ABSTRACT
Water stress affects plant growth and development, leading to agricultural crop losses in maize cultivation. It also threatens food security in economical crops such as maize, one of the major crops produced worldwide. Transcriptomic studies associated with morphological assessments have been widely conducted on the mechanisms of crop development and stress response; however, data on maize is still very much limited. Hence herein, we used both the morphological and proteomic analyses to investigate and establish physical features and proteins associated with maize in response to osmotic stress. In addition, proteomic analysis (1DE and 2DE techniques) was used to separate and enumerate water stress responsive proteins. Morphologically, a decrease in the overall growth of the maize plant as a result of water stress was observed, whereby features such as leaf colour and size, shoot height and stem diameter were negatively affected. Through proteomics analyses, a total of nine expressed proteins were revealed in response to water stress. Overall, this work, has successfully profiled the water stress responsive proteins and specifically indicating the efficiency of proteomic tools in the detection and analysis of qualitative proteins from maize.
Key words: Zea mays, water stress, induced proteins, proteomics, plant response, crop losses.
Climate change and global warming accelerates the risk of drought, which has several detrimental effects on various organisms including humans, animals and plants (Dai, 2013). However, plants as the primary producers are constantly exposed to various abiotic stresses, which affect their essential roles in the general life systems of mankind (Jin et al., 2015). Due to climate change, some regions on earth are not receiving enough rainfall, thus such regions do experience drought. Soil water supply is an important environmental factor, controlling seed germination and seedling establishment (Kramer and Kozlowski, 1980; Bargali and Bargali, 2016). Hence, when water potential is reduced, seed germination will be delayed or halted depending on the extent of its reduction (Hegarty, 1977; Zobel et al., 1995). Seed germination and early seedling growth are considered the most critical phases for the establishment of any plant species (Bargali and Singh 2007; Pratap and Sharma, 2010; Vibhuti et al., 2015; Pantola et al., 2017).
Water deficiency is one of the major abiotic stresses that affect plant growth, development and productivity worldwide (Zhao et al., 2011; Shi et al., 2014). With such effects, it is estimated that by the end of the 21st century, drought terrestrial areas will increase and threatens food security (Zhao et al., 2011). Hence, it is imperative to determine and understand the mechanisms that are employed by plants when experiencing drought, in order to improve their tolerance to water stress.
To deal with water-deficit stress, plants have developed various mechanisms to regulate the balance of cells, through optimization of their morphology, physiology and metabolism at a cellular level (Boyer, 1982). Previous studies have shown various signal responses to drought, where a plant would undergo leaf abscission, while in other plants, the response may be deadly (Chaves et al., 2002). In some cases, slow water loss results in acclimation to water stress condition thus limiting the drastic effects of plant damage (Bray, 1997), whereas in rapid water loss, acclimatization is prevented because plants have limited time. In addition, water stress results as a physiological condition, where plants have less than full turgor pressure, due to the transpiration demand exceeding root water uptake (Dejonge et al., 2012). The physiological impact of water stress at both the tissue and cellular levels in plants have been shown to result in new metabolic and structural abilities mediated by the changed gene and protein expression that will assist in plant functioning (Bray, 1997; Kasuga et al., 1999; Hasegawa et al., 2000; Seki et al., 2003; Shinozaki et al., 2003). In addition, some of the physiological and biochemical modifications that are involved during water stress include growth inhibition as a result of stomatal closure, which affects photosynthesis and respiration (Fathi and Tari, 2016).
Maize (Zea mays L.) is one of the major cultivated crops in South Africa, which serves as a staple food to many homes. Maize being a thermophilous crop, requires temperatures that exceed 10°C for its proper growth as well as related physiological processes such as canopy photosynthesis and root system activities (Liu et al., 2010). However, it needs to be produced under optimal conditions for maximum production and the coordination of its high sensitivity to harsh environmental conditions such as water deficit (Lobell et al., 2011). Maize exhibits varying physiological and biochemical effects during water stress such as development and growth inhibition during the early growth stages, structural damage, reduction in kernel number and ear size (Bassetti and Westgate, 1993; Farré and Faci, 2006). In addition, water stress induces stomatal closure, which results in decreased CO2 absorption that reduces photosynthetic activity (Nayyar and Gupta, 2006).
