ABSTRACT
The prospects for using Moringa oleifera leaves extract (MLE) supplemented with different concentrations of kanwa or sodium bicarbonate (NaHCO3) as a low-cost alternative growth medium of Spirulina platensis were evaluated in a small-scale outdoor cultivation system. The present study was aimed to evaluate the potential of MLE growth medium enriched with different concentrations (4 or 8 g L-1) of kanwa and NaHCO3 on growth, chlorophyll content, biochemical characteristics, antioxidant compounds and some physico-chemical factors. Jourdan’s standard medium was taken as control. The results showed that the growth parameters such as cell population, biomass dry weight, cell productivity and specific growth rate were positively affected in MLE cultivation medium enriched with kanwa or NaHCO3 at different concentration levels. The addition of urea, kanwa or NaHCO3 in MLE cultivation medium at different concentration levels increased significantly (p< 0.05) the protein content, the peroxidase and polyphenol oxidase activity, the conductivity, pH, total dissolved solids and salinity from 20 to 25 days of cultivation whereas a decrease in carbohydrate and phenol content was recorded during all the period of the experimentation. The highest values of growth parameters were notably in MLE medium supplemented with urea and kanwa at 8 g L-1. The MLE medium enriched with urea and kanwa at 8 g L-1 was shown to be an appropriate growth medium that can be used as a low-cost alternative growth medium for commercial cultivation of S. platensis.
Key words: Antioxidant compounds, biochemical traits, growth, Moringa oleifera leaves extract, physico-chemical factors, Spirulina platensis.
Microalgae is in high demand in many biotechnology sectors such as bioremediation, biofuels, biofertilizers (Kosamia et al., 2020; Markou et al., 2021), cosmetics, biomedicals (Mesquita et al., 2017; Mellou et al., 2019; Wen et al., 2020), aquaculture (Shah et al., 2018) and animal and human nutrition (Molino et al., 2018) because of the biological and commercial value of its products. In this respect, Spirulina platensis is one of the most promising microalgae (Lupatini et al., 2017). It represents the most abundant and common photosynthetic, filamentous, multicellular and microscopic microalgae in the tropics and subtropics (Nyabuto et al., 2015). S. platensis is commercially produced because of its high digestibility and interesting protein content (46-71%) of the dry weight of the algae, as well as high amounts of essential fatty acids and amino acids, vitamins, pigments (phycobiliproteins and carotenoids) and polysaccharides (Zhu et al., 2018; Corrêa and Teixeira, 2021). This cyanobacterium has gained in importance and international demand not only for its nutritional and therapeutic properties but also for its applications in human and animal nutrition, therapeutics and diagnostics (Panjiar et al., 2017; Ama Moor et al., 2020). It is generally recognized as safe from the US Food and Drug Administration and considered as the most complete food for the future by the Food and Agriculture Organization of the United Nations (Goulamabasse, 2018; Branyikova and Lucakova, 2020).
The growth and the biochemical composition of the biomass produced by S. platensis depend on many factors, the most important of which are temperature, nutrient availability and light (Madkour et al., 2012; Soni et al., 2019). The temperature of the culture medium of S. platensis was positively influenced by the addition of FeSO4.7H2O and NaCl to Jourdan’s medium, while MgSO4.7H2O, CO(NH2)2 and NaHCO3 lead to a decrease of the temperature from 31.66 to 25.90°C (Ndjouondo et al., 2017). Production of spirulina with reduced costs is necessary when considering large-scale cultivation for industrial purposes. The cost of nutrients and availability are considered the second major factors influencing the cost of spirulina biomass production after harvesting (Vonshak, 1997). Zarrouk’s medium has successfully served as the standard medium for S. platensis culture for many years (Zarrouk, 1966). Consequently, many media synthetic have been developed such as CFTIR medium (Venkataraman et al., 1995), OFERR medium (Singh, 2006) and Jourdan medium (Jourdan, 2013).
However, they are expensive, require rapidly depleting minerals, and not readily available. The change of the nutrients in Jourdan’s medium has the potential to produce a large scale biomass of S. platensis and could be suitable for its optimal growth culture conditions that could be beneficial for human’s health. According to Ndjouondo et al. (2017), the dry weight, the specific growth rate and the cell productivity of S. platensis were positively influenced by the addition of FeSO4.7H2O, NaCl, MgSO4.7H2O, CO(NH2)2 and NaHCO3 to Jourdan’s medium at 0.01, 2.5, 0.1, 0.02 and 4 g L-1, respectively. Nutrients such as phosphorus, nitrogen, calcium, potassium and iron present in agro-industrial effluents and vegetables can be used to increase microalgae growth. A number of green algal species have been shown to be able to utilize carbonates such as Na2CO3 and NaHCO3 for cell growth (Emma et al., 2000).
Kanwa, a type of salt, is formed when salt water from a sea or lake evaporates and leaves behind colorful crystals of sodium chloride. It is also called halite or rock salt. Rock salts offer numerous health benefits, such as treating colds and coughs, as well as aiding digestion and contain various levels of trace minerals, such as manganese, copper, iron, and zinc (Nafee et al., 2013). Rock salt contains natural impurities having calcium sulfate (CaSO4) and potassium chloride (KCl) as impurities. It is found in deposits of rock salt, brines, saline lakes, marshes, seawater and saline earth (Nafee et al., 2013).
