ABSTRACT
Plant extracts have been lately used by the population to treat various types of diseases, and this has been notably encouraged by the World Health Organization (WHO). Curcuma zerumbet (Zingiberaceae) belonging to the family of the Zingiberaceae, is herbaceous, perennial and, utilized by the population to treat gastric disorders. However, data on the subacute toxicity of this species are scarce in the literature. Therefore, the present work aimed to ascertain the subacute toxicity of different doses of the aqueous and hydroalcoholic extracts from C. zerumbet. These extracts were orally administered through gavage in Wistar rats for 28 consecutive days. This study followed the instructions put forth by Guideline 407 (subacute toxicity) of Organization for Economic Cooperation and Development (OECD). In specific pharmacological tests for acute and subacute toxicity in rats, it can be stated that C. zerumbet extracts doses greater than 5 g/kg neither caused mortality nor presented oral toxicity. Therefore, the extracts toxicity parameters analyzed in different doses on the groups of animals have shown no significant difference from those found in the control group. This allows one to conclude C. zerumbet (Zingiberaceae) rhizome aqueous and alcoholic extracts in high doses to harbor very low toxicity within a short time.
Key words: Curcuma, subacute toxicity, extracts.
The knowledge regarding the medicinal properties of plants has been passed and enhanced from generation to generation in rural and urban communities. The diversity of medicinal plant species has fostered the use of the ones harboring phytotherapy properties due to the belief that all that is “natural” causes no ill effects (Oliveira et al., 2011). However, the use of plant extracts is not totally free of risks, since in addition to harbouring bioactive compounds, the same plant may contain toxic substances in different amounts and concentrations.
The species Curcuma zerumbet (Zingiberaceae) is a perennial herb belonging to the family Zingiberaceae,
spontaneously occurring in Southeast Asia and Northeast India (DAS et al. 2013). In the state of Amazonas, this plant is found in some municipalities, being utilized in ornamentation or in the form of teas for treating gastric disorders (Castro et al., 2017).
In Brazil, most genera are used as ornamentation, food and pharmaceuticals. They stand out in the treatment of cervical cancer (Epstein et al., 2010), flatulencies (Alsarhan et al., 2012), gastritis (Neamsuvan, 2012), hepatic disorders (Singh et al., 2012), cough (Abe and Ohtani, 2013), rashes skin infections (Rahmatullah et al., 2011) hepatitis and Inflammation (Schaffer et al., 2011). C. zerumbet subacute toxicity phamacological studies have shown very few scientific evidences. Thus, the toxicological study, regardless of pharmacological results, is essential on account of the existing ethnopharmacological and toxicological data. This is why the present work was undertaken with the objective to assess the subacute toxicity of C. zerumbet extracts, being orally administered to rats through gavage.
Collection and herbization of C. zerumbet Roscoe (Zingiberaceae)
The C. zerumbet species samples were collected in the community of Tarumã-mirim, Ramal do Pau Rosa, Latitude 2°43ʹ17ʺS, Longitude 60°08ʹ19ʺW, located in the state of Amazonas/Brazil. The exsiccate was sent to the Botany unit of the National Research Institute of Amazonia (INPA) to be identified and deposited in its Herbarium under No. 265800. The rhizomes were hygienized, washed in running water and dried in an air circulating oven at 45°C for 24 h.
How extracts were obtained
The aqueous extract was obtained by the infusion method using 50 g of Curcuma diluted in 1000 mL of distilled water, while the hydroalcoholic extract was obtained by the maceration method using 200 g of Curcuma in 2000 ml ethanol: water (1: 1) for one period of 72 h. After extractions, the extracts were dehydrated through lyophilization using the LS 3000 (TERRONI®) lyophilized apparatus for 48 h (Castro et al., 2017).
Extracts sub-acute toxicity analysis
The determination of the pharmacological activity was done by a test model established by the World Health Organization (WHO) and in accordance with the technical Standards established and approved by the Commission for Ethics in Research on the Use of Animals (CEUA / INPA) under protocol number 003/2013; the animals used in the experiments were albino rats weighing 200 - 400 g, from the Central Bioterium of INPA.
