ABSTRACT
The bacterial genus Xanthomonas consists of several species of economic importance, among which Xanthomonas campestris pv.musacearum (Xcm), the cause of enset and banana wilt is the most important in tropical Africa. However, the natural occurrence and host range of this species is yet to be clarified. The objectives of this study were to verify the presence of Xanthomonas bacteria on plants growing in and around enset gardens in South and Southwest Ethiopia, and to elucidate the pathogenicity of Xcm strains to cultivated and wild plants. Several economical and ornamental plants were assessed for wilting in South and Southwest Ethiopia. Wilting was visible on Canna spp. with 9.8% incidence and 30% prevalence, while reddish streak symptoms (typical of Xanthomonas bacteria) were observed on the leaves of sugarcane, sorghum and wild sorghum with disease incidence ranging from 20 to 80%, and prevalence varying from 30 to 100%. The pathogenicity of three Xcm isolates to five plant species was tested in a factorial experiment arranged in CRD with five replications. All the tested Xcm isolates were found to be pathogenic to banana, cultivated and wild enset, Canna indica, Canna orchoides, maize, sorghum and finger millet. The analysis of variance for incubation period and disease incidence revealed significant differences (p<0.05) among test plants and isolates. Results suggest marked variations among test plants’ ability to resist the bacterium. Variations were also evident in the aggressiveness of the bacterial isolates. On the other hand, enset and banana did not show any symptom after being inoculated with four Xanthomonas isolates from other crops.
Key words: Enset, incubation period, wilt incidence, Xanthomonas campestris pv. musacearum.
The genus Xanthomonas is composed of several species of economic importance as they affect the production of different crops all over the world. A member of this genus, Xanthomonas campestris pv. musacearum (Xcm), has been implicated in threatening the crop enset (Ensete ventricosum (Welw.) Cheesman) in Ethiopia since the 1960s (Yirgou and Bradbury, 1968; Dereje, 1985; Weldemichael, 2000).
The disease has also been implicated as causing a serious threat on banana production thereby the
livelihood of thousands of people throughout the Great Lake Region of Africa (Wasukira et al., 2012). Previous work based primarily on DNA sequences and fatty acid data has shown that strains of X. campestris pv. musacearum have very close homology to strains of Xanthomonas vasicola and most likely belong to this species. Accordingly, the name X. vasicola has been proposed for X. campestris pv. musacearum (Aritua et al., 2007). However, we will use the previous name Xcm (currently still the official name) throughout this paper.
The initial symptoms by Xcm on enset and banana occur on the central leaf and spread to all parts. The earliest symptoms are usually loss of turgor and wilting in the spear (youngest emerging leaf) or one or more of the young leaves, sometimes preceded by yellowing and distortion, especially in young plants. Bacterial ooze exudes when a non-dry part of the plant is cut. A cut made through the petioles of newly infected enset plants reveals browning of the vascular bundles and yellowish or grayish masses of bacterial ooze come out from the vascular bundles (Tripathi et al., 2009). Cross sections at the base of the pseudostem and corm show discoloration of the vascular bundles with large bacterial pockets and grayish or yellowish exudates with brownish to black spots, respectively (Wondimagagne, 1981; Ashagari, 1985).
The main known natural host plants to X. campestris pv. musacearum are Banana (Musa spp.) and cultivated enset (Ensete ventricosum) both of which belong to the Musaceae family and order zingiberales (Yirgou and Bradbury, 1968, 1974). However, the host range of this pathogen appears rather controversial. While Ssekiwoko et al. (2006) reported Xcm as being able to only infect monocots that belong to the families Musaceae and Cannaceae, Mwangi et al. (2006) ruled out grasses like maize, sorghum and napier grass along with such crops as common beans, cassava, taro, sweet potato and tobacco as hosts to the pathogen. On the other hand, Xanthomonas species have been reported in sweet potato, sugar cane, maize, common beans and sorghum (Hernandez and Trujillo, 1990; Destefano et al., 2003; Mkandawire et al., 2004; De Cleene, 2008; Todorović et al., 2008). Xcm was also found to be pathogenic to maize and sugarcane (Aritua et al., 2008; Karamura, 2012).