Plants subject to stress conditions produce cytotoxic activated oxygen that can seriously disrupt normal metabolism, through oxidative damage of lipids, proteins, and nucleic acids (Abbaspour, 2012). In response to stress, plants activate powerful antioxidant systems, both enzymatic (e.g., SOD, POD, catalase, glutathione reductase) and non-enzymatic (flavonoid, PC, carotenoids, vitamins C and E) (Ashraf, 2009; Kahrizi et al., 2012). According to Kasote et al. (2015) and Mostafa et al. (2016), this increase of PPO and POD activity could be correlated to a decrease in oxidative stress and reactive oxygen derivatives produced during photosynthesis and to the high content of phenols which would act as antioxidant by producing an enzymatic substrate to alleviate the harmful effects of reactive
oxygen species.
The increase of some physico-chemical parameters such as salinity and total dissolved solids in S. spirulina cultivation media had being previously explained by the presence of electrically charged atoms which increase with the evaporation of water in media and to the change of the other variables of the culture media due to uptake of nutrients brought by the different concentrations of NaHCO3 in MLE media or by the increase in alkalinity and concentration of dissolved ionic salts resulting from MLE media (Mutanda et al., 2014; Rusydi, 2018). Soni et al. (2019) reported that for flourishing and optimal growth, temperature for S. platensis is between 30 and 35°C and pH value between 8.5 and 10.5.
The appropriate organic waste collected from digested sago starch (Miah et al., 2000), molasses (Andrade and Costa, 2007), rice mill effluents (Usharani et al., 2012), palm oil empty fruits bunches (Suharyanto et al., 2014), and digested rotten apple (Mia et al., 2019) were also used as growth media for S. platensis culture. Thus, for the mass production of S. platensis particularly in developing countries as Cameroon, there is a need to find an effective, cheaper and readily available alternative cultivation media.
Moringa (Moringa oleifera Lam.) is a highly valued plant, distributed in many countries of the tropics and subtropics. It has a high nutritional value and an impressive range of medicinal and industrial purposes (Khalafalla et al., 2010; Adebayo et al., 2011; Moyo et al., 2011). Moreover, Moringa leaves extract (MLE) has received enormous attention from the scientific community because of its rich source in hormones, antioxidants, vitamins and minerals such as iron, calcium and potassium as well as vitamins and macronutrients which have plant growth-promoting capabilities and often applied as exogenous plant growth enhancers (Rady et al., 2013; Yasmeen et al., 2013; Khan et al., 2017a, b). Thus, MLE contains appreciable amounts of macro and micronutrients and readily available and cost-effective feed to substitute inorganic fertilizers and support good S. platensis growth. To the best of our knowledge, few information is so far available for the use of MLE as S. platensis growth media.
The present study was aimed to evaluate the effect of MLE medium supplemented with different concentrations of kanwa or bicarbonate on the growth, chlorophyll content, biochemical characteristics, antioxidant compounds and some physico-chemical factors of S. platensis in order to define the optimal growth cultivation conditions.
Plant material and growth medium
The cyanobacterium S. platensis strain used in this study was obtained from the culture pond of SAGRIC Common Initiative Group Farm, Douala-Cameroon. S. platensis was grown on Jourdan’s modified medium consisting of (per liter): 8 g NaHCO3, 5 g NaCl, 3 g Na2CO3.10H2O, 2 g KNO3, 0.15 g MgSO4. 7H2O, 0.12 g(NH4)2HPO4, 0.05 g CO(CH2)2, 0.02 g FeSO4. 7H2O and 0.02 g CaCl2 and maintained at pH 8 by use of dilute 0.2 M NaOH solution.
Jourdan’s medium was taken as control. For the five experimental groups, the M. oleifera leaves extracts (MLE) used as growth medium, was supplemented with 0.05 g L-1 of urea (U) and 4 or 8 g L-1 of kanwa (NaCl: K) or sodium bicarbonate (NaHCO3: B), respectively as follow: MLE+U; MLE+U+B4; MLE+U+K4, MLE+U+B8 or MLE+U+K8. The depth of the culture in the open concrete tanks was 15 cm with 15 L of MLE. For preparation of M. oleifera leaves extracts (MLE), the young moringa leaves were collected from a mature moringa tree from SAGRIC Common Initiative Group Farm. An amount of 40.0 g of young moringa leaves was suspended in 1.0 L of distilled water for 7 days. The suspension was stirred using a homogenizer to help maximize the amount of the extract. The solution was then sieved and filtered through a 30-mesh Tylor net. The extract diluted with distilled water at a 1:8 ratio (v/v) was used as MLE medium. The algae S. platensis cells were inoculated at a concentration of 15% (V inoculation/V media). The initial pH of all culture media was adjusted to 8 with 0.2 M NaOH before addition of S. platensis cells. Cultures were carried out in 25-L open concrete tanks under daylight in a greenhouse for 25 days. Growth and maintenance of the culture was done at 30 ± 2°C under 12/12 h light-dark cycles. Cultures were agitated by aeration at a flux of 20 L/h provided by a diaphragm pump. Samples were collected every 5 days for assessment of the cyanobacteria growth as well as estimation of biochemical components status. All experiments were carried out with three replicates.
Growth and productivity parameters determination
S. platensis cell populations were determined by direct microscopic counting method described by Usharani et al. (2012). The number of filaments was evaluated using a light microscope (Cyscope® HP, Sysmex-Partec, Japan).