Water deficiency intensely affects the agricultural sector, thus limiting total crop yield, which in turn, affects food security and pose a serious threat to the growing population. An increase in crop production is hindered by drought stress (Fathi and Tari, 2016). Various studies are being conducted on maize that includes the mechanisms of crop development and the environmental adaption of crops to stress, in order to improve quality and yield. This raises a need to better understand the mechanisms used by crop plants when they are exposed to drought stress. Thus, our study, reported herein aimed to profile water stress induced proteins from leaf extracts of a Z. mays cultivar (R450 w/uo2550 CML550).
Transcriptomic studies have previously been carried out to reveal the large-scale drought modulated gene expression in leaf meristem and reproductive tissues and seedling shoots of maize (Zheng et al., 2010; Kakumanu et al., 2012). Recently, proteomics analyses have been performed on various maize tissues to study water stress responsive protein expression in drought-tolerant and drought sensitive genotypes (Riccardi et al., 2004). Although, proteomics approaches have been studied in various plant species (Cui et al., 2005; Dani et al., 2005; Ndimba et al., 2005), the published proteomic data on responses of maize to water stress is still limited (Yoshimura et al., 2008). Ultimately, information on such studied genes/proteins can then be possibly genetically transferred into other maize varieties and/or related crops that exhibit sensitivity to water stress.
Plant material and treatment growth conditions
The R450w/uo2250w CML550 Z. mays seeds cultivar used in this study were obtained from Molelwane Farm, Department of Crop Science, North-West-University, RSA. The seeds were selected for size homogeneity in terms of size and physical appearance for each pot. The seeds were surface-sterilized with 70% (v/v) ethanol for a minute, followed by decontamination with 1.25% sodium hypochlorite solution (bleach) for 10 min. Immediately after sterilization, the seeds were rinsed three times with sterile distilled water. Three seeds were sown in each of the 12 plastic plant pots, filled with a 3:2 (v/v) mixture of sterilized organic soil (Levington F2, seed and modular compost) and vermiculite. The intended maize plants were grown in a randomized design to eliminate the effect of variations in environmental conditions at different positions. Thereafter, plants at the same developmental stage and of similar height, were selected for all experiments. The sown seeds were watered daily with 100 ml of sterile tap water up until germination begun on day 7. Germinated plants were grown under greenhouse conditions of 16-h days and 8-h nights, day/night air temperature of 26/22°C and relative humidity of 75%. Treatment of plants began when seedlings were 8 days old, whereby plants were divided into two groups: well-watered plants irrigated after every 2 days (control) with 100 ml sterile tap water while the water-stressed plants did not receive any water up until the recovery period (16 days) (treatment). On the 16th day, leaves of both the control and treatment plants were harvested, rinsed with sterile distilled water and immediately snap-frozen in liquid nitrogen. Each treatment group was conducted in three independent biological replicates.
Total protein extraction from maize leaf tissues
Maize leaf protein extracts were prepared from sixteen, 16-day old maize seedlings. The snap-frozen leaf material was ground into fine powder using pre-chilled sterile mortar and pestle. The powdered tissues were precipitated with 10% (w/v) trichloroacetic acid (TCA). The generated precipitate for each sample was individually washed 3 times with 1 ml of ice-cold 80% (v/v) acetone through centrifugation at 13 400 g for 10 min at room temperature. Immediately after washing, the pellet was air-dried for 5 min at room temperature. The air-dried pellet was solubilized in 1 ml of lysis buffer (9 M urea, 2 M thiourea and 4% (w/v) 3-cholamidopropyl dimethylammonio 1-propanesulfonate (CHAPS)) through vigorous vortexing at room temperature for an hour. After an hour of vortexing, the homogenate was centrifuged at 15 700 g at room temperature for 10 min. The supernatants for each sample were then transferred into fresh sterile Eppendorf tubes and stored at -20°C. Total protein concentration of the leaf extracts were quantified using a 2000 Nanodrop spectrophotometer (Thermo Scientific Inc., California, USA). One-dimensional (1D) sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) or 1DE of about 10 μg protein on a 12% (w/v) was performed to evaluate the quality of the obtained protein extracts.