Wild Musa zebrina, Musa ornata and Canna indica were also reported as potential alternative hosts for this pathogen (Ssekiwoko et al., 2006).
Enset bacterial wilt caused by X. campestris pv. musacearum was first reported in Ethiopia by Yirgou and Bradbury (1968) and has since spread to all the enset growing regions in Ethiopia (Brandt et al., 1997). However, most of the studies conducted in Ethiopia thus far focus on surveying the disease in some areas and characterizing the pathogen based on biochemical tests (Wondimagagne, 1981; Ashagari, 1985; Spring et al., 1996; Bobosha, 2003; Addis et al., 2004).
Screening some cultivated enset clones for wilt resistance and studying the survival and dispersal of the pathogen have also been investigated although not thoroughly (Weldemichael, 2000; Addis et al., 2006; Weldemichael et al., 2008a and b). Studies on the occurrence of the disease on plants other than enset and banana are lacking under Ethiopian conditions, and are very limited even throughout Africa. Besides, the pathogenicity of Xcm isolates to plants growing in and around enset gardens has not been well established.
Therefore, this study was designed to: 1) verify the presence of Xanthomonas bacteria on plants growing in and around enset gardens in South and Southwest Ethiopia, and 2) elucidate the pathogenicity of Xcm strains to various plants.
Assessing plants around enset gardens for Xanthomonas spp. infection
A field survey was carried out to assess some crops that is, Canna spp., sugar cane (Sacharum officinarum), cultivated sorghum (Sorghum bicolor), and wild sorghum (Sorghum halepense). The survey was carried out by visiting enset and banana producing areas in South and Southwest Ethiopia. During the survey, data were collected on the type of plants growing in and around each field; incidence of disease on each of the above plants as proportion of plants with visible symptoms. Besides, specimens were collected from each plant and brought to the laboratory for verification. Identity of the isolated bacteria was confirmed following colony growth on semi selective medium (sucrose peptone agar medium: 20 g sucrose, 5 g peptone, 0.5 g K2H3PO4, 0.25 g MgSO4 and 15 g agar in 1l sterilized distilled water) (Mwebaze, 2007; Mwangi et al., 2007), catalase (Dickey and Kelman, 1988) and Gram staining reaction tests (Schaad, 1988). In addition, physiological tests that is, gelatin liquefaction and starch hydrolysis testes as well as catalase reaction were carried out.
Pathogenicity tests
Pathogenicity tests were carried out to determine the possible host range of the pathogenic Xcm and the reaction of various plant species. The experiment had a factorial design with isolates as sub-factors and test plants as main factors. It was arranged in a completely randomized design with five replications.
Three Xcm isolates (I1, I2 and I3) were isolated from naturally infected cultivated enset, wild enset and banana, respectively (Table 1), and used for the pathogenicity test on cultivated enset, wild enset, banana, Canna species and cereal crops (maize, sorghum and finger millet) collected from different areas (Table 2). Each isolate was collected by taking bacterial ooze in the field using a toothpick and then suspending the ooze in a test tube half filled with sterilized distilled water according to Weldemichael (2000). Before inoculation of test plants, the concentration of each bacterial suspension was adjusted using a spectrophotometer to 0.3OD at 460 nm, which is equivalent to 108cfu/ml bacteria cells.
Seedlings of banana, enset and Canna spp. were transplanted into pots (22×22 cm), filled with a sun-dried mixture of soil, sand and manure at a ratio of 3:1:1 (Quimio, 1992), then allowed to establish for three months (four to seven leaf stage). Inoculation of test plants with each bacterial isolate was done by injecting an aliquot of 3 ml of the bacterial suspension into the petiole base of the newly expanding central leaf using a 10 ml sterile hypodermic syringe (Ashagari, 1985). Inoculated plants were then covered with a wet plastic bag for 48 h.