The aqueous and hydroalcoholic extracts subacute toxicity was analyzed using male albino Wistar rats, from the Inpa’s Central Bioterium. During the experimental period (28 days), the animals were kept in polypropylene boxes, under controlled photoperiod (12 h light / 12 h dark) at 23 ± 2°C. These animals were fed ration and water with extracts (aqueous or hydroalcoholic)
ad libitum orally, through gavage (OECD, 2008b). During this period, we analyzed the ration and water consumption variation as well as the body mass of the animals (
OECD, 2008a). The animals were randomly divided into seven groups, with each group containing six rats, according to the following protocol: (i) Group 1: control; (ii) Group 2: fed with 100 mg/Kg of aqueous extract; (iii) Group 3: fed with 1000 mg/Kg of aqueous extract; (iv) Group 4: fed with 5000 mg/Kg of aqueous extract; (v) Group 5: fed with 100 mg/Kg of hydroalcoholic extract; (vi) Group 6: fed with 1000 mg/Kg of hydroalcoholic extract; (vii) Group 7: fed with 5000 mg/Kg of hydroalcoholic extract;
After 28 days, the animals were submitted to 12 h of fasting, and blood collection by intracardiac puncture was performed after anesthesia with intraperitoneal administration of ketamine / xylazine (10/10 mg/kg). The blood was stored in collection tubes with 10% sodium EDTA and the following parameters were analyzed:
(i) Hematological: total leukocyte, neutrophils, lymphocytes, eosinophilia, monocytes, red blood cells, hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red blood cells distribution slope according to the erythrocyte volume (RDW-SD), mean red blood cell volume coefficient of variance (RDW-CV) and mean platelet volume (MPV) platelet count.
(ii) Biochemical: aspartate aminotransferase (AST or TGO), alanine minotransferase (ALT or TGP), albumin, glucose, urea, creatinine, total cholesterol, triglycerides, uric acid.
At the end of the experimental period, all animals were sacrificed by decapitation and the following organs were removed, weighed, measured and observed macroscopically: liver, small intestine, kidneys, spleen, heart, and lung.
Statistical analysis
Data were represented as means ± standard deviation of the means of five animals per group of experiment in vivo. The differences between the means were determined by one way analysis of variance (ANOVA), followed by the Tukey test. Analyses were performed using the Windows program, Graph Pad Prism version 6.0 (Graph Pad Software). The value of less than 0.05 was considered significant.
Extracts sub-acute toxicity analysis
Subacute toxicity assessment is a widely used methodology to ascertain and classify substances as to their ability to cause acute damage to living organisms in high doses, especially pathological and pathological injuries, and may contribute to establish safety parameters - together with other toxicity data – pertaining to human health (Zatta et al., 2009). As regards plants, this method is useful for identifying the toxicity it may present and thereby prevent people from believing natural products to be devoid of toxic or adverse effects (Silveira et al., 2008; Cunha et al., 2009; Farsi et al., 2013). The present work, through experimental trials with rats being orally administered with aqueous and alcoholic extracts at doses of 100 to 5 g/kg, has demonstrated the lack of pharmacological parameters or toxicological effects prone to lead them to mortality. This indicates that extracts from C. zerumbet harbor very low toxicity. In Table 1, the results of the biochemical parameters after the application of the extracts for a period of 28 days of experiment. Table 1 shows that the study groups total cholesterol did not differ significantly but triacylglycerol differ significantly when hydroalcoholic extract was administered orally at the dose of mg/kg whereas no change was observed when the rats were fed with the aqueous extract.
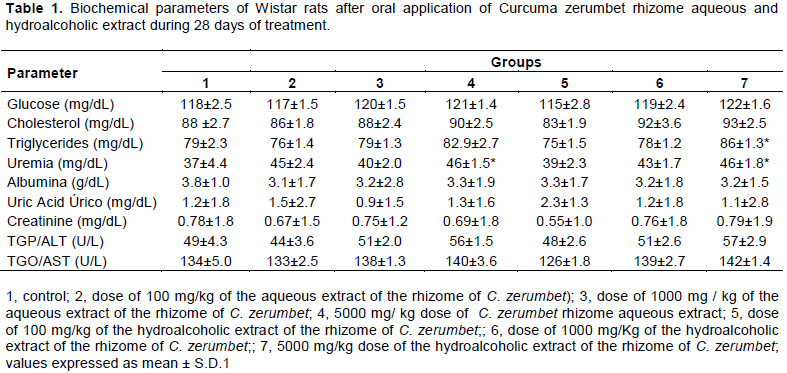
This finding may be both related to hydroalcoholic extract concentration and the animals feeding, since the latter was not controlled.
Urea and/or creatinine are eliminated by the kidneys and; in chronic kidney disease there is reduced functioning of the kidneys, with both substances being accumulated in the blood (Dusse and Freitas, 2015). Many factors can significantly change the plasma urea values without being related to the renal function especially protein-rich diet, such as the one found in rodents ration. Elevated urea values ​​in conjunction with creatinine indicate processes that lead to decreased renal blood flow or to gastrointestinal bleeding with obstructive post-renal processes such as tumors and stenosis of the urinary tract (Sodré et al., 2007). Since there were no significant increases on urea levels, uremia is possibly related to total protein content and not to renal disease. Proteins are degraded to urea and as still observed in Table 1. The latter had their levels significantly increased for groups (4) and (7) as compared to control (1).
Renal (pre-renal, renal and post-renal) and extra-renal (protein intake) factors may interfere with increased serum urea concentration. Therefore, this increase in urea levels in the animals that consumed higher doses of the extracts, both aqueous and hydroalcoholic, may be suggested by extra renal factors such as the ingestion of dietary protein by animals, since urea is the main end product of protein catabolism or toxicity index for the
high dose groups of aqueous and hydroalcoholic extracts.
Plasma albumin is a good indicator of nutritional status and, in this case, the values ​​obtained in this research are within the normality standards for the species under study. According to the literature, healthy rats bear albumin content in the range of 3.4 to 4.3 g dL-1 (Gautier et al., 2014). There was no effect on AST and ALT levels, which are considered sensitive indicators of hepatocellular damage and when within limits may provide a quantitative assessment of the degree of liver damage (Al-habori et al., 2002). Therefore, it is possible to deduce, that the aqueous and hydroalcoholic extract caused no damage to the kidneys or liver, at doses below 5000 mg / kg. The hematological parameters assessment can be used to determine the extent of deleterious effects brought about by strange compounds, including plant extracts, on an animal’s blood constituents (Ashafa et al., 2012).
Low hemoglobin, erythrocytes and hematocrit concentrations may indicate anemia, recent bleeding or fluid retention, causing hemodilution. Yet, a decreased platelet count (thrombocytopenia) can result from a series of pathological situations, such as the increased destruction of these cells, due to the use of certain drugs, immune disorders, disseminated vascular coagulation and even mechanical lesions (Dougan et al., 2008). The leukocyte differential is used to assess the distribution and morphology of white blood cells, providing more specific information about the immune system than the leukocyte count alone does (Prinyakupt and Pluempitiwiriyawej, 2015).
Hematological parameters were measured in this study after 28 days of administration of the extracts. The treated animals showed no significant decreases in red blood cell, hemoglobin, hematocrit and platelets levels(Table 2) and the differential count of white cells and lymphocytes, as compared to control’s.
In the study by Hossen et al. (2017), the MCV and HCM concentration levels increased when animals were fed for a period of 28 days with species Curcuma Longa. While the other hematological data remained unchanged. In this study, this was not observed. In the study by Salama et al. (2013) this species biochemical and hematological presented no significant difference between the control groups. Thus, corroborating the findings put forth by the present work.
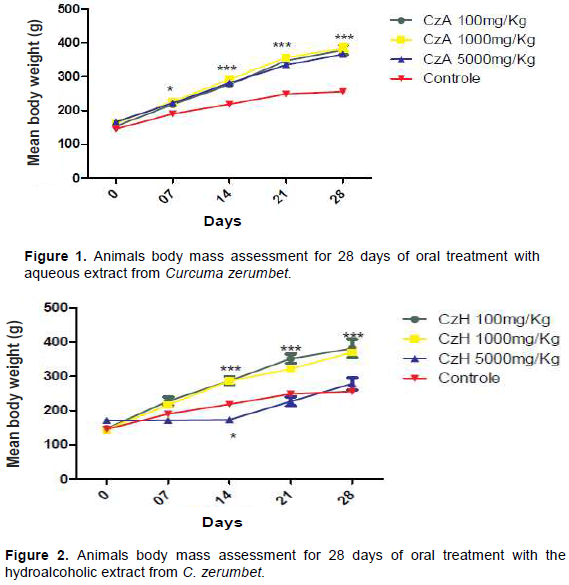
For daily habits (feed consumption and fecal production), there was no significant variation between the groups treated with the aqueous and hydroalcoholic extract and the control group. However, there was change in the body mass parameter, when compared to that shown by the control. The mean body mass presented by the animals treated with the aqueous extract at doses of 100, 1000 and 5000 mg/Kg increased from 155 ± 5.8 to 363 ± 7.8; 166 ± 8.4 to 370 ± 7.5 and 166 ± 6.9 to 350 ± 8.7 respectively; while that presented by the control increased from 145 ± 5.7 to 241 ± 6.5 showing a statistical difference from the seventh day of application onwards (Figure 1) while the mean body mass of the animals observed in the hydroalcoholic extract experiment increased from 147 ± 8.9 to 366 ± 7.8 at the dose of 100 mg / kg; 144 ± 8.8 to 345 ± 9.8 at the dose of 1000 mg / kg and 171 ± 7.0 to 252 ± 7.8 at the dose of 5000 mg / kg. These findings may be related to the extracts-borne protein and carbohydrate levels. Figure 2, shows the animals to present a significant difference in the body mass from the 14th day of the experiment onwards, as compared to that of the control at doses of 100 and 1000 mg/kg. The animals evaluated at the dose of 5000 mg/kg exhibited a constant body mass in the first weeks of the experiment. They exhibited the same gain of body mass as that of control animals, from the 14th day onwards. It is known that systemic toxicity can be identified by the decrease in the body mass of the animals and by changes in water and feed intake, which have shown to be paramount when undertaking a substance or extract’s toxicity assessment, since it provides data pertaining to the animals’ health status as a whole (Valadares, 2006). Nevertheless, these animals showed no changes on their food and water intake. The macroscopically analyzed and weighed organs exhibited no statistical differences between the control groups and the experimental doses. Thus, the tested C. zerumbet extracts showed no evidence of harboring any toxicity.
It can be concluded that the aqueous and hydroalcoholic extract from the rhizome of C. zerumbet (Zingiberaceae) harbors low, short term toxicity in high doses. In cases of prolonged use, further studies are required to ascertain the outcome of such histopathologic changes.
The authors have not declared any conflict of interests.
The authors thank Capes for its financial support.
REFERENCES
|
Abe R, Ohtani K (2013). An ethnobotanical study of medicinal plants and traditional therapies on Batan Island. the Philippines. Journal of Ethnopharmacology 145(2):554-565.
Crossref
|
|
|
|
Al-habori AM, Al-aghbari M, Al-mamary M, Baker (2002). Toxicological evaluation of Catha edulis leaves: a long term feeding experiment in animals. Journal of Ethnopharmacology 83(3):209-217.
Crossref
|
|
|
|
|
Alsarhan A, Sultana N, Kadir MRA, Aburjai T (2012). Ethnopharmacological survey of medicinal plants in malaysia. the kangkar pulai region. International Journal of Pharmacology 8(8):679-686.
Crossref
|
|
|
|
|
Ashafa AOT, Orekoya LO, Yakubu MT (2012). Toxicity profile ethanolic extract of Azadirachtaindica stem bark in male Winstar rats. Asian Pacific Journal of Tropical Biomedicine 2(10):811-817.
Crossref
|
|
|
|
|
Castro MS, Pinheiro CCS, Marinho HA (2017). Phytochemical and Physical-chemical Screening of Curcuma Zerumbet Roscoe (Zingiberaceae) Extracts From Amazonas for the Production of Therapeutic Foods. Amazonian Biota 7(1):6-11.
|
|
|
|
|
Cunha LC, Azeredo FS, Mendonça ACV, Vieira MS, Pucci LL, Valadares MC, Freitas HOG, Sena AAS, Lino JRS (2009). Acute and subacute toxicity studies of the latex and of the ethanolic extract of the leaves of Synadenium umbellatum Pax in rats. Brazilian Journal of Pharmacognosy 19(2):403-11.
Crossref
|
|
|
|
|
Das A, Kesari V, Nath A, Khare A, Rangan L (2013). Antimicrobial and Micro Raman Spectroscopy of Selected Zingiberaceae Species from Northeast India. Journal Crop Science Biotechnology 16(1):75-81.
Crossref
|
|
|
|
|
Dougan S, Turkoglu I, Agacı K, Kullanarak Y, Verileri B, TeÅŸhisi A, Kelimeler A, Tanıma O, Madenciligi V, Çıkarma O, Parametreler H, Sistem U (2008). Iron-deficiency anemia detection from hematology parameters by using decision trees. International Journal of Science Technology3(1):85-92.
|
|
|
|
|
Dusse LMS, Freitas LG (2015). Clinical Applicability of Reticulated Platelets. Clinica Chimica Acta. 439(15):143-147.
Crossref
|
|
|
|
|
Epstein J, Sanderson I, Macdonald T (2010). Curcumin as a therapeutic agent: The evidence from in vitro animal and human studies. British Journal of Nutrition 103(11):1545-1557.
Crossref
|
|
|
|
|
Farsi E, Shafaei A, Hor SY, Ahamed MB, Yam MF, Asmawi MZ, Ismail Z (2013).Genotoxicity and acute and subchronic toxicity studies of a standardized methanolic extract of Ficus deltoidea leaves. Clinics 68(6):865-875.
Crossref
|
|
|
|
|
Gautier JC, Gury T, Guffroy M, Khan-Malek R, Hoffman D, Pettit S, Harpur E (2014). Normal ranges and variability of novel urinary renal biomarkers in Sprague-Dawley Rats: comparison of constitutive values between males and females and across assay platforms. Journal of Toxicologic Pathology 42(7):1092-1104.
Crossref
|
|
|
|
|
Hossen MS, Tanvir EM, Maruf BP, Sudip P, Moumoni Md, Yousuf A, Siew Hua G, Md, Khalil I, Nurul K(2017) Protective mechanism of turmeric (Curcuma longa) on carbofuran-induced hematological and hepatic toxicities in a rat model. Pharmaceutical Biology 55(1):1937-1945.
Crossref
|
|
|
|
|
Neamsuvan O, Tuwaemaengae T, Bensulong F, Asae A, Mosamae K, (2012). A survey of folk remedies for gastrointestinal tract diseases from Thailand's three southern border provinces. Journal of Ethnopharmacology 144(1):1-21.
Crossref
|
|
|
|
|
OECD (2008b). Guidelines for testing of chemical. Repeated dose 28-day oral toxicity study in rodents. OECD (Ed.): Paris.
Crossref
|
|
|
|
|
OECD (2008a). Guidelines for testing of chemical. Acute oral toxicity – up-and-down-procedure. OECD (Ed.): Paris.
Crossref
|
|
|
|
|
Oliveira AKM, Oliveira NA, Resende UM, Martins PFRB (2011). Ethnobotany and traditional medicine of the inhabitants of the Pantanal Negro sub-region and the raizeiros of Miranda and Aquidauna. Mato Grosso do Sul. Brazilian Journal of Biology 71(1):176-179.
Crossref
|
|
|
|
|
Prinyakupt J, Pluempitiwiriyawej C (2015). Segmentation of white blood cells and comparison of cell morphology by linear and naïve Bayes classifiers. BioMedical Engineering OnLine 14(63):1-19.
Crossref
|
|
|
|
|
Rahmatullah M, Ishika T, Rahman M, Swarna A, Khan T, Monalisa MN, Seraj S, Mou SM, Mahal MJ, Biswas KR (2011). Plants prescribed for both preventive and therapeutic purposes by the traditional healers of the bede community residing by the turag river. dhaka district. American-Eurasian Journal of Sustainable Agriculture 5(3):325- 331.
|
|
|
|
|
Salama SM, Abdulla MA, AlRashdi AS, Ismail S, Alkiyumi SS, Golbabapour S (2013). Hepatoprotective effect of ethanolic extract of Curcuma longa on thioacetamide induced liver cirrhosis in rats. BMC Complementary and Alternative Medicine 13(56):811-817.
Crossref
|
|
|
|
|
Schaffer M, Schaffer MP, Zidan J, Sela GB (2011). Curcuma as a functional food in the control of cancer and inflammation. Current Opinion in Clinical Nutrition and Metabolic Care. 14(1):588-597.
Crossref
|
|
|
|
|
Silveira PF, Bandeira MAM, Arrais PSD (2008). Pharmacovigilance and adverse reactions to the medicinal plants and herbal drugs: a reality. Brazilian Journal of Pharmacognosy 18(4):618-626.
Crossref
|
|
|
|
|
Singh AG, Kumar A, Tewari DD (2012). An ethnobotanical survey of medicinal plants used in Terai forest of western Nepal. Journal of Ethnobiology Ethnomedicine 8(19):2-14.
Crossref
|
|
|
|
|
Sodré FL, Costa JCB, Lima JCC (2007). Evaluation of renal function and damage: a laboratorial challenge. Brazilian Journal of Pathology Laboratory Medicine 43(5):329-337.
Crossref
|
|
|
|
|
Valadares MC (2006). Acute Toxicity Assessment: Strategies After the "LD50 Test Age. Electronic Pharmacy Magazine 3(2):93-98.
|
|
|
|
|
Zatta DT, Pimenta FC, Tresvenzol LMF, Fiuza TS, Bara MTF, Cunha LC, Pucci LL, Garrote CFD, Oliveira FNM, Paula JR (2009). Study of Antibacterial Activity against Pseudomonas aeruginosa strains and Acute Toxicity of Jacaranda decurrens leaves. Latin American Journal Pharmacology 28(4):485-489.
|
|