Two-dimensional electrophoresis (2-DE) of total soluble proteins
Prior to further analysis with 2-dimensional gel electrophoresis (2DE), the resolved total protein samples were further purified using the ReadyPrep™2-D Clean-up kit (catalog # 163-2130, Bio-Rad Laboratories Inc., California, USA) following the manufacturer’s instructions to improve the quality of proteins. Good quality resolved proteins from the 1DE samples were then selected for further analysis through a 2DE. The protein samples were mixed with a rehydration buffer (8 M urea, 2% (w/v) CHAPS, 50% (w/v) dithiothreitol (DTT), 0.2% (v/v) ampholytes, 0.1% (w/v) of bromophenol blue (Bio-Rad Laboratories Inc., California, USA) to make a final volume of 125 μl. The immobilized pH gradient (IPG) strips (7 cm), pH 3 - 10 (Bio-Rad Laboratories Inc., California, USA) were passively rehydrated overnight in an equilibration tray with the rehydration solution containing equal amounts (150 μg) of protein samples at room temperature on a flat surface. Subsequently, the strips were then subjected to an isoelectric focusing (IEF) on a PROTEAN i12 IEF cell (Bio-Rad Laboratories Inc., California, USA) in a step-wise program. Focusing was carried out at 20°C and 50 µA current per IPG strip, following the set procedure of: 250 V for 20 min, followed by 4 000 V for 2 h and finally, 4,000 V until it reached 10,000 Vh.
The focused IPG strips were then equilibrated with 2.5 ml of SDS containing equilibration buffers as described by Ngara and Ndimba (2011). The equilibrated IPG strips were dipped into a 100 ml of SDS-PAGE running buffer and loaded onto the 10% (w/v) polyacrylamide resolving gels of 1-mm thickness. The strips were then overlaid with pre-warmed overlay agarose solution (100 ml 1 x SDS-PAGE running buffer; 0.5% (w/v) agarose; 0.002% (w/v) bromophenol blue), which was allowed to cool and solidify. The gels were electrophoresed on a Mini-PROTEAN Tetra hand cast system (Bio-Rad Laboratories Inc., California, USA) at a constant voltage of 150 V for 45 min or until the dye front had reached the bottom of the gel. Immediately, the electrophoresis was complete, gels were stained in a Coomassie staining buffer solution followed by de-staining (100% (v/v) ethanol, 100% (v/v) methanol, 100% (v/v) acetic acid) for 50 min, shaking on an ultra-rocker (Bio-Rad Laboratories., USA) until the protein spots were visualized. The de-stained gels were then image-captured on a Chemi DOC™ Imaging system (Bio-Rad Laboratories Inc., California, USA) using the Bio-Image Lab™ software.
Morphological responses of Zea mays to water stress
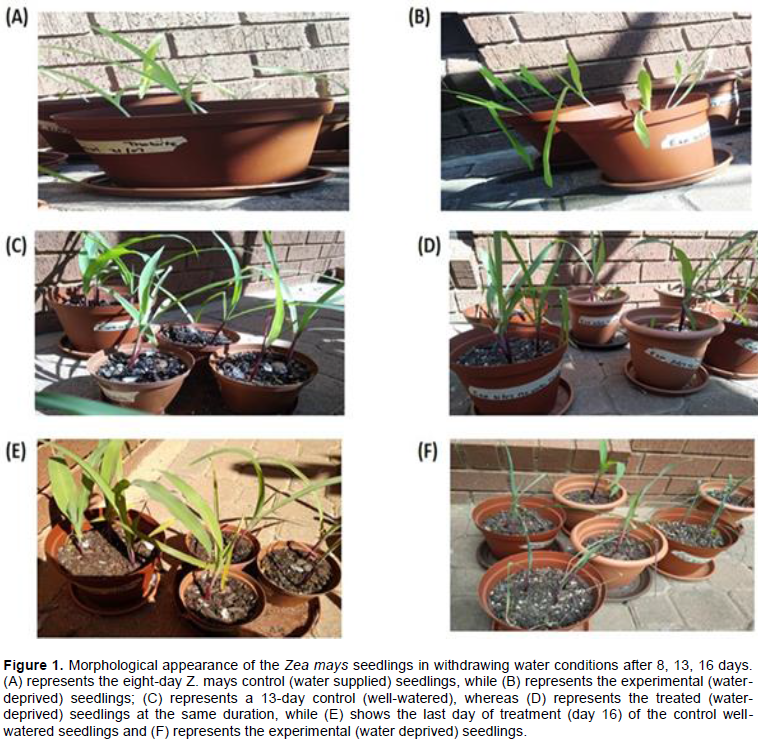
After the successful growth of the Z. mays cultivar (R450w/uo2250w CML550), morphological differences in the appearance of the control (water supplied plants) and experiment (water deprived plants) were documented. The resultant phenotypic changes between the two sets of plants were recorded for 16 days as illustrated in Figure 1. Water stress treatment resulted in noticeable phenotypic changes as shown by the gradual effects on the plants. Reduction in the overall plant growth was exhibited in treated plants as compared to the control treatment. In addition, leaf discoloration was also evident, whereby all leaves of the experimental plants had a dull green appearance while those of the control were somewhat bright green (Figure 1C and D). Control (well-watered) plants showed fully expanded leaves (Figure 1E) as compared to the experiment (water deficit) leaves that revealed a rolled morphology (Figure 1F). The width of the leaves showed a detectable difference, with the control leaves having a larger width than the experiment (Figure 1E and F). Number of leaves per plant was reduced, with the control having larger number of leaves than the experiment (Figure 1). Also, a decrease in shoot height and stem diameter (Figure 1) was evident in the experimental plants (Figure 1B, D and F), while shorter and thin in the controls (Figure 1A, C and E).