For dry weight concentrations measurement, homogenous suspension of S. platensis sample (200 ml) was filtered through Whatman no. 1 filter paper and oven dried at 50°C for 48 h. The dry filter containing S. platensis dry weight was cooled and weighed. The difference between the initial and final weight was taken as dry weight of S. platensis biomass.
The cell productivity of S. platensis was calculated according to the formula described by Jarisoa (2005):
P = X2 –X1/ t2 – t1
Where X2 and X1 represent the biomass concentrations at the times t2 and t1.
The specific growth rate (µ) was calculated as follows (Göksan et al., 2007):
µ = ln X2 – ln X1/t2-t1
where X2 and X1 represent the biomass concentrations at the times t2 and t1 respectively.
Biochemical characteristics determination
Total soluble carbohydrates were estimated by phenol-sulphuric acid method (Dubois et al., 1956). 1 g of plant fresh materials was weighed and digested by hot ethanol 80% two times, each time by 5 mL ethanol and then filtered by Whatman No. 2. Filter paper and the extracts were diluted by distilled water to the volume of 50 mL. 1 ml for each sample was placed in the test tube and then 1 mL phenol solution added. The procedure was followed by adding 5 mL of sulphuric acid by shaking well. The yellow-orange colour was pipetted off and the wavelength was read in 490 nm by spectrophotometer (Pharmaspec UV-1700 model). The amount of carbohydrates was presented from the glucose standard curve.
Total soluble protein content was measured according to the method described by Bradford (1976) using bovine serum albumin (BSA) as a protein standard. Fresh leaf samples (100 mg) were homogenized with 4 mL Na-Phosphate buffer (pH 7.2) and then centrifuged at 13000 g for 4.5 min at 4°C. 1 ml of supernatant is added to the Bradford reagent (5 mL) and the mixture was incubated thereafter in the dark for 15 min. Then, it was pipetted in spectrophotometer cuvettes and absorbance was measured at 595 nm using a UV spectrophotometer (PG instruments T60).
Chlorophyll content determination
Total leaf chlorophyll (CHL) of plants was extracted in 80% (v/v) aqueous acetone and absorption was measured in spectrophotometer (Thermospertronic Heλios β) at 645 and 663 nm (Arnon, 1949). CHL was calculated using the formula:
Total leaf chlorophyll = (20.2 x D645 + 8.02 x D663) x (50/1000) x 100/5) x ½
Where, D: Absorbance
Antioxidant compounds determination
The activity of peroxidase (POD) and polyphenol oxidase (PPO) were determined according to Thorpe and Gaspar (1978) and Van Kammen and Broumer (1964) methods, respectively. For the assay of POD and PPO, a fresh S. platensis sample was extracted in 10 mL potassium phosphate buffer (50 mM, pH 6.0). The homogenate was subsequently centrifuged (6000 g, 30 min at 4°C) and the supernatant was collected. The pellet was re-suspended in the same buffer centrifuged under the same conditions as previously. The second supernatant was added to the first to obtain extract which was used for PPO and peroxidase POD activity. POD activity was determined by measuring the oxidation of guaiacol and the increase in absorbance at 420 nm was recorded in 3 min. PPO activity was assayed by measuring the decomposition of H2O2 by following the decline in its absorbance at 330 nm for 30 s. The activity was defined as Unit/µg of proteins contents.
The phenol content (PC) was determined according to the method described by Singleton and Rossi (1965). Ethanol extracts (0.2 mL) were added to 1.6 mL of H2O and 0.5 mL of Folin-Ciocalteu reagent and incubated at 25°C for 10 min. Afterwards, 1 mL of a 7.5% solution of Na2CO3 was added to each sample and left at 40°C for 20 min in a water bath, with intermittent shaking. The absorbance of the sample was recorded at 760 nm. The calibration curve was performed with gallic acid and the results were expressed as mg of gallic acid equivalents per g of dry weight.
Physico-chemical parameters determination
The conductivity, temperature, pH, total dissolved solids and salinity of media were measured according to the methods described by Rodier et al. (2009). The physico-chemical parameters were recorded daily using multi-parameters (HI 98130, HANNA Instruments, Rhodes Island, USA).
Statistical analysis
The data obtained were represented as the mean ± standard error. All of the statistical analyses were conducted using SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). The one-way analysis of variance (ANOVA) with Duncan's Multiple Range Tests was used to compare differences between treatment means when significant F values were observed at p <0.05.
Growth parameters
Growth of S. platensis was expressed in terms of cell population (CP), biomass dry weight (DW), cell productivity (CPr) and specific growth rate (SGR) (Table 1). The highest values of CP (90037 ± 2363 cp mL-1), DW (1.01 ± 0.42 mg/L), CPr (0.25 ± 0.002 g L-1 day-1) and SGR (0.028 ± 0.009 cell day-1) were recorded in the Jourdan medium (control) compared to all other treatments comprising moringa leaf extract (MLE) during all the period of experimentation (Table 1). The highest values of CP (67825 ± 815 cp mL-1), DW (0.86 ± 0.02 mg L-1), CPr (0.16 ± 0.006) and SGR (0.022 ± 0.005) registered in MLE enriched with urea and kanwa at 8 g L-1 were also significantly (p <0.05) higher than those grown in other MLE media compared to control (Table 1). Kanwa, a type of salt, is formed when salt water from a sea or lake evaporates and leaves behind colorful crystals of sodium chloride. According to Nafee et al., (2013), it also contains natural impurities such as calcium sulfate, potassium chloride and various levels of trace minerals, such as manganese, copper, iron, and zinc. Moreover, MLE has received enormous attention from the scientific community because of its rich source in hormones, antioxidants, vitamins and minerals such as iron, calcium and potassium as well as vitamins and macronutrients which have plant growth-promoting capabilities and often applied as exogenous plant growth enhancers (Rady et al., 2013; Yasmeen et al., 2013; Khan et al., 2017a, b). In this study, the CP, DW, CPr and SGR were significantly (p <0.05) increased when the MLE cultivation medium was enriched with NaHCO3 or kanwa compared to those supplemented only with urea (Table 1). These results could be explained by the fact that the supply of MLE cultivation medium NaHCO3 or kanwa can improve water quality and increase the quality of S. platensis growth and it will also cause good removal of turbidity in the cultivation media so that the light penetration increases will improve photosynthesis and production of S. platensis (Ogbonna and Chukukwu, 2018; Silva et al., 2020). This may be also due to uptake of nutrients (carbohydrates and ash) and minerals (Na, K, Ca, Mg) brought by MLE and supplemented NaHCO3 or kanwa which increase cell growth and the metabolism of carbon in the photosynthetic activity of S. platensis (Nweze and Nwafor, 2014a,b). A number of green algal species have been shown to be able to utilize carbonates such as Na2CO3 and NaHCO3 for cell growth (Emma et al., 2000). Large-scale production of S. platensis biomass is essentially a complex process involving a large number of variables and for their successful growth; the nutrient sources and the temperature needs to be conditioned to meet as many of the essential requirements of the organism (Ndjouondo et al., 2017). On the other hand, the values of CP (20783 ± 1258 cp mL-1) and DW (0.40 ± 0.26 mg L-1) and those of CPr (- 0.04 ± 0.003) and SGR (- 0.09 ± 0.004) of S. platensis were negatively affected by MLE supplemented only by urea (Table 1). These low values of CP, DW, CPr and SGR were influenced by nutrients found in S. platensis cultivation media (Magwell, 2017).
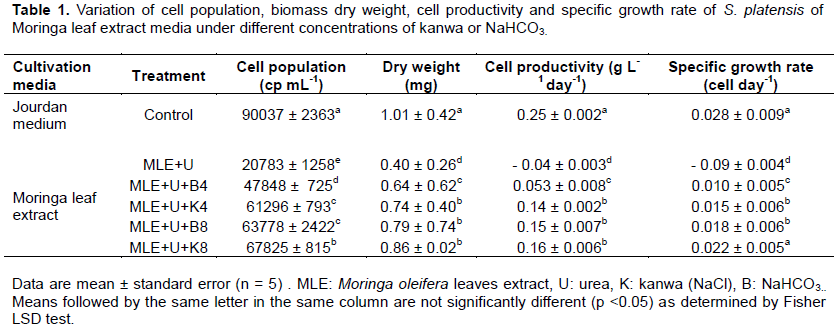
Biochemical characteristics
In this study, the presence of kanwa or NaHCO3 in MLE cultivation medium of S. platensis at different concentration levels resulted in a significant (p <0.05) increase in proteins content compared to Jourdan standard medium (control) except in the MLE medium supplemented only with urea while a significant (P <0.05) decrease in carbohydrate and phenol contents was recorded during all the period of experimentation (Figure 1b and c). The results showed that the MLE medium enriched with urea and kanwa (8 g L-1) was significantly (P <0.05) higher than other treatments. This could be related to the high amount of nutrients in the medium such as colorful crystals of sodium chloride and natural impurities having calcium sulfate and potassium chloride (Nafee et al., 2013). The effect of the MLE ‘supplemented with urea and kanwa could be also explained by the increase of nitrogen assimilation due to the high amount of inorganic carbon provided by NaHCO3 in the medium. A number of green algal species have been shown to be able to utilize carbonates such as Na2CO3 and NaHCO3 for cell growth (Emma et al., 2000). In this study, the carbohydrate content remains very low compared to the protein content (Figure 1a and b). Depraetere et al., (2015) reports that when the amount of nitrogen is high or excessive, it would lead to carbohydrate hydrolysis. The present study also revealed significant (P<0.05) decrease in MLE medium supplemented only with urea for all the treatments compared to Jourdan standard medium (Figure 1a, b and c). This effect of urea could be attributed to the low concentration used for this experiment (50 mg L-1). According to Rangel-Yagui et al. (2004), the best cellular growth for S. platensis was observed with 500 mg L-1 of urea at a light intensity of 5600 lux.
Chlorophyll content
The enrichment of MLE cultivation medium with urea, kanwa or NaHCO3 at different concentration levels led to a significant decrease (p< 0.05) in chlorophyll content during all the cultivation period of S. platensis compared to Jourdan standard medium (Figure 2). This depressive effect may be attributed to salt-induced weakening of protein-pigment-lipid complex or increased chlorophyllase (Strogonov, 1970). The significant (P< 0.05) decrease of chlorophyll content could be also due to low (50 mg L-1) concentration of urea supply. According to Rangel-Yagui et al. (2004), the highest concentration of chlorophyll in the biomass was observed with 500 mg L-1 at a light intensity of 1400 lux.