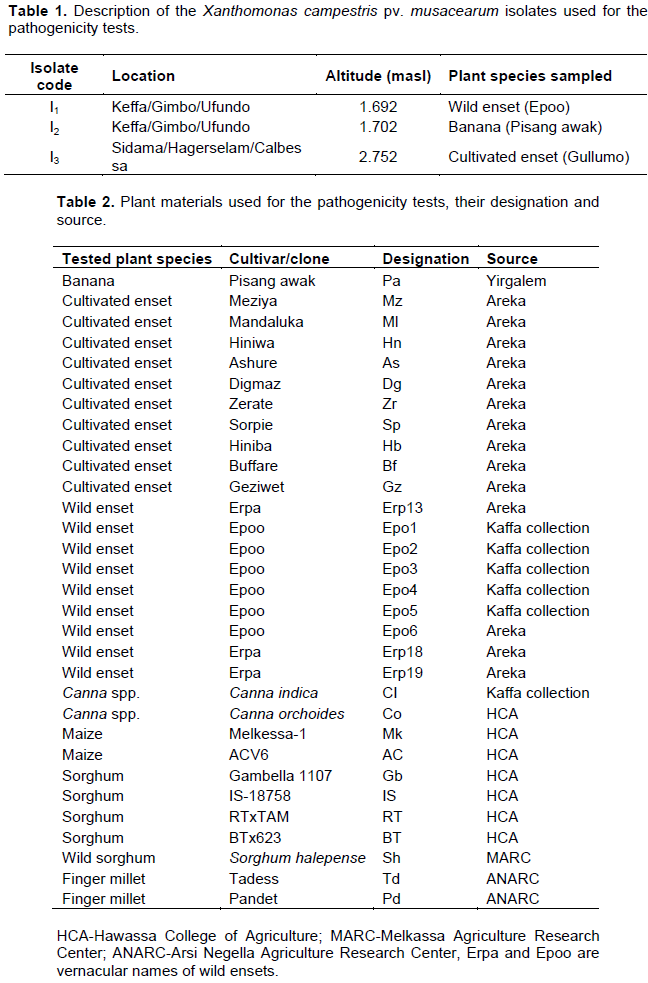
For the cereals, seeds of each species were planted in plastic pots (18x18 cm) filled with a sun-dried sterile mixture of soil, sand and manure (3:1:1) and 324 mg of urea per pot; this amount of urea was re-applied six weeks after planting. About 10 seeds were planted in drills and thinned to five plants/pot two weeks after planting. The cereals were inoculated at one month old (three to four leaf stage) by wounding and spraying techniques, that is, their leaves were physically struck with very fine sterile sand paper, sprayed with 3 ml of each bacterial isolate suspension and covered with a transparent plastic bag for 48 h (Hussien, 2001). Negative controls of each plant species were inoculated with the same quantity of sterile distilled water.
Disease assessment
Data were collected on incubation period (period between inoculation and first wilting symptom) and the number of plants showing disease symptoms was recorded weekly starting from one week after inoculation for four consecutive months. Disease incidence was calculated according to the following formula:
Where, DI: disease incidence, NPCW: number of plants completely wilted, NPPT: number of plants assessed.
In addition, disease severity was assessed using a standard disease scale of 0 to 5 (Winstead and Kelman, 1952) where 0: no symptom; 1: only the inoculated leaf wilted; 2: 2 to 3 leaves wilted; 3: four leaves wilted; 4: all leaves wilted and 5: plant dead). The severity grades were converted into percentage severity index for analysis (Cooke, 2006).
Where, PSI is percent severity index; SNR is the sum of the numerical rating; NPR is number of plant rated; MSS is the maximum score of the scale. Severity from each scoring date was converted to area under percent severity index progress curve (AUPSiPC) using the formula by Jerger and Vijanen-Rollinson (2001) as follows:
Where, n is total number of assessments, ti is the time of the ith assessment in weeks from the first assessment date, xi is the percentage of the disease severity or disease incidence at ith assessment. AUPSIC is the area under percent severity index progress curve was expressed in percent-weeks.
Data analysis
Analysis of variance was performed for data on disease parameters (wilt incidence and incubation period) using the General Linear Model of SAS computer package (SAS, Institute Inc., 2003). Means were separated by least significant difference (LSD) at 5% probability level.
Disease incidence on plants around enset garden and bacterial isolates characterization
Visible disease symptoms (yellowing of the leaf at margin side and tip, wilted leaf and blade folded upward and inward and also dry leaf) were evident on diseased plants. Reddish-brown streaks were also recorded on the grasses that is, cultivated and wild sorghum, and sugar cane. Disease incidence (proportion of infected plants in a field) varied from 10% on Canna sp. to 80% on sugar cane (Figure 1). Disease prevalence (proportion of fields with at least one diseased plant) ranges between 30% on Canna sp. and 100% on wild sorghum.
When diseased (wilted) plants were plated on sucrose peptone agar medium (a semi selective medium for Xanthomonas), deep yellow colonies grew out from sugarcane and sorghum, and yellow colored colonies were observed growing from Canna. All of these isolates were found to possess negative reaction to Gram staining, and positive reaction to catalase reaction. Inoculation of the isolates to enset and banana did not induce any symptom and hence the isolates were considered non-pathogenic to enset and banana.
Pathogenicity of Xcm isolates to various plants
The pathogenicity of Xcm isolates to various plants was tested in two experiments. Although, the results of both experiments were consistent; the average of the two experiments was presented in the current report.
Banana and cultivated enset
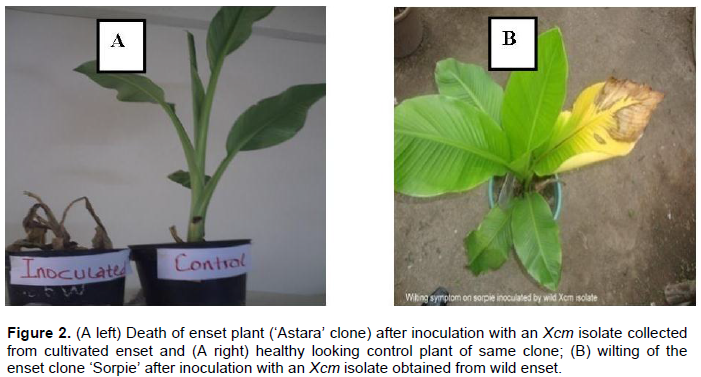
Disease assessment started a week after inoculation and the earliest typical external disease symptoms were observed two to four weeks post inoculation on ‘Pisang awak’ and enset clones. These included folding down of the leaf blade along the midrib, followed by scalding and dull green appearance of the central inoculated leaf. This was followed by yellowing, starting at the apex, sequential wilting of leaves, drying and wilting of the whole plant and finally plant rotting and death (Figure 2). Yellowish bacterial ooze was observed when pseudostem and leaf petiole were cut. In the current experiment, there were significant variations among clones and isolates in terms of incubation period, disease incidence and area under disease severity index progress curve (Tables 3 to 5). However, the interaction was not significant (data not shown).
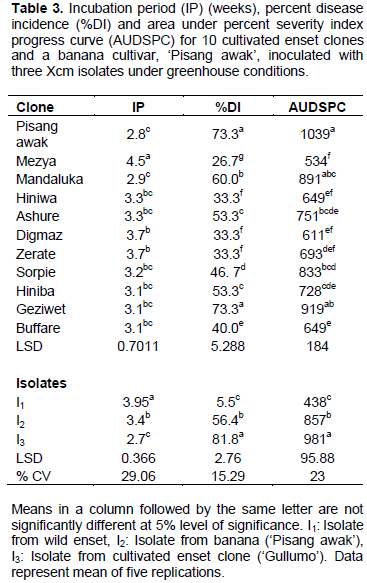
Among the tested plants, the banana cultivar ‘Pisang awak’ was found to have the shortest incubation period followed by the enset clone ‘Mandaluka’. Enset clone ‘Mezya’ had the longest incubation period. This clone also had the lowest average wilt incidence (27%) across the three isolates, while ‘Pisang awak’ and ‘Geziwot’ had the highest wilt incidence (73%), followed by ‘Mandaluka’ (60%). Furthermore, the highest AUPSPC (1039) was recorded on ‘Pisang awak’ followed by ‘Geziwet’ and ‘Mandaluka’ in that order, while the lowest AUPSPC (534) was recorded on ‘Mezya’. Thus, the banana cultivar ‘Pisang awak’ and enset clone ‘Geziwot’ were suggested to be highly susceptible to Xcm as compared to the other clones tested in the current experiment.
When comparisons were made across isolates, isolate I3 caused wilting the earliest (2.7 weeks after inoculation) while the other two isolates, I1 and I2, took about four weeks and three weeks, respectively, to induce symptoms (Table 3). Most plantlets inoculated with isolates I2 and I3 completely wilted but most of the enset clones and some ‘Pisang awak’ plantlets inoculated with isolate I1 did not wilt completely. Moreover, wilt incidence and area under the disease severity index progress curve were significantly the lowest for isolate I1. On the other hand, isolate I3 caused the earliest wilting and disease parameters after inoculation with this isolate were significantly greater than for the others. As a result, among the three isolates of Xcm used in this study, the wild Xcm isolate I1 was found to be a weaker pathogen as compared to isolates I3 and I2. In contrast, isolate I3, which was obtained from cultivated enset in Sidama zone of southern Ethiopia, was the most virulent and aggressive.
Wild enset
The first disease symptoms on wild enset plants were recorded a week after inoculation as yellowing from the apex to the edge of the inoculated leaf and water-soaked lesions along the inoculated leaf’s midrib. Two to five weeks after inoculation leaf wilting and yellowing symptoms were observed on most plantlets (Figure 2). Yellowish bacterial ooze was observed when pseudostem and leaf petiole were cut. Such symptoms are similar to typical Xanthomonads bacterial wilt symptoms described on the banana cultivars and cultivated enset under field and experimental conditions.
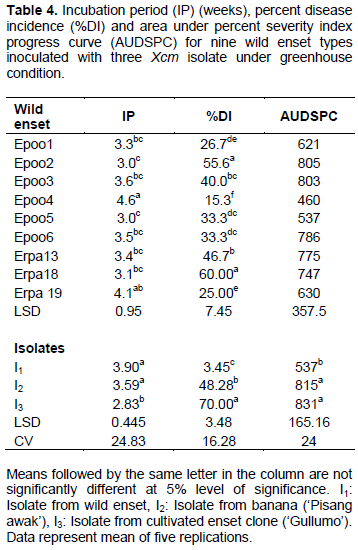
Like the cultivated enset clones, the wild enset types also reacted differentially to the isolates of Xcm. Significant variations were observed among the wild enset types and Xcm isolates in terms of incubation period, disease incidence and area under disease severity index progress curve (Table 4).
The mean number of weeks required for the appearance of initial symptoms on wild enset clones varied between three and five. The incubation period was shorter on wild ensets clones such as ‘Epoo5’, ‘Epoo2’, ‘Erpha18’ and ‘Erpha13’, while ‘Epoo4’ had the longest incubation period among the tested wild enset clones. None of the nine wild enset types tested in the current experiment showed complete resistance to Xcm isolates used in this study. Among wild enset type tested in the current experiment, wilt incidence was the highest (60%) on ‘Erpa18’ followed by ‘Epoo2’, which had the highest AUPSPC (805). Thus these two wild enset types were found to be highly susceptible to Xcm. On the other hand, the wild enset ‘Epoo4’ had significantly the lowest wilt incidence and AUDSPC, making it relatively more tolerant to the pathogen.
In this experiment too, incubation period was the longest for isolate I1, while isolate I3 had the shortest incubation period. Symptom appearance after inoculation with I1 was delayed by one to two weeks compared to the other two isolates. Most of the plantlets inoculated with isolates I2 and I3 were completely wilted 10 weeks after inoculation. On the other hand, only one plantlet of ‘Epoo3’ inoculated with isolate I1 completely wilted at the same time of assessment. This difference between isolates in inducing symptoms on tested plants indicates variations in aggressiveness among the isolates. Disease incidence and severity were also high for most wild enset after inoculation with isolates I2 and I3. One hundred percent disease severity indexes were recorded at 5 to 9 weeks after inoculation on wild enset with isolate I3 (data not shown). Isolate I2 caused 60 to 100% severity at 7 to 11 weeks after inoculation, while isolate I1 resulted in 40 to 60% severity at 8 to 14 weeks after inoculation. On average, 70% disease incidence and AUDSPC value of 831 were caused by isolate I3. In contrast, isolate I1 had significantly lower disease incidence and AUDSPC. This further confirmed the most aggressive nature of isolate I3 as compared to the remaining two isolates.
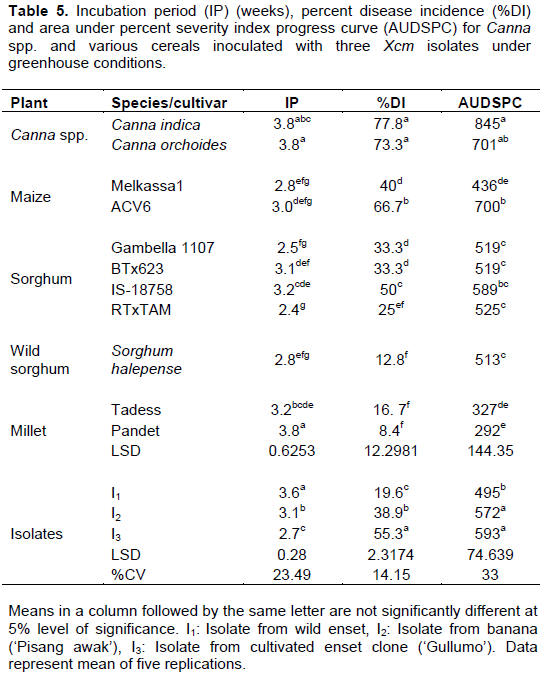
Canna spp. and cereals
Among the suspected alternative host plants, Canna spp., maize, sorghum and finger millet varieties were tested for the reaction to three Xcm isolates. Two to three weeks after inoculation, typical external disease symptoms were observed on some plantlets of these suspected plants. On Canna plantlets, water soaked lesions developed along the inoculated leaf’s midrib within two weeks after inoculation and after three to four weeks some inoculated leaves wilted and leaf blade folded upward and inward, turned yellow, dried and died. However, new suckers that emerged from the corm after the inoculated plantlet kept growing. This may be related to inability of the bacteria to colonize the corm of the Canna plants.
In maize, the first symptom observed on the inoculated leaf was necrosis and discoloration or yellowing of the leaf, starting from the tip to the bottom of the leaf, three to four weeks after inoculation. Gradual wilting along the midrib to the edge of the inoculated leaf was also observed. In sorghum varieties, lesions or discoloration initially developed at the tip of the inoculated leaf two weeks after inoculation. Thereafter, the lesions at the tip of the leaf gradually elongated to the midrib and then to the leaf blade. Eventually, a yellowing symptom appeared on the leaf blade and, in severe cases, a burned appearance at the margin of the leaf. In addition, leaves withered and turned brown, wilted, dried and dropped off. The observed symptoms on finger millet varieties were discoloration starting from the tip to bottom of the leaf and finally turning yellow and dried.
The analysis of variance for incubation period, disease incidence and AUDSPC revealed significant differences among varieties and isolates (Table 5). The number of weeks to the appearance of first disease symptoms varied between two and half, and four among cereal cultivars. Among the tested plants, initial symptoms appeared the earliest on the sorghum cultivar ‘RTxTAM’ and the latest on the finger millet cultivar ‘Pandet’. Each of the inoculated plant species reacted differently to the three isolates of Xcm. Disease incidence was in excess of 70% on C. indica and C. orchoides and reached 67% on the maize variety ‘ACV6’ (Table 5). Disease incidence was negligible on the finger miller variety ‘Pandet’. This variety showed initial symptoms but then the disease progressed quite slowly. The second longest incubation period and lowest AUDSPC were recorded from the other finger millet variety, ‘Tadess’. The current results may suggest the more resistant nature of finger millets as compared to the other cereals.
Isolates of Xcm differed in their ability to cause the disease on Canna spp. and the various cereals. Disease symptoms were induced the earliest by isolate I3 followed by isolate I2. The highest disease incidence was induced by isolate I3 on Canna indica and Canna orchoides. The same isolate caused up to 60% disease incidence on maize, cultivated and wild sorghum and finger millets. On average, the highest AUDSPC value of 593 was recorded when plants were inoculated by isolates I3 (Table 5). This was significantly higher than the AUDPSC from isolate I1. In a trend similar to that from cultivated and wild enset, and banana, isolate I3 was found to be the most aggressive on Canna spp. and cereals.
Bacterial wilt caused by X. campestris pv. musacearum is considered as one of the major biotic stresses threatening enset and banana (Thwaites et al., 2000). The enset-Xcm pathosystem remains one of the least studied pathosystems to date. The current study objectives were to determine enset bacterial wilt occurrence on various plants commonly grown in and around enset farms in South and Southwest Ethiopia and elucidate the pathogenicity of Xcm isolated from different group of plants.
During the field survey, different plants that is, Canna sp., cultivated and wild sorghum, and sugarcane were assessed for symptoms associated with the Xanthomonas bacteria. Results reveal the prevalence of symptoms associated with the Xanthomonas bacteria ranging from 30 to 100% with disease incidence varying from 10 to 80%. Thus, these plants were considered as possible alternate hosts to the Xcm bacteria. Ssekiwoko et al. (2006) also reported 80 to 100% disease incidence on C. indica in a pot experiment, while Ashagari (1985) identified C. orchoides as a host for the Xcm pathogen. In the present study, Xanthomonas from any of these plants did not induce observable symptoms on enset and banana, and hence the isolates were considered as non-pathogenic to enset and banana. On the other hand, the Canna spp. and all the aforementioned cereals crops were found to be susceptible to Xcm from enset and banana.
The current study reveals the pathogenicity of Xcm to cultivated and wild enset, banana, Canna spp., and several grasses. In contrast, Ssekiwoko et al. (2006) reported that Xcm infects only monocots belonging to the two families Musaceae and Cannaceae. Mwangi et al. (2006) has also excluded maize and sorghum from possible hosts of Xcm. On the other hand, Aritua et al. (2008) and Karamura (2012) have reported maize and sugarcane developing disease after being artificially inoculated with Xcm. Aritua et al. (2008) even reported genetic similarities between Xcm isolates on one hand, and isolates of Xanthomonas vasicola pv holcicola from sorghum and Xanthomonas vasicola pv vasculorum from sugarcane on the other. Inoculation of maize with Xcm resulted in the development of full blown yellow-brown streaks (Karamura, 2012), a result that coincides with this study findings.
Significant variations (p<0.05) existed among the isolates in terms of incubation period, wilting incidence and severity. In general, isolate I3 from cultivated enset in South Ethiopia was found to be the most aggressive, while I1 from wild enset plant was the least aggressive. Variability in terms of pathogenicity among Xcm isolates was also reported by Weldemichael (2000). This was contrary to a report by Aritua et al. (2007) that revealed low level of genetic variation among the pathogen isolates collected from different African countries.
However, the current pathogenicity test findings contradict those of Tripathi et al. (2009), who reported no significant differences in pathogenicity among Xcm isolates. Our findings thus call for more research in diversity of the pathogen populations. Besides, the results confirm the need to consider isolate variation in breeding for bacterial wilt resistance. We recommend this isolate be used in future resistance screening trials. We also suggest that molecular studies including sequencing be carried out to understand the genetic basis of variation in pathogenicity of the isolates.
The test plants also differed significantly in their degree of susceptibility to Xcm. The banana cultivar Pisang awak, C. indica, and enset clones Geziwot, Mandaluka, wild enset Epoo2 and Erpa18 showed high disease incidence and severity, and short incubation period, and hence were considered as most susceptible. Enset clones Mezya, wild enset Epoo4, and finger millet cultivar Pandet had lower disease severity and longer incubation period, and hence were considered relatively tolerant to the pathogen. While not much work has been done to assess the susceptibility of wild enset to Xcm, the enset clone ‘Mezya’ was also found to be more tolerant to Xcm infection by Ashagari (1985) and Weldemichael (2000).
The current work reveals the potential various plants including wild enset may play in harboring Xcm pathogenic to both cultivated enset and banana. Hence, care must be taken to minimize the risk of the pathogen being spread from the wild to agricultural fields. Further characterization of the X. campestris pv.musacearum strains from wild enset, cultivated enset and banana should be carried out by using the existing available detection methods. In addition, the genetic diversity among both the host and the pathogen should be investigated further. Additional tests on the Xcm isolates to different plant species should be carried out to elucidate the potential of wild and cultivated plants in harboring and disseminating the pathogen.
The authors have not declared any conflict of interests.
The authors acknowledge the Directorate General for Development (DGD-Belgium) through the Bioversity International component of the CIALCA project for sponsoring the research.
REFERENCES
|
Addis T, Handoro F, Blomme G (2004). Bacterial wilt (Xanthomonas campestris pv. musacearum) on Enset and banana in Ethiopia. InfoMusa 13:44-45.
|
|
|
|
Addis T, Weldemichael G, Bobosha K, Blomme G, Mekonnen S, Mengesha T (2006). Screening banana cultivars for resistance to bacterial Bacterial wilt. InfoMusa 15(1 -2):10-12.
|
|
|
|
|
Aritua V, Nanyonjo A, Kumakech F, Tushemereirwe W (2007). Rep-PCR reveals High genetic homogeneity among Ugandan isolates of Xanthomonas campestris pv. musacearum. Afr. J. Biotechnol. 6:179-183.
|
|
|
|
|
Aritua V, Parkinson N, Thwaites R, Heeney JV, Jones DR, Tushemereirwe W, Crozier J, Reeder R, Stead DE, Smith J (2008). Characterization of the Xanthomonas sp. causing wilt of enset and banana and its proposed reclassification as a strain of X. vasicola. Plant Pathol. 57:170-177.
|
|
|
|
|
Ashagari D (1985). Studies on the bacterial wilt of enset (Ensete ventricosum) and prospects for its control. Ethiop. J. Agric. Sci. 7(1):1-14.
|
|
|
|
|
Bobosha K (2003). Characterization of Xanthomonas campestris pv. musacearum isolates causal agent of Enset bacterial wilt disease. Addis Ababa, Ethiopia, Addis Ababa University, MSc. Thesis.
|
|
|
|
|
Brandt SA, Spring A, Hiebsch C, McCabe ST, Tabogie E, Diro M, Weldemichael G, Yintiso G, Shigeta M, Mekonnen S (1997). The 'Tree Against Hunger'. Enset based Agricultural Systems in Ethiopia. Washington DC, USA, American Association for the Advancement of Science.
|
|
|
|
|
Cooke BM (2006). Disease assessment and yield loss. In. Cooke BM, Jone DG, Kaye B. (eds) The Epidemiology of plant diseases, 2nd edition. Dorchert, Springer. pp. 43-80.
Crossref
|
|
|
|
|
De Cleene M (2008). Scanning electron microscopy of the establishment of compatible and incompatible Xanthomonas campestris pathovars on the leaf surface of Italian ryegrass and maize. EPPO Bull. 19:81-88.
Crossref
|
|
|
|
|
Destefano SAL, Almeida IMG, Rodrigues Neto J, Ferreira M, Balani DM (2003). Differentiation of Xanthomonas species pathogenic to sugarcane by PCR-RFLP analysis. Eur. J. Plant Pathol. 109:283-288.
Crossref
|
|
|
|
|
Dickey RS, Kelman A (1988). 'Caratovora' or soft rot group. In: Schaad NW (ed) Laboratory guide for identification of plant pathogenic bacteria, 2nd edition. St Paul, MN, USA, APS Press. pp. 81-84.
|
|
|
|
|
Hernandez Y, Trujillo GE (1990). A new leaf disease in sweet potato caused by Xanthomonas campestris. J. Agron. Trop. 40:291-307.
|
|
|
|
|
Hussien T (2001). Evaluation of Inoculation Methods and Resistance of some Sorghum Genotypes to Bacterial Leaf Streak (Xanthomonas campestris pv.holcicola). Pest Manage. J. Ethiop. 5:21-28.
|
|
|
|
|
Jerger MJ, Viljanen-Rollion SLH (2001). The use of the area under the disease progress curve to assess quantitatively resistant in crop cultivators. Theor. Appl. Gene 102(1):32-40.
Crossref
|
|
|
|
|
Karamura G (2012). Development of field diagnostic tools for and characterisation of Xanthomonas Campestris pathovar musacearum, causal agent of banana Xanthomonas wilt. