ABSTRACT
Inflammation is the body’s immune response to combat suspected harmful effect by a stimulus. Antioxidants prevent chain of reaction that would result in the production of free radicals. Prolonged usage of steroids as anti-inflammatory agents is unsafe, demanding search for anti-inflammatory compounds with sustainable future. In this study, seeds and leaves of Solanum xanthocarpum were explored in vitro for their anti-inflammatory and antioxidant activity. The ethanolic extract of seeds and leaves of S. xanthocarpum was prepared by maceration. Anti-inflammatory activity of ethanolic extract of seeds (SE) and leaves (LE) was evaluated in terms of percent inhibition of albumin denaturation, membrane stabilization and protease inhibition. The antioxidant activity was estimated by 2,2-diphenyl-1-picrylhydrantiiazyl (DPPH) free radical scavenging assay by exploiting 1,1-diphenyl-2-picrylhydrazine. Successive solvent extraction with maceration of seeds was carried out by using solvents ethanol, ethyl acetate, acetone and water. The ethanolic extracts of seeds and leaves of S. xanthocarpum demonstrates anti-inflammatory and antioxidant activity. The acetones extract exhibited potent anti-inflammatory activity than ethyl acetate and aqueous extract. Results presented here suggest that the S. xanthocarpum exhibits anti-inflammatory and antioxidant potentiality.
Key words: Solanum xanthocarpum, anti-inflammatory, phytochemical and antioxidant activity.
Inflammation is a complex response to local injury or other trauma; it is characterized by redness, heat, swelling, and pain. Inflammation is a normal protective response to tissue injury caused by physical trauma, noxious chemical and/or microbial agents. It is the body’s response to inactivate or destroy the invading organisms, to remove the irritants and set the stage for tissue repair (Leelaprakash and Dass, 2011; Chandra et al., 2012).
The response can sometimes be alarming including allergies; autoimmune diseases, microbial infections, transplants, and burns may initiate a chronic inflammatory response. Various therapeutic approaches are available for reducing long-term inflammatory responses and thus the complications associated with them. The major approach used presently includes use of steroidal and non-steroidal anti-inflammatory drugs (NSAIDs). The duration of their use is limited by gastrointestinal side effects that include unease and abdominal pain and in a
few serious cases bleeding or perforation of the stomach or upper GI tract (Karthik et al., 2013; Bacchi et al., 2012). The NSAIDs has been reported to cause transient imbalance in electrolyte and water levels as well as liver and renal toxicity (Bacchi et al., 2012; Feldman et al., 1997).
In many inflammatory disorders there is excessive activation of phagocytes, free radical, hydroxyl radical as well as nonâ€free radical species, which can harm surrounding tissue either by powerful direct oxidizing action or indirectly with hydrogen peroxide (H2O2) and hydroxyl radicals; those that initiates lipid peroxidation and membrane destruction. Tissue damage then provokes inflammatory response by production of mediators and chemotactic factors. The reactive oxygen species are also known to activate matrix metalloprotease (MMP), causing increased destruction of tissues for example collagenase damage seen in various arthritic reactions. Hence, agents that can scavenge these reactive oxygen species would be beneficial in treatment (Sakat et al., 2010). Plants have been used as traditional medicinal source. Plants have bioactive compounds with medicinal value and with low side effects.
The Solanum xanthocarpum is a very prickly diffused bright-green, perennial herb of the family Solanaceae. It is found abundantly throughout India in plains of dry regions, by roadsides, wastelands and rubbish heaps (Gangwar et al., 2013). The S. xanthocarpum, a plant from dashmula (More et al., 2013) of Ayurvedic system has been found to have anti-asthematic, hypoglycemic, hepatoprotective, anti-inflammatory, antipyretic and nephron-protective activities (Hussain et al., 2012; Solapure et al., 2016). This work was aimed to estimate whether or not seeds from raw fruits and leaf of S. xanthocarpum in vitro exhibits anti-inflammatory and antioxidant activity.
Collection of plant
Plant material was collected from Varud, Aurangabad (MS, India) from its natural habitat during May 2017. Authentication of plant was conducted by Herbarium center at Department of Botany, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad. The leaves of S. xanthocarpum were washed by distilled water and dried under shade. Fine powder was made in mixer grinder (Kenstar). The seeds were collected from the fruit and washed with distilled water to remove fruit impurities. The seeds were then dried at 40°C for overnight. Fine powder of dried seeds was prepared in mortar and pestle.
Ethanolic extraction
Three grams powder of seed and leaf was extracted with 30 ml ethanol. Maceration was carried out for 24 h. The extracts were filtered through muslin cloth and afterwards by Whatman paper 1. Extract was dried in oven at 40°C for 1 h. Dried extract was dissolved in 1% DMSO. The stock of extract 1 mg/ml was prepared in 1% DMSO.
Solvent extraction and phytochemical analysis
Three grams of dried seeds were extracted with 30 ml of various solvents successively in the order ethyl acetate, acetone and distilled water. The maceration was carried out for 24 h for each solvent at room temperature. The extracts for each solvent were collected separately, filtered and dried in oven at 40°C for 1 h and re-suspended in 1% DMSO. Phytochemical analysis was carried out for detection of carbohydrate, phenolics. Tannins, falvonoids, terpenoids, glycosides, alkaloids and saponins were determined as described by More and Kharat (2013).
Inhibition of albumin denaturation
The method given by Govindappa et al. (2011) with slight modification was used. The reaction mixture contained 500 µl of 1% aqueous solution of bovine serum albumin fraction prepared in phosphate buffer saline of pH 6.4 and extract at concentration 100 µg/ml. The reaction mixture was incubated at 37°C for 20 min and then heated to 50°C for 20 min, reaction mixtures were cooled to room temperature and turbidity was measured spectrophoto-metrically at 660 nm. Diclofenac was used as the comparative standard (Geetha et al., 2013; Osman et al., 2016). The experiment was performed in triplicate. Percent inhibition of protein denaturation was calculated as follows:
% inhibition= [{Abs control- Abs sample}/Abs control] ×100
Where, Abs control is the absorbance of the bovine serum albumin fraction, Abs sample is the absorbance of bovine serum albumin fraction with extract/standard (Govindappa et al., 2011).
Membrane stabilization
Fresh whole human blood (10 ml) from volunteers as per University guidelines was collected and transferred to centrifuge tubes, mixed with equal volume of Alsever solution (2% dextrose, 0.8% sodium citrate, 0.5% citric acid and 0.42% NaCl) and was centrifuged at 3,000 rpm. The packed cells were washed with isotonic saline for three times and a 10% suspension was made in isotonic saline (Pant et al., 2012). Heat induced hemolysis studied with modified method of Govindappa (2011) and Kamlesh Pant (2012) was carried out. The reaction mixture consisted of 100 µg/ml of extract and 500 µl of 10% RBCs suspension; instead of test sample only saline was added to the control test tube. Diclofenac was taken as a standard drug (Osman et al., 2016). All the tubes containing reaction mixture were incubated in water bath at 50°C for 30 min. At the end of the incubation the tubes were cooled. 500 µl of isotonic saline was added to reaction mixture. The reaction mixture was centrifuged at 3000 rpm for 5 min and the absorbance of the supernatants was taken at 560 nm. The experiment was performed in triplicates for all the extracts. Percent membrane stabilization activity was calculated by the formula mentioned as follow:
% Protection = 100-(Optical density of drug treated sample/Optical density of control) × 100 (Chowdhury et al., 2013)
Protease inhibitory action
Modified method of Govindappa (2011) was carried out. The reaction mixture contained 0.06 mg trypsin, 500 µl of 20 mM Tris- HCl buffer (pH 7.4) and 100 µg/ml extract. The action mixture was incubated at 37°C for 5 min and then 1 ml of 0.8% (W/V) casein was added. The mixture was inhibited for an additional 20 min, 2 ml of 70% acetic acid was added to terminate the reaction. Cloudy suspension was centrifuged and the absorbance of the supernatant was read at 210 nm against buffer as a blank (Govindappa et al., 2011). The experiment was performed in triplicate. The percentage of inhibition of protease inhibitory activity was calculated as follow:
Percentage inhibition = (Abs control –Abs sample) × 100/ Abs control (Leelaprakash and Dass, 2011)
Free radical scavenging activity
Modifications in method given by Kumar et al., 2012 were used. The free radical scavenging activity of the extract was measured in vitro by using 1,1diphenyl2 picryl hydrazine (DPPH). The reaction mixture contained 1 ml of DPPH (0.004% in methanol), 100 µg/ml of extract in DMSO (Kumar and Pandey, 2014). The content was mixed and allowed to stand at room temperature for 30 min in dark. The reduction of DPPH free radical was measured by recording the absorbance at 517 nm (Kumar et al., 2012; Patil, 2013) ascorbic acid served the comparison standard (Kumar et al., 2012; Maharana et al., 2010). The percentage scavenging activities (% Inhibition) of the extract was calculated using the following formula.
(%) I = [(AC - AS) / AC] ×100
Where, I is inhibition, AC and AS are the absorbance values of the control and the sample respectively. For each sample, three replicates were made, standard deviation were calculated, and indicated as standard error on the graph.
The leaves and fruit of S. xanthocarpum showed evidence of anti-inflammatory activity, which was reported by careenage induced mice (Poongothai et al., 2011). The leaves were raised to form callus and its methanolic extract was used to detect antioxidant activity by thiobarbituric acid reactive substances (Poongothai et al., 2011).The whole plant was evaluated for anti-inflammatory activity. The sequential extraction with hexane, benzene, chloroform, ethyl acetate, acetone, ethyl alcohol and water of S. xanthocarpum roots exhibited antioxidant activity (Kumar et al., 2012). In this study for the first time, S. xanthocarpum raw seeds were evaluated for their anti-inflammatory as well as antioxidant potentiality.
Phytochemical analysis
The S. xanthocarpum ethanolic extract of leaf (LE), seed (SE), acetone (AC) and aqueous (Aq) seed extracts were subjected for phytochemical analyses. Results shown in Table 1 indicates LE contained phenolics, tannins and alkaloids, SE contained phenolics, quinols and flavonoids, the AC contained phenolics, alkaloids whereas aqueous seed extracts Aq contained phenolics, flavonoids, alkaloids and quinols.
Invariably in all of the extracts, existence of phenolic compounds was confirmed. With an exception of seed ethanolic extract, all other extracts contained alkaloids. Curiously, carbohydrates were not found to exist in the detectable threshold concentration.
Inhibition of albumin denaturation
The denaturation of proteins as one of the causes as inflammation has been well documented. A number of anti-inflammatory drugs have been known to inhibit protein denaturation (Perumal et al., 2008). To investigate the ability of anti-inflammatory activity of S. xanthocarpum, albumin denaturation inhibition assay was performed. The inhibition of albumin denaturation by S. xanthocarpum at concentration 100 µg/ml for leaf ethanolic extract was effective (25%) than seed ethanolic extract (8.33%) (Figure 1). The standard drug used diclofenac, which served a positive control could efficiently inhibit bovine serum albumin denaturation than that of ethanolic seed extract (13.88%). Successive solvent extraction of seeds with ethyl acetate, acetone and water of seeds was also found to be inhibiting albumin denaturation. It is evident from Figure 2, acetone extract has maximum inhibition (61.11%) compared to ethyl acetate (27.77%) and aqueous extract (16.66%).
Membrane stabilization
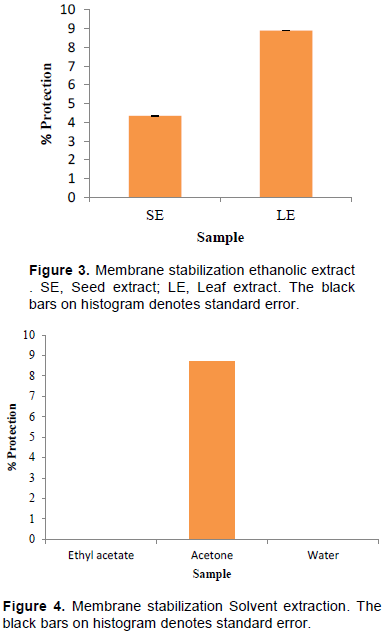
Inflammation is a complex response to local injury or other trauma; it is characterized by redness, heat, swelling, and pain. Lysosomal enzymes released during inflammation produces a variety of disorders which leads to the tissue injury by damaging the macromolecules and lipid peroxidation of membranes. Stabilization of lysosomal membrane is important in limiting the inflammatory response by inhibiting the release of lysosomal constituents of activated neutrophil such as bactericidal enzymes and proteases, which cause further tissue inflammation and damage upon extra cellular release or by stabilizing the lysosomal membrane. Human red blood cells (HRBC) or erythrocyte membrane is analogous to the lysosomal membrane and its stabilization implies that the extract may as well stabilize lysosomal membranes. Stabilization of HRBC by heat induced membrane lysis can be taken as an in vitro measure of anti-inflammatory activity of the drugs or plant extracts (Chippada et al., 2011. Erythrocyte membrane stabilization was studied by incubating erythrocyte with SE and LE. Results shown in Figure 3 denote that LE was able to stabilize erythrocyte membrane better to that of SE. In a parallel experiment, erythrocyte membrane stabilization studies were carried with acetone, ethyl acetate and aqueous extracts prepared from seeds. Results shown in Figure 4 demonstrate that membrane stabilization was found with acetone extracts only whereas both ethyl acetate and aqueous extracts failed to stabilize erythrocyte membrane.
Protease inhibition
Proteases play a crucial role in inflammation process. The serine proteases have been known to be involved in tissue damage thus triggering inflammatory response. The protease inhibitors thus were anticipated to reduce inflammatory response which would be caused by mediators released by leukocytes in response to proteases. To investigate serine protease inhibitor activity, we sought to test anti-trypsin activity in S. xanthocarpum extracts. Results depicted in Figure 5 indicate that anti-trypsin activity was noticed more for SE (3.14%) than for LE (1.26%). The experiment with successive solvent extraction of seeds with acetone, ethyl acetate and water illustrated 4% protease inhibitory activity for acetone and aqueous extract while poor 1% activity was seen for ethyl acetate extract, shown in Figure 6.
Free radical scavenging activity
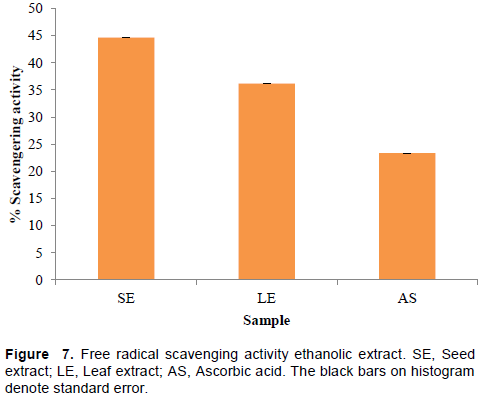
It is known that during inflammatory disorders there is an excessive activation of phagocytes, production of reactive oxygen species, nascent oxygen, hydroxyl and hydrogen peroxide, which could harm surrounding tissue. This in turn would initiate lipid peroxidation resulting in membrane destruction. Tissue damage then provokes inflammatory response by production of mediators and chemotactic factors. The reactive oxygen species are also known to activate MMP. The collagenase and MMP is known to cause increased tissue destruction demonstrated in various arthritic reactions. Hence, the agents that can scavenge these reactive oxygen species would be of greater interest in treatment (Sakat et al., 2010). The DPHH assay for free radical scavenging activity by the first time as per references cited using 1,1-diphenyl-2-picrylhydrazine was performed. The scavenging of free radicals results shown in Figure 7 indicates highest activity for SE (44.68%) in comparison to LE (36.17%) and data were parallelized with a standard compound ascorbic acid (23.4%). Curiously, both LE and SE exhibited superior activity than that of the positive control ascorbic acid.
Solanum species have been known to exhibit various bioactivity in leaf, root and ripened fruits. Our preliminary results suggest that raw fruits and in particular seeds of S. xanthocarpum exhibit strong anti-inflammatory and anti-oxidation potentiality. Our experimental data suggest that either phenolic compounds or alkaloids are likely to be the active pharmaceutical ingredient (API). Experiments are underway to dissect more on biochemical characterization of an API from the SE and acetone seed extracts.
The authors have not declared any conflict of interests.
The authors wish to express their gratitude to S. A. Kate, Head of Department of Biotechnology Shivchhatrapati College, Aurangabad for the facilities extended to complete this research work.
REFERENCES
|
Bacchi S, Palumbo P, Sponta A, Coppolino MF (2012). Clinical Pharmacology of Non-Steroidal Anti-Inflammatory Drugs: A Review. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry 11(1):52-64.
Crossref
|
|
|
|
Chandra S, Chatterjee P, Dey P, Bhattacharya S (2012). Evaluation of In vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pacific Journal of Tropical Biomedicine pp. 178-180.
Crossref
|
|
|
|
|
Chippada SC, Volluri SS, Bammidi SR, Vangalapati M (2011). In vitro anti- inflammatory activity of methanolic extract of Centella asiatica by HRBC membrane stabilisation. Rasayan Journal of Chemistry 4(2):457-460.
|
|
|
|
|
Chowdhury A, Azam S, Jainul MA, Faruq KO, Islam A (2013). Antibacterial Activities and In vitro anti-Inflammatory (Membrane Stability) Properties of Methanolic Extracts of Gardenia coronaria Leaves. International Journal of Microbiology 2014:1-5.
Crossref
|
|
|
|
|
Feldman H, Kinman J, Berlin J, Hennessy S, Kimmen SE, Farrar J (1997). Parenteral Ketorolac: The risk of acute renal failure. Annals of Internal Medicine 126(3):193-199.
Crossref
|
|
|
|
|
Gangwar AK, Ghosh AK, Saxena V (2013). Phytochemical Screening and Analgesic activity of "Kantkari", International Journal of Herbal Medicine 1(2):177-186.
|
|
|
|
|
Geetha N, Anitha S, Shashidhara S (2013). Evaluation of Anti-Inflammatory Activity of Abroma augusta Linn. Journal of Pharmaceutical Sciences 3(3):29-34.
|
|
|
|
|
Govindappa M, Channabasava R, Sowmya DV, Meenakshi J, Shreevidya MR, Lavanya A, Gustavo Santoyo, SadanandaTS (2011). Phytochemical Screening, Antimicrobial and in vitroAnti-inflammatory Activity of Endophytic Extracts from Loranthus sp. Pharmacognocy Journal 3(25):82-90.
Crossref
|
|
|
|
|
Govindappa M, Naga Sravya S, Poojashri MN, Sadananda TS, Chandrappa CP (2011). Antimicrobial, antioxidant and in vitro anti-inflammatory activity of ethanol extract and active phytochemical screening of Wedelia trilobata (L.). Journal of Pharmacognosy and Phytotherapy 3(3):43-51.
|
|
|
|
|
Hussain T, Gupta RK, Sweety K, Khan MS, Hussain MS, Arif M, Hussain A, Faiyazuddin M, Rao CV (2012). Evaluation of antihepatotoxic potential ofSolanum xanthocarpum fruit extract against antitubercular drugs induced hepatopathy in experimental rodents. Asian Pacific Journal of Tropical Biomedicine 2:(6):454-460.
Crossref
|
|
|
|
|
Journal of Pharmacy and Pharmaceutical Sciences 2(1):146-155.
|
|
|
|
|
Karthik K, Kumar P, Venu PR, Kumar S K,Singh R, RathoreB(2013). Evaluation of Anti-Inflammatory activity of Canthium parviflorum By In-vitro method. Indian Journal of Research in Pharmacy and Biotechnology 1(5):729-730.
|
|
|
|
|
Kumar S, SharmaUK, Sharma AK, Pandey AK (2012). Protective efficiency of Solanum xanthocarpum root extracts against free radical damage: phytochemical analysis and antioxidant effect. Cellular and Molecular Biology 58(1):174-181.
|
|
|
|
|
Kumar SK, Pandey AK (2014). Medicinal attributes of Solanum xanthocarpum fruit consumed by several tribal communities as food: an in vitro antioxidant, anticancer and anti HIV perspective, BMC Complementary and Alternative Medicine 14:112.
Crossref
|
|
|
|
|
Leelaprakash G, Das SM (2011). In vitro Anti-Inflammatory Activity of methanol extract of Enicostemma axillare. International Journal of Drug Development & Research 3(3)189-196.
|
|
|
|
|
Maharana L, Kar DM, Pattnaik S, Sahu PK, Sudam CS (2010). In vitro antioxidant activity of aqueous leaf extract of Solanum nigrum Linn. Pharmacology online 3:333-345.
|
|
|
|
|
More SK,Lande AA, Jagdale PG, Adkar PP, Ambavade SD(2013). Evaluation of anti-inflammatory activity of Solanumxanthocarpum Schrad and Wendl (Kaṇá¹akÄri) extract in laboratory Animals. Ancient Science of Life 32(4):222-226.
Crossref
|
|
|
|
|
Osman NI, Sidik NJ, Awal A, Adam NA, Rezali NI (2016). In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L. and total phenolic content analysis for potential anti-inflammatory use in gouty arthritis. Journal of Intercultural Ethnopharmacology 5(4):343-349.
Crossref
|
|
|
|
|
Pant K, Agarwal K, Saini P (2012). To study in vitro anti-inflammatory activity of Anthracephalus cadamba leaves extract. DHR International Journal of Pharmaceutical Sciences 3(1):55-60.
|
|
|
|
|
Patil DD (2013). Antioxidant Effect of the Stem and Leaves of Solanum xanthocarpum. Unique Journal of Ayurvedic and Herbal Medicines 1(3):68-70.
|
|
|
|
|
Perumal R, Deya A, Manavalana R, Prakasam K, Jayachandran E, Sreenivasa GM (2008). Inhibition of albumin denaturation and Anti Inflammatory activity of furfuryl substituted pyrimidinoimidazolinones. International Journal of Chemical Sciences 6(4):2016-2022
|
|
|
|
|
Poongothai K, Ponmurugan P, Ahmed KS, Kumar BS, Sherif SA (2011). Antihyperglycemic and antioxidant effects of Solanum xanthocarpumleaves (field grown & in vitro raised) extracts on alloxan induced diabeticrats. Asian Pacific Journal of Tropical Medicine 4(10):778-785.
Crossref
|
|
|
|
|
Sakat SS, Juvekar AR, Gambhire MN (2010). In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. International Journal of Pharmacy and Pharmaceutical Sciences 2(1):146-55.
|
|
|
|
|
Solapure P, Pradeep RM, Hegde PL (2016). Comparative anti-inflammatory activity of Clerodendrum serratum (Linn) Moon and Solanum xanthocarpum Schrad and Wendl in wistar ablino rats. The Journal of Phytopharmacology 5(2):38-44.
|
|