct effects contribute to the encapsulation and especially X3 which contributes strongly, but its quadratic effect contributes negatively. This confirms that the longer the agitation takes place, the more the microcapsules are not broken by the shocks of the agitation.
Encapsulation optimization
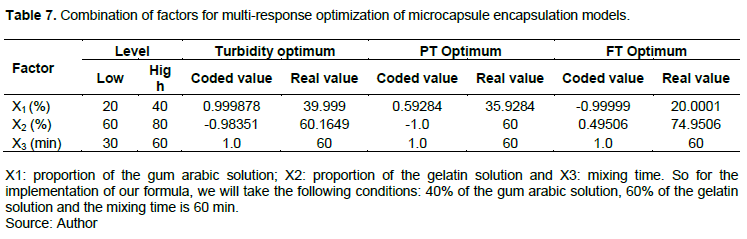
The results of the optimization of the encapsulation by complex coacervation are reported in Table 6. The optimum values of responses such as turbidity, total phenols and total flavonoids were measured in two trials and then compared to the values calculated by the equations of the models found. Let's remember that the higher the values of turbidity, total phenols and total flavonoids, the greater the encapsulation yield and these are the best responses. At each optimal condition, for the responses, experimental tests were carried out and the results obtained are recorded in Table 6. The experimental results are almost similar to the calculated results, which is why the design of these experiments is validated.
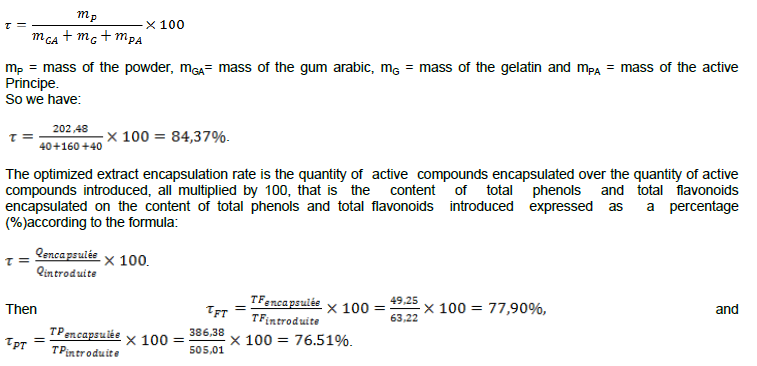
In addition, a multi-response optimization was performed. Indeed, the mixture is obtained from the multi-response optimization of the microcapsules. The combination of the different factors is shown in Table 7. Under these conditions, we represent the combination of factors for optimizing the encapsulation of microcapsules by coacervation of the models of turbidity, total phenols and total flavonoids in Table 7.
Evaluation of the powder obtained
Formula yield obtained
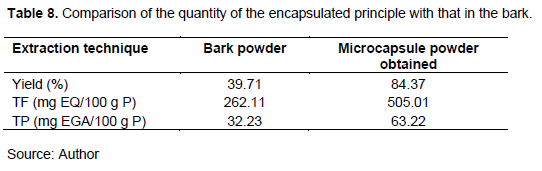
The mixture is made according to the experimental plan, by adding in 2 L of distilled water, 40 g of gum arabic, 160 g of gelatin and 40 g of the active Principe (methanol extract from the bark of the trunk of the cashew). After lyophilization, we obtained 202.48 g of powder from the microcapsules. The yield is calculated according to the following formula:
These values ??are acceptable because the complex coacervation method encapsulates between 70 and 90%. It is the best chemical method of encapsulation. These results corroborate those of Annalisa et al. (2017).
So the amount of the active ingredient in the powder obtained and the bark powder of the plant used in this study can be compared. Table 8 gives the results of the comparison.
The results show that the powder obtained contains twice as much of the active principle as the powder of the bark of the trunk of the cashew. So the study microcapsule powder is rich and can be valued.
CONCLUSION
This study has shown and confirmed previous studies which attest that the rate of encapsulation of the active compounds depends on the proportion of gum arabic, the proportion of gelatin and also on the mixing time. The kinetics of encapsulation follows kinetics of order 2 which gives polynomial equations of the second degree. The best conditions for encapsulation by complex coacervation are as follows: 40% of the gum arabic solution, 60% of the gelatin solution and the mixing time is 60 min. They give the values ??of total phenols and total flavonoids of 689.82 mg EAG / 100 g DM and 88.64 mg EQ/100 g DM, respectively. The evaluation of the powder gives us a yield of the powder formulation of 84.37% and the encapsulation rate of 77.9% for the total flavonoids and 76.5% for the total phenols. Finally, the powder obtained is twice as rich in active principle as the plant material used.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Alfa BHG, Moussa AA, Aristide T, Sylvin O (2017). Etude des effets vermicide et anti-diarrhéique du macéré aqueux des feuilles de Salvadora persica L. (Salvadoraceae). International Journal of Biological and Chemical Sciences 11(1):54-66. |
|
|
Annalisa D, Sara C, Gaetano L, Anna AB (2017). Encapsulation of Active Molecules in Microparticles Based on Natural Polysaccharides. Natural Product Communications 12(6):863-866. |
|
|
Deepak M, Sheweta B (2020) Mesquite Gum (Prosopis Gum): Structure, Properties & Applications- A Review. International Journal of Biological Macromolecules 159(2020):1094-1102. |
|
|
Dharamveer D, Bharat M, Siddiqui HH (2013). Pharmacognostical and phytochemical studies on Anacardium occidentale Linn. Leaves. Research Journal of Pharmacy and Technology 6(1):75-79. |
|
|
Farid D, Giorgia S, Kamal M, Hocine R, Asma C, Khodir M (2014). Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Industrial Crops and Products 61(1):31-40. |
|
|
Jeansheng G, Cheng Z (2018). Secretory Structures of Pogostemon auricularius: Morphology, Development, and Histochemistry. Symmetry 11(1):13. |
|
|
Jing S, Wenlong W, Qinyan Y (2016). Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials 9(4):231. |
|
|
Jothi V, Vijay KT, Vasudev B, Giliyar S (2013). Antimicrobial effect of Anacardium occidentale leaf extract against pathogens causing periodontal disease. Advances in Bioscience and Biotechnology 4(1):15-18. |
|
|
Lv Y, Zhang X, Abbas S, Karangwa E (2012). Simplified optimization for microcapsule preparation by complex coacervation based on the correlation between coacervates and the corresponding microcapsules. Journal of Food Engineering 111(2010):225-233. |
|
|
Madjitoloum BS, Talla E, Ngassoum MB, Tsatsop TRK, Nyemb JN, Mahmout Y (2018a). Optimization of microwave-assisted extraction of total phenol content and total flavonoids content from Anacardium occidental L. (Anacardeaceae) using response surface methodology. International Journal of Biochemistry and Biotechnology 7(4):800-809. ISSN : 2169-3048. |
|
|
Madjitoloum BS, Talla E, Nyemb JN, Ngassoum MB, Tsatsop TRK, Mahmout Y (2018b). Comparative survey of three processes used for the extraction of total phenol content and total flavonoid content of Anacardium occidentale L and the assessment of its antioxidant activity. African Journal of Biotechnology 17(40):1265-1273. |
|
|
Marta PD, Javier FEC, Raúl PG, Antonio G, Emilia MG (2020). Optimization of the Emulsifying Properties of Food Protein Hydrolysates for the Production of Fish Oil-in-Water Emulsions. Food 9(636):1-16. |
|
|
Mohammadinejad R, Karimi S, Iravani S, Varma RS (2016). Plant-derived nanostructures: types and applications. Green Chemistry 18(1):20-52. |
|
|
Ngolo E, Etou Ossibi AW, Epa C, Loubanou CAC, Samba Ndossi ASF, Ouamba JM, Abena AA (2018). Effet hypotenseur de l'extrait aqueux de la recette à base de Brillantaisia patula T. Anderson (Acanthaceae) et de Desmodium velutinum (Willd) D.C (Fabaceae) chez le rat. International Journal of Multidisciplinary and Current Research 6(1):1415-1423. ISSN: 2321-3124 |
|
|
Oumarou PM, Régis P, Oumarou B, Mama N, Nicole S (2010). Abandon ou extension des plantations d'acacias au Nord-Cameroun : tout dépendra du fonctionnement des filières gomme arabique. BOIS ET FORÊT S DES TROPIQUES (306):1-14. |
|
|
Raoufou Radji, Kouami Kokou (2013).Classification et valeurs thérapeutiques des plantes ornementales du Togo. Vertigo la Revue électronique en sciences de l'environnement 13(3). |
|
|
Sarunyoo S, Pichayakarn Y, Pimrat P, Duangkhae M, Prapaporn B (2018). Microencapsulation of citronella oil for mosquito repellent: Preparation and evaluation of release characteristics. Songklanakarin Journal of Science & Technology (4):767-775. |
|
|
Tsatsop RKT, Djiobie GT, Kenmogne BS, Regonne KR, Ngassoum MB (2016). Optimization of Microwave-Assisted Extraction of Bioactive Compounds from Anogeissus leiocarpus Guill. and Perr. Stem Bark Using Response Surface Methodology. International Journal of Scientific and Technology Research 5(5):103-112. |
|
|
Vázquez-González Y, Prieto C, Filizoglu MF, Ragazzo-Sánchez JA, Calderón-Santoyo M, Furtado RF, Cheng HN, Biswas A, Lagaron JM (2021). Electrosprayed cashew gum microparticles for the encapsulation of highly sensitive bioactive materials. Carbohydrate Polymers 152(64):118060. |
|
|
Yuksel H, Dirim SN (2018). Agglomeration process in the fluidized bed, the effecting parameters and some applications. Croatian Journal of Food Technology, Biotechnology and Nutrition 13(3-4):159-163. |
|
|
Zhang S, Zhou W, Qin H (2013). Inverse Gaussian process-based corrosion growth model for energy pipelines considering the sizing error in inspection data. Corrosion Science 73(2013):309-320. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0