Antioxidant compounds
In the present study, antioxydant compounds of S. platensis were expressed in terms of polyphenol oxidase (PPO), peroxidase (POD) and phenol content (PC) (Table 2). The highest values of PPO (6.02 ± 0.044 UE µg-1) and POD (0.63 ± 0.03 UE µg-1) were recorded in the medium which contains MLE enriched with urea compared to Jourdan standard medium (control) which showed the lowest values (2.00 ± 0.024 UE µg-1) and (0.03 ± 0.03 UE µg-1), respectively (Table 2). On the other hand, the highest value of PC was noted in Jourdan standard medium (2.09 ± 0.07 mg L-1) while the lowest value was observed in MLE cultivation medium supplemented only with urea (1.00 ± 0.010 mg L-1) (Table 2). In response to stress, plants activate powerful antioxidant systems, both enzymatic (e.g., SOD, POD, catalase, glutathione reductase) and non-enzymatic (flavonoid, PC, carotenoids, vitamins C and E) (Ashraf, 2009; Kahrizi et al., 2012). The activity of antioxidant enzymes (PPO and POD) was notably increased with the cultivation period of S. platensis in all MLE medium supplemented with different concentrations of kanwa or NaHCO3 compared to Jourdan standard medium (Table 2). The use of MLE based medium supplemented with a high concentration of kanwa or NaHCO3 may cause the increasing trend of the amount of PPO and POD activity. According to Kasote et al. (2015) and Mostafa et al. (2016), this increase in enzymatic activity could be correlated to a decrease in oxidative stress and reactive oxygen derivatives produced during photosynthesis and to the high content of phenols which would act as antioxidant by producing an enzymatic substrate to alleviate the harmful effects of reactive oxygen species.
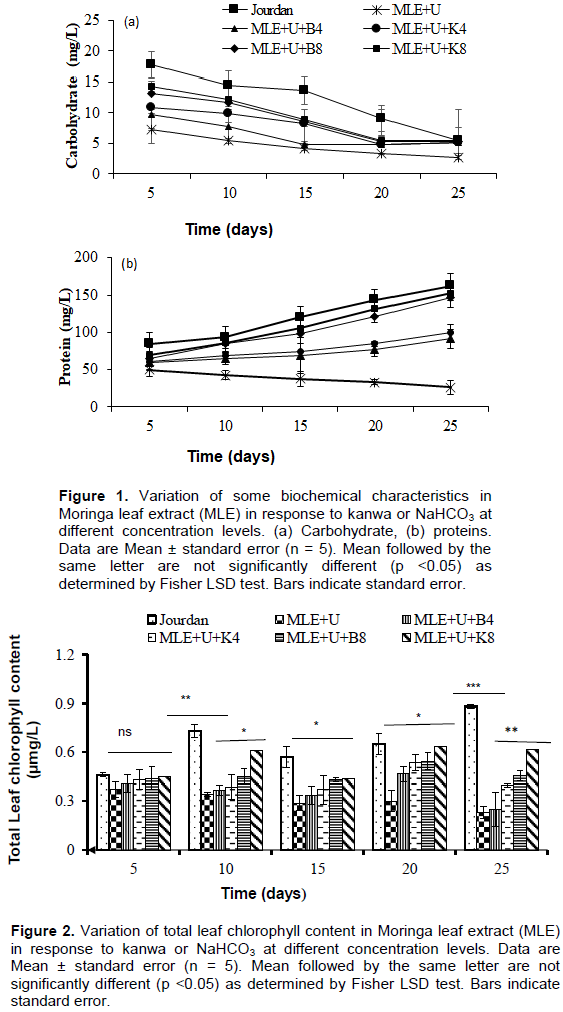
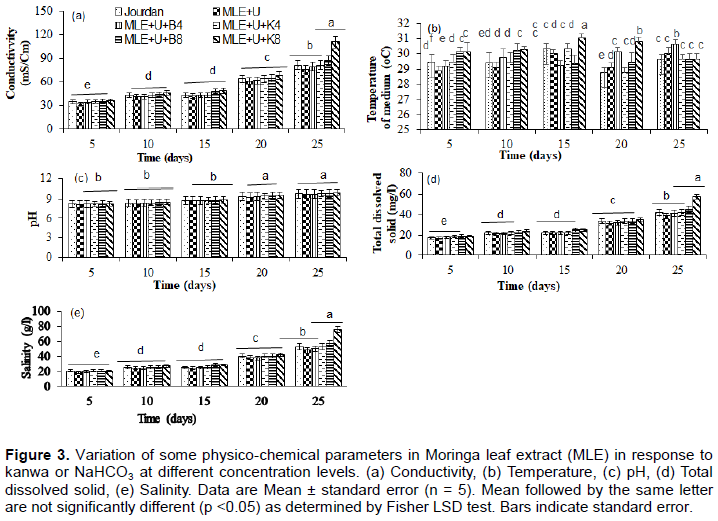
Physico-chemical parameters
In this study, some physico-chemical parameters (conductivity, pH, total dissolved solids and salinity) of S. spirulina cultivation media led to a significant (p < 0.05) increase in MLE media enriched with different concentrations of urea, kanwa or NaHCO3 from 20 to 25 days of cultivation compared to Jourdan medium and others treatments (Figure 3a, c, d and e). This increase of salinity and total dissolved solids could be explained by increase with the evaporation of water in media and to the change of the other variables of the culture media due to uptake of nutrients brought by the different concentrations of kanwa or NaHCO3 in MLE media (Rusydi, 2018). This progressive increase in total dissolved solids and salinity could be explained by the increase in alkalinity and concentration of dissolved ionic salts resulting from MLE media with different concentration of kanwa or NaHCO3 following the evaporation of water from the media as reported by Mutanda et al. (2014). The temperature of S. platensis cultivation media varied significantly (p < 0.05) between 29 and 31°C and the pH between 8 and 10 with the highest value of temperature (31°C ) recorded in MLE medium supplemented with urea and Kanwa at 8 g L-1 and the lowest (29°C) was noted in Jourdan standard medium (Figure 3b). The results obtained are in agreement with those reported by Soni et al. (2019) which indicated that for flourishing and optimal growth, temperature for S. platensis is between 30 and 35°C and pH value between 8.5 and 10.5.
In general, the results of this study showed that the growth parameters such as cell population, biomass dry weight, cell productivity and specific growth rate were positively affected in MLE cultivation medium enriched with kanwa or NaHCO3 at different concentration levels. This result may be due to uptake of nutrients (carbohydrates and ash) and minerals (Na, K, Ca, Mg) brought by MLE and supplemented NaHCO3 or kanwa which increase cell growth and the metabolism of carbon in the photosynthetic activity of S. platensis. The addition of kanwa or NaHCO3 in MLE cultivation medium at different concentration levels increased significantly the presence of electrically charged atoms which physico-chemical parameters (conductivity, pH, total dissolved solids and salinity) from 20 to 25 days of cultivation, the protein content and antioxidant enzymes (PPO and POD) activity whereas a decrease in carbohydrate content was recorded during all the period of the experimentation. This increase in enzymatic activity could be correlated to a decrease in oxidative stress and reactive oxygen derivatives produced during photosynthesis and to the high content of phenols which would act as antioxidant by producing an enzymatic substrate to alleviate the harmful effects of reactive oxygen species. This study draws attention to a good view on MLE as a cheaper and easily available organic fertilizer that need to be enriched for the culture of S. platensis. Thus, further investigations should be done in the commercial cultivation of S. platensis using MLE in agriculture, food industry, cosmetics, pharmaceutics and medicine.
The authors have not declared any conflict of interests.
The authors thank the SAGRIC Common Initiative Group (CIG) Farm, Douala-Cameroon who graciously provided us with the Spirulina strain.
REFERENCES
|
Abbaspour H (2012). Effects of salt stress on lipid peroxidation, antioxidative enzymes and proline accumulation in pistachio plants. Journal of Medicinal Plant Research 6(3):526-529.
|
|
|
|
Adebayo AG, Akintoye HA, Olufolaji OO, Aina MT, Olatunji MT, Shokalu AO (2011). Assessment of organic amendments on vegetative development and nutrient uptake of Moringa oleifera Lam. in the nursery. Asian Journal of Plant Sciences 10(1):74-79.
|
|
|
|
|
Ama Moor VJ, Pieme CA, Nkeck JR, Nya Biapa CP, Ikomey MG, Kouanfack C, Okomo Assoumou MC, Ngogang J (2020). Spirulina platensis enhances immune status, inflammatory and oxidative markers of HIV patients on antiretroviral therapy in Cameroon. Research Square pp. 1602-6494.
|
|
|
|
|
Andrade MR, Costa JAV (2007). Mixotrophic cultivation of microalga Spirulina platensis using molasses as organic substrate. Aquaculture 264(1-4):130-134.
|
|
|
|
|
Arnon DI (1949). Copper enzymes in isolated chloroplasts. Polyhenoloxidases in Beta vulgaris. Plant Physiology 24(1):1-15.
|
|
|
|
|
Ashraf M (2009). Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnology Advances 27(1):84-93.
|
|
|
|
|
Bradford M (1976). A rapid and sensitive method for the quantitative of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72(1-2):248-254.
|
|
|
|
|
Branyikova I, Lucakova S (2020). Technical and physiological aspects of microalgae cultivation and productivity-spirulina as a promising and feasible choice. Organic Agriculture 11(2):269-276.
Crossref
|
|
|
|
|
Corrêa PS, Teixeira CMLL (2021). Polyhydroxyalkanoates and pigments coproduction by Arthrospira (Spirulina) platensis cultivated in crude glycerol. Journal of Applied Phycology 33:1487-1500.
Crossref
|
|
|
|
|
Depraetere O, Deschoenmaeker F, Badri H, Monsieurs P, Foubert I, Leys N, Wattiez R, Muylaert K (2015). Trade-off between growth and carbohydrate accumulation in nutrient-limited Arthrospira sp. PCC 8005 studied by integrating transcriptomic and proteomic approaches. PLoS ONE 10(7):e0132461.
|
|
|
|
|
Dubois M, Gilles KA, Hamil JK, Rebers PA, Smith F (1956). Colorimetric method for determination of sugars and related substances. Analytical Biochemistry 28(3):350-356.
|
|
|
|
|
Emma Huertas I, Colman B, Espie GS, Lubian LM (2000). Active transport of CO2 by three species of marine microalgae. Journal of Phycology 36(2):314-320.
|
|
|
|
|
Göksan T, Zekeriyaoglu A, Ak I (2007). The growth of Spirulina platensis in different culture systems under greenhouse condition. Turkish Journal of Biology 31(1):47-52.
|
|
|
|
|
Goulamabasse R (2018). Spirulina: Therapeutic activities and its interest in the fight against malnutrition in Madagascar, Ph.D Thesis, UFR pharmaceutical and biological sciences, University of Lille 135 p.
|
|
|
|
|
Jarisoa T (2005). Adaptation of Spirulina from southern Madagascar to seawater culture. Development of production structures at the village level. Ph.D, Thesis University of Toliara, Madagascar, 129p.
|
|
|
|
|
Jourdan JP (2013). Grow your Spirulina. Manual of artisanal culture for the production of Spirulina. Antenna Technology, 143p.
|
|
|
|
|
Kahrizi S, Sedghi M, Sofalian O (2012). Effect of salt stress on proline and activity of antioxidant enzymes in ten durum wheat cultivars. Annals of Biological Research 3(8):3870-3874.
|
|
|
|
|
Kasote DM, Katyare SS, Hegde MV, Bae H (2015). Significance of antioxidant potential of plants and its relevance to therapeutic applications. International Journal of Biological Sciences 11(8):982-991.
|
|
|
|
|
Khalafalla MM, Abdellatef E, Dafalla HM, Nassrallah AA, Aboul-Enein KM, Lightfoot DA, Eldeeb FE, El-Shemy HA (2010). Active principle from Moringa oleifera Lam. leaves effective against two leukemais and a hepatocarcinoma. African Journal of Biotechnology 9(49):8467-8471.
|
|
|
|
|
Khan S, Basra SMA, Afzal I, Wahid A (2017a). Screening of moringa landraces for leaf extract as biostimulant in wheat. International Journal of Agriculture and Biology 19:999-1006.
|
|
|
|
|
Khan S, Basra SMA, Afzal I, Nawaz M, Rehman HU (2017b). Growth promoting potential of fresh and stored Moringa oleifera leaf extracts in improving seedling vigor, growth and productivity of wheat crop. Environmental Science and Pollution Research 24:7601-27612.
|
|
|
|
|
Kosamia NM, Samavi M, Uprety BK, Rakshit SK (2020). Valorization of biodiesel by product crude glycerol for the production of bioenergy and biochemicals. Catalysts 10(6):609.
|
|
|
|
|
Lupatini AL, Colla LM, Canan C, Colla E (2017). Potential application of microalga Spirulina platensis as a protein source. Journal of the Science of Food and Agriculture 97(3):724-732.
|
|
|
|
|
Madkour F, Abd El-Wahab K, Hodam S (2012). Production and nutritive value of Spirulina platensis in reduced cost media. The Egyptian Journal of Aquatic Research 38(1):51-57.
|
|
|
|
|
Magwell PFR (2017). Influence of sulphate salts on growth performance and biochemical analysis of Spirulina platensis. Master thesis, University of Douala. Douala-Cameroon, 111p.
|
|
|
|
|
Markou G, Diamantis A, Arapoglou D, Mitrogiannis D, Gonzalez- Fernandez CUA (2021). Growing Spirulina (Arthrospira platensis) in seawater supplemented with digestate: Trade-offs between increased salinity, nutrient and light availability. Biochemical Engineering Journal 165:107815.
|
|
|
|
|
Mellou F, Varvaresou A, Papageorgiou S (2019). Renewable sources: applications in personal care formulations. International Journal of Cosmetic Science 41(6):517-525.
|
|
|
|
|
Mesquita SDS, Teixeira CMLL, Servulo EFC (2017). Carotenoids: properties, applications and market. Revista Virtual de Quimica 9:672-688.
|
|
|
|
|
Miah MS, Phang SM, Chu WL, Hashim MA (2000). Spirulina culture in digested sago starch factory wastewater. Journal of Applied Phycology 12(3):395-400.
|
|
|
|
|
Mia ML, Habib MAB, Hoque N, Islam MS (2019). A study on growth performance of Spirulina platensis in different concentrations of rotten apple as a carbon source. International Journal of Excellence Innovation and Development 2(1):29-40.
|
|
|
|
|
Molino A, Iovine A, Casella P, Mehariya S, Chianese S, Cerbone A, Rimauro J, Musmarra D (2018). Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. International Journal of Environmental Research and Public Health 15(11):2436.
Crossref
|
|
|
|
|
Mostafa MS, Yassin EM, Michele PN (2016). Role of pH on antioxidants production by Spirulina (Arthrospira) platensis. Brazilian Journal of Microbiology 47(2):298-304.
|
|
|
|
|
Moyo B, Masika PJ, Hugo A, Muchenje V (2011). Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. African Journal of Biotechnology 10(60):12925-12933.
|
|
|
|
|
Mutanda T, Ranjith RK, Bux F (2014). Physico-chemical and biotic factors influencing microalgal seed culture propagation for inoculation of a large scale raceway pond. African Journal of Biotechnology 13(35):3607-3616.
|
|
|
|
|
Nafee M, Nazish K, Shah R, Adila B (2013). Analysis of rock and sea salts for various essentials and inorganic elements. Journal of Science and Technology University of Peshawar 37(1):9-20.
|
|
|
|
|
Ndjouondo JP, Dibong SD, Wamba FO, Taffouo VD (2017). Growth, productivity and some physico-chemical factors of Spirulina platensis cultivation as influenced by nutrients change. International Journal of Botany 13(2):67-74.
|
|
|
|
|
Nweze NO, Nwafor FI (2014a). Phytochemical, proximate and mineral composition of Leaf extracts of Moringa oleifera Lam. from Nsukka, South-Eastern Nigeria. IOSR Journal of Pharmacy and Biological Sciences 9(1):99-103.
|
|
|
|
|
Nweze NO, Nwafor FI (2014b). Promoting increased Chlorella sorokiniana Shih and Krauss (Chlorophyta) biomass production using Moringa oleifera Lam. leaf extracts. African Journal of Biotechnology 13(53):4720-4725.
|
|
|
|
|
Nyabuto D, Cao K, Mariga A, Kibue G, He M, Wang C (2015). Growth performance and biochemical analysis of the genus Spirulina under different physical and chemical environmental factors. African Journal of Agricultural Research 10(36):3614-3624.
|
|
|
|
|
Ogbonna NC, Chukukwu EI (2018). Harvesting Chlorella variabilis biomass using Moringa oleifera seed-induced sedimentation. Journal of Advances in Biology & Biotechnology 18(4):1-11.
|
|
|
|
|
Panjiar N, Mishra S, Yadav AN, Verma P (2017). Functional foods from cyanobacteria. in microbial functional foods and nutraceuticals (Gupta VK, Treichel H, Shapaval V, Antonio de Oliveira L, Tuohy MG eds, 314 p.
Crossref
|
|
|
|
|
Rady MA, Varma BC, Howladar SM (2013). Common bean (Phaseolus vulgaris L.) seedlings overcome NaCl stress as a result of presoaking in Moringa oleifera leaf extract. Scientia Horticulturae 162:63-70.
|
|
|
|
|
Rangel-Yagui CO, Danesi EDG, de Carvalho JCM, Sato S (2004). Chlorophyll production from Spirulina platensis: Cultivation with urea addition by fed-batch process. Bioresource Technology 92(2):133-141.
|
|
|
|
|
Rodier J, Legube B, Marlet N, Brunet R (2009). Water analysis. 9th edition DUNOD, Paris, 1579p.
|
|
|
|
|
Rusydi AF (2018). Correlation between conductivity and total dissolved solid in various type of water: A review. IOP Conference Series: Earth and Environmental Science 118(1):012019.
Crossref
|
|
|
|
|
Shah MR, Lutzu GA, Alam A, Sarker P, Kabir CMA, Parsaeimehr A, Liang Y, Daroch M (2018). Microalgae in aquafeeds for a sustainable aquaculture industry. Journal of Applied Phycology 30:197-213.
|
|
|
|
|
Silva DF, Speranza L, Quartaroli L, Moruzzi R, Silva GH (2020). Separation of microalgae cultivated in anaerobically digested black water using Moringa Oleifera Lam. seeds as coagulant. Journal of Water Process Engineering 39:2214-7144.
|
|
|
|
|
Singh S (2006). Spirulina: A Green gold mine. Paper presented at: Spirutech 2006. Spirulina cultivation: Potentials and prospects. Jabalpur, Madhya Pradesh.
|
|
|
|
|
Singleton VL, Rossi JA (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture 16(3):144-158.
|
|
|
|
|
Soni RA, Sudhakar K, Rana RS (2019). Comparative study on the growth performance of Spirulina platensis on modifying culture media. Energy Reports 5:327-336.
|
|
|
|
|
Suharyanto TP, Permatasari S, Syamsu K (2014). Production of Spirulina platensis in continuous photobioreactor using palm oil mill effluent media. Menara Perkebunan 82(1):1-9.
|
|
|
|
|
Thorpe TA, Gaspar T (1978). Changes in isoperoxydase during shoot formation in tobacco callus. In Vitro 14(6):552-539.
|
|
|
|
|
Usharani G, Saranraj P, Kanchana D (2012). In vitro cultivation of Spirulina platensis using rice mill effluent. International Journal of Pharmaceutical and Biological Archives 3(6):1518-1523.
|
|
|
|
|
Van Kammen A, Broumer D (1964). Increase of polyphenol oxidase activity by the local virus infection in uninoculated parts of leaves. Virology 22(1):9-14.
|
|
|
|
|
Venkataraman LV, Bhagyalakshmi N, Ravishankar GA (1995). Commercial production of micro and macro algae problems and potentials. Indian Journal of Microbiology 35:1-19.
|
|
|
|
|
Vonshak A (1997). Spirulina: Growth, physiology and biochemistry. In: Vonshak A (Ed) Spirulina platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology. Taylor and Francis, London, 233p.
|
|
|
|
|
Wen Y, Wen P, Hu T, Linhardt RJ, Zong M, Wu H, Chen Z (2020). Encapsulation of phycocyanin by prebiotics and polysaccharides based electrospun fibers and improved colon cancer prevention effects. International Journal of Biological Macromolecules 149(2):672-681.
|
|
|
|
|
Yasmeen A, Basra SMA, Farooq M, Rehman H, Hussain N, Athar HR (2013). Exogenous application of moringa leaf extract modulates the antioxidant enzyme system to improve wheat performance under saline conditions. Plant Growth Regulation 69:225-233.
Crossref
|
|
|
|
|
Zarrouk C (1966). Contribution to the study of a cyanophyceae. Influence of various physical and chemical factors on the growth and photosynthesis of Spirulina maxima (Setch.Et Gardner) Geitler. Ph.D Thesis. University of Paris, France 412 p.
|
|
|
|
|
Zhu C, Zhu H, Cheng L, Chi Z (2018). Bicarbonate-based carbon capture and algal production system on ocean with floating inflatable membrane photobioreactor. Journal of Applied Phycology 30(2):875-885.
|
|