One-dimensional gel electrophoresis (1DE) expression profile of maize proteome
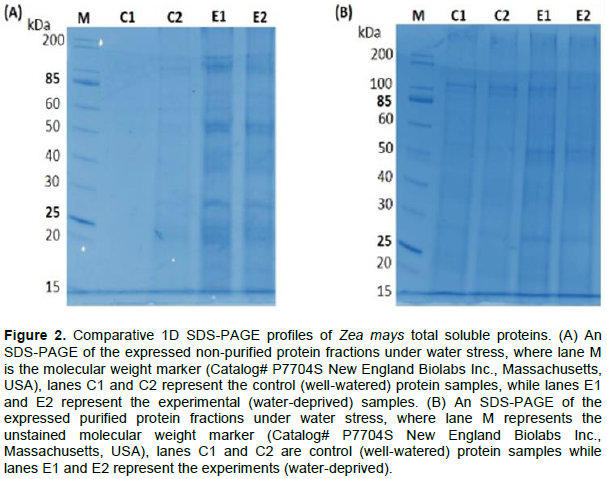
In order to investigate the changes in the maize leaf proteome in response to water stress, 1DE analysis of the total soluble proteins was undertaken. Maize total soluble leaf protein extracts were separated by 1DE to evaluate the quality of the extracts and visualized after staining with Coomassie (Figure 2). The total soluble protein expression profiles exhibited a mixture of numerous higher and lower abundant proteins (Figure 2). The protein extracts exhibited a relatively uniform protein expression, abundance and loading across biological replicates for both the control and water stressed treatment (Figure 2A). In addition, newly synthesized water stress proteins were observed in E1 and E2 (25, 27, 55 and 120 kDa), as compared to the control, where they were absent (Figure 2A). In order to eliminate contaminants, the protein extracts were further purified. No evident differences were detected in protein profiles (Figure 2B) between the control and water-stressed treatment.
Two-dimensional (2D) gel electrophoresis expression profile of maize proteome
Purified total soluble proteins were separately (two treatment groups) subjected to 2D gel electrophoresis or 2DE analysis to evaluate the changes in 16-day old maize leaf proteome in response to water deficit using the 7 cm IPG strips, pH 3-10. The resolved control (well-watered) protein profile (Figure 3A) produced a minimal number of Coomassie stained spots, while the treated group (water deficit) exhibited an increased number of induced protein spots (Figure 3B), which indicate the effect of water deficit on the expression of most proteins. A total of nine differentially expressed protein spots were visualized through a comparison between the well-watered and water deficit leaf extracts (Figure 3).
Water deficit is one of the most serious abiotic factors that threaten the agricultural sector since it limits crop production especially in maize worldwide (Farooq et al., 2009; Raos et al., 2016). Many research groups have invested most of their time in attempting to discover the various complex mechanisms by which plants can cope with the different biotic and abiotic stress factors. Hence, in our study, we focused on the effects of abiotic stress on maize (Z. mays), specifically water deficit after day seven to the sixteenth-day of water-deficit exposure period at the seedling stage. During water stress, plants are subjected to a multiplex of biochemical, physiological and molecular influences, which ultimately affect their growth, development and osmotic homeostasis (Zhu, 2002).
Combined morphological and proteomics approaches were used in our study to investigate the responses of water stress in Z. mays. Leaves are the most essential organ of a developing plant due to their role in photosynthesis; as they are the main indicators of the plant’s health. Various studies support the fact that water deficit stress causes a reduction in the overall plant growth as indicated in Brassica species (Hasanuzzaman et al., 2014) and other plant species (Rizhsky et al., 2002; Jaleel et al., 2009). Our experimental findings concur with the previous investigations, where a decrease in the overall plant growth was evident under water deficiency (Figure 1). Water stress induces various morphological changes that includes modifications in leaf anatomy and ultrastructure, shrinkage in leaf size, decrease in stomata number; thickening of leaf cell walls, cutinization of leaf surface, and induction of early senescence (Seyed et al., 2012).
In our study, leaf rolling and reduced leaf area, were evident in the water deficient treated plants as compared to the well-watered control, which remained unrolled with expanded leaf area (Figure 1E and F) that clearly indicates the effect of water stress with longer exposure. Similarly, the rolling of leaf, reduction in leaf area and low rate of transpiration, were reported as coping mechanism employed by plants in arid areas against water loss (Clarke, 1986). The same leaf morphological changes were observed as a result of water loss (Kadioglu et al., 2012; Kim et al., 2014).
A notable difference was observed in the leaf width, with the control leaves having a larger width than the water deficit experiment (Figure 1E and F). Our findings concurred with previous experiments, which indicated inhibition of leaf expansion during water stress (Salazar et al., 2015; Fathi and Tari, 2016). In addition, water stress decreased the number of leaves per plant and shoot height, with the control plants having larger number of leaves than the experimental plants (Figure 1C and D). A decrease of shoot height in water stressed plants demonstrates that drought stress has an apparent effect on plant height (Hasanuzzaman et al., 2014). According to Fathi and Tari (2016), water deficit has a negative impact onto the development of shoot/root, thus affecting the height of the plant. The stem diameter was relatively thicker for control plants as compared to the experimental plants, which was thin but strong enough to support the whole plant. The obtained results firmly agree with other studies conducted on crop species such as tomato (Gallardo et al., 2004) and pepper (Cohen et al., 1998).
Maize total soluble leaf protein extracts were separated by 1DE to evaluate the quality and clear visualization with Coomassie staining (Figure 2). A relatively uniform protein expression, abundance and loading across the three biological replicates for both the control and water stressed treatments was observed (Figure 2A). In addition, water stress led to the observation of newly synthesised and/or more pronounced proteins that were clearly detected in E1 and E2 (25, 27, 55 and 120 kDa) as compared to the control, where the protein were absent (Figure 2A). In order to eliminate phenolic and ionic contaminants that normally associate with extracted protein samples, the protein extracts were further subjected to purification. Our findings showed no apparent difference in purified protein profiles (Figure 2B) between the control and water stressed treatment. The obtained results contrast those of a similar study conducted on plant seeds by Parchin and Shaban (2014), which found that there is always higher protein abundance in irrigated plants than those that are not irrigated. In general, our total soluble protein expression profiles exhibited a mixture of higher and lower abundant proteins (Figure 2).
In our study, the purified total soluble proteins from the stressed and unstressed groups were subjected to 2D gel analysis (2DE) on 7 cm IPG strips, pH 3-10, to profile water stressed proteins in maize. Notably, 2DE remains one of the highly recommended techniques for the identification of the total expressed proteins in both the stressed and unstressed treatment groups, due to its advantage in providing an overview of proteome separation in terms of their isoelectric point (pl) and molecular mass (Kim et al., 2015). In our case therefore, the separation on 2DE was carried out to determine the expression profiles of leaf protein extracts between the stressed (water deficit) and unstressed (well-watered) 16-day old maize plants, and depending on the nature, composition and complexity of the protein mixture.
The protein profile for the control (well-watered) (Figure 3A) produced a minimal number of Coomassie stained spots, while the experiment (water deficit) demonstrated an increase in the number of induced protein spots (Figure 3B), which indicates the effect of water deficit on the general expression of most proteins. A total of nine differentially expressed protein spots were visualized through a comparison between the well-watered and water deficit leaf extracts (Figure 3). From the control, four proteins were expressed (Figure 3A), while in the experiment, about five proteins were newly induced (spots 5, 6, 7, 8 and 9) under water deficit stress (Figure 3B). Our results indicate that water stress induced the abundance of several proteins in Z. mays leaves, and some of the affected proteins were either up-regulated (spots 1, 3) or down-regulated (spot 4) when water was withdrawn for days. Generally, most protein spots were however, confined between an experimental IEF pH restriction of 3-10. Nonetheless, our findings concur with previous studies carried out on drought stress in various plant species (Ngara et al., 2012; Kim et al., 2015; Cao et al., 2017).
Water stress induced a number of morphological and molecular changes in the R450w/uo2250w CML550 Z.mays cultivar. Our study, has in this regard, established and profiled the total soluble stress responsive proteins in the maize leaf proteome using 2DE. A total of nine differentially expressed proteins were identified, indicating that the proteomic tools used herein were able to separate and allow for the detection of qualitative proteins in Z. mays.
In addition, proteins profiled in this study with their probable associated biochemical pathways provide new information regarding the response of maize to water stress, since maize is known to be highly sensitive to water stress. Findings of this study aid insights regarding molecular pathway responses in an understanding of the morphological and molecular mechanisms used by maize cultivars in response to water deficit. Future work on further identification of the profiled water stress proteins by mass spectrometry, iTRAQ and bioinformatics analyses will strongly assist in confirming the response mechanisms employed by the R450w/uo2250w CML550 cultivar against water stress.
This project was supported by the North-West University, South Africa. The authors acknowledge and express their gratitude for the support.
The authors have not declared any conflict of interests.
REFERENCES
|
Bargali K, Bargali SS (2016). Germination capacity of seeds of leguminous plants under water deficit conditions: implication for restoration of degraded lands in Kumaun Himalaya. Tropical Ecology 57(3):445-453.
|
|
|
|
Bargali K, Singh SP (2007). Germination behavior of some leguminous and actionorhizal plants of Himalaya. Effect of temperature and medium. Tropical Ecology 48:99-105.
|
|
|
|
|
Bassetti P, Westgate ME (1993). Water deficit affects receptivity of maize silks. Crop Science 33:279-282.
Crossref
|
|
|
|
|
Boyer JS (1982). Plant productivity and environment. Science 218:443-448.
Crossref
|
|
|
|
|
Bray EA (1997). Plant responses to water deficit. Trends in Plant Science 2(2):48-54.
Crossref
|
|
|
|
|
Cao J, Packer JS, Ramani V, Cusanovich DA, Huynh C, Daza R, Qiu X, Lee C, Furlan SN, Steemers FJ, Adey A (2017). Comprehensive single-cell transcriptional profiling of a multicellular organism. Science 357(6352):661-667.
Crossref
|
|
|
|
|
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osório ML, Carvalho I, Faria T, Pinheiro C (2002). How plants cope with water stress in the field? Photosynthesis and growth. Annals of Botany 89(7):907-916.
Crossref
|
|
|
|
|
Clarke JM (1986). Effect of leaf rolling on leaf water loss in Triticum spp. Canadian Journal of Plant Science 66:885-891.
Crossref
|
|
|
|
|
Cohen M, Save R, Biel C, Marfa O (1998). Simultaneous measurements of water stress with LVDT sensors and electrotensiometers: Application in pepper plants grown in two types of perlites. Acta Horticulturae 421:193-199.
Crossref
|
|
|
|
|
Cui S, Huang F, Wang J, Ma X, Cheng Y, Liu J (2005). A proteomic analysis of cold stress responses in rice seedlings. Proteomics 5(12):3162-3172.
Crossref
|
|
|
|
|
Dai A (2013). Increasing drought under global warming in observations and models. Nature Climate Change 3(1):52-58.
Crossref
|
|
|
|
|
Dani V, Simon WJ, Duranti M, Croy RR (2005). Changes in the tobacco leaf apoplast proteome in response to salt stress. Proteomics 5(3):737-745.
Crossref
|
|
|
|
|
DeJonge KC, Ascough II JC, Andales AA, Hansen NC, Garcia LA. Arabi M (2012). Improving evapotranspiration simulations in the CERES-Maize model under limited irrigation. Agricultural Water Management 115:92-103.
Crossref
|
|
|
|
|
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009). Plant drought stress: Effects, mechanisms and management. Agronomy for Sustainable Development 29:185-212.
Crossref
|
|
|
|
|
Farré I, Faci JM (2006). Comparative response of maize (Zea mays L.) and sorghum (Sorghum bicolor L. Moench) to deficit irrigation in a Mediterranean environment. Agricultural Water Management 83:135-143.
Crossref
|
|
|
|
|
Fathi A, Tari DB (2016). Effect of drought stress and its mechanism in plants. International Journal of Life Sciences 10(1):1-6.
Crossref
|
|
|
|
|
Gallardo M, Thompson RB, Valdez LC, Fernández MD (2004). Use of stem diameter variations to detect plant water stress in tomato. Irrigation Science 24(4):241-255.
Crossref
|
|
|
|
|
Hasanuzzaman M, Nahar K, Alam MM, Fujita M (2014). Modulation of antioxidant machinery and the methylglyoxal detoxification system in selenium-supplemented Brassica napus seedlings confers tolerance to high temperature stress. Biological Trace Element Research 161(3):297-307.
Crossref
|
|
|
|
|
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000). Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology 51:463-499.
Crossref
|
|
|
|
|
Hegarty TW (1977). Seed activation and seed germination under moisture stress. New Phytologist 78:349-359.
Crossref
|
|
|
|
|
Jaleel CA, Wang G, Ahmad P (2009). Changes in the photosynthetic characteristics of 'Catharanthus Roseus' L. as a result of exogenous growth regulators. Plant Omics 2(4):169.
|
|
|
|
|
Jin R, Shi HT, Han CY, Zhong B, Wang Q, Chan ZL (2015). Physiological changes of purslane (Portulaca oleracea L.) after progressive drought stress and rehydration. Scientia Horticulturae 194:215-221.
Crossref
|
|
|
|
|
Kadioglu A, Terzi R, Saruhan N, Saglam A (2012). Current advances in the investigation of leaf rolling caused by biotic and abiotic stress factors. Plant Science 182:42-48.
Crossref
|
|
|
|
|
Kakumanu A, Madana MR, Curtis-Klumas A, Krishan A, Batlang U, Myers E, Grene R, Pereira A (2012). Effects of drought on gene expression in maize reproductive and leaf meristem tissue revealed by RNA-Seq. Plant Physiology 160:846-867.
Crossref
|
|
|
|
|
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999). Improving plant drought, salt and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nature Biotechnology 17(3):287-291.
Crossref
|
|
|
|
|
Kim S, Kim D, Cho SW, Kim J, Kim JS (2014). Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Research 24(6):1012-1019.
Crossref
|
|
|
|
|
Kim SG, Lee JS, Kim JT, Kwon YS, Bae DW, Bae HH, Son BY, Baek SB, Kwon YU, Woo MO, Shin S (2015). Physiological and proteomic analysis of the response to drought stress in an inbred Korean maize line. Plant Omics 8(2):159.
|
|
|
|
|
Kramer PJ, Kozlowski TT (1980). Physiology of Trees. Mc Graw- Hill, New York.
|
|
|
|
|
Liu W, Lü P, Su K,Yang JS, Zhang JW, Dong ST, Liu P, Sun Q (2010). Effects of planting density on the grain yield and source-sink characteristics of summer maize. Ying Yyong Sheng Ttai Xue Bbao 21(7):1737-1743.
|
|
|
|
|
Lobell DB, Bänziger M, Magorokosho C, Vivek B (2011). Nonlinear heat effects on African maize as evidenced by historical yield trials. Nature Climate Change 1:42-45.
Crossref
|
|
|
|
|
Nayyar H, Gupta D (2006). Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. Environmental and Experimental Botany 58(1):106-113.
Crossref
|
|
|
|
|
Ndimba BK, Chivasa S, Simon WJ, Slabas AR (2005). Identification of Arabidopsis salt and osmotic stress responsive proteins using twoâ€dimensional difference gel electrophoresis and mass spectrometry. Proteomics 5(16):4185-4196.
Crossref
|
|
|
|
|
Ngara R, Ndimba B (2011). Mapping and characterisation of the sorghum cell suspension culture secretome. African Journal of Biotechnology 10 (2):253-266.
|
|
|
|
|
Ngara R, Ndimba R, Borch-Jensen J, Jensen ON, Ndimba B (2012). Identification and profiling of salinity stress-responsive proteins in Sorghum bicolor seedlings. Journal of Proteomics 75(13):4139-4150.
Crossref
|
|
|
|
|
Pantola S, Vibhuti, Bargali K, Bargali SS (2017). Screening of three leguminous crops for drought stress tolerance at germination and seedling growth stage Indian Journal of Agricultural 87(4):467-472.
|
|
|
|
|
Parchin RA, Shaban M (2014). Study on protein Changes in wheat under drought stress. International Journal of Advanced Biological and Biomedical Research 2(2):317-320.
|
|
|
|
|
Pratap V, Sharma YK (2010). Impact of osmotic stress on seed germination and seedling growth in black gram (Phaseolus mungo). Journal of Environmental Biology 31:721-726.
|
|
|
|
|
Raos GJN, ReddyJN, Variar M, Mahender A (2016). Molecular breeding to improve plant breeding to improve plant resistance to abiotic stresses. Advances in Plant Breeding Strategies 2:283-326.
Crossref
|
|
|
|
|
Riccardi F, Gazeau P, Jacquemot MP, Vincent D, Zivy M (2004). Deciphering genetic variations of proteome responses to water deficit in maize leaves. Plant Physiology and Biochemistry 42:1003-1011.
Crossref
|
|
|
|
|
Rizhsky L, Liang H, Mittler R (2002). The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiology 130(3):1143-1151.
Crossref
|
|
|
|
|
Salazar C, Hernández C, Pino MT (2015). Plant water stress: Associations between ethylene and abscisic acid response. Chilean Journal of Agricultural Research 75:71-79.
Crossref
|
|
|
|
|
Seki M, Kamei A, Yamaguch-Shinozaki K, Shinozaki K (2003). Molecular responses to drought, salinity and frost: common and different paths for plant protection. Current Opinion in Biotechnology 14:194-199.
Crossref
|
|
|
|
|
Seyed YSL, Rouhollah M, Mohammad MH, Ismail MMR (2012). Water stress in plants: causes, effects and responses. Drought Stress Tolerance in Plants 1:1-16.
|
|
|
|
|
Shi H, Ye T, Zhong B, Liu X, Chan Z (2014). Comparative proteomic and metabolomic analyses reveal mechanisms of improved cold stress tolerance in Bermuda grass (Cynodon dactylon (L). Pers.) by exogenous calcium. Journal of Integrative Plant Biology 56(11):1064-1079.
Crossref
|
|
|
|
|
Shinozaki K, Yamaguch-Shinozaki K, Seki M (2003). Regulatory network of gene expression in the drought and cold stress responses. Current Opinion in Plant Biology 6 (5):410-417.
Crossref
|
|
|
|
|
Vibhuti CS, Bargali K, Bargali SS (2015). Seed germination and seedling growth parameters of rice (Oryza sativa L.) varieties as affected by salt and water stress. Indian Journal of Agricultural Sciences 85(1):102-108.
|
|
|
|
|
Yoshimura K, Masuda A, Kuwano M, Yokota A, Akashi K (2008). Programmed proteome response for drought avoidance/tolerance in the root of a C3 xerophyte (wild watermelon) under water deficits. Plant and Cell Physiology 49(2):226-241.
Crossref
|
|
|
|
|
Zhao Y, Du H, Wang Z, Huang B (2011). Identification of proteins associated with water-deficit tolerance in C4 perennial grass species, Cynodon dactylon × Cynodon transvaalensis and Cynodon dactylon. Physiologia Plantarum 141:40-55.
Crossref
|
|
|
|
|
Zheng J, Fu J, Gou M, Huai J, Liu Y, Jian M, Huang Q, Guo X, Dong Z, Wang H, Wang G (2010). Genome-wide transcriptome analysis of two maize inbred lines under drought stress. Plant Molecular Biology 72:407-421.
Crossref
|
|
|
|
|
Zhu JK (2002). Salt and drought stress signal transduction in plants. Annual Review of Plant Biology 53(1):247-273.
Crossref
|
|
|
|
|
Zobel DB, Jeet R, Bargali SS (1995). Structural and physiological changes in Quercus leucotrchophora and Pinus roxburghii associated with stand disturbance in the Kumaun Himalaya, India. International Journal of Ecology and Environmental Sciences 21:45-66.
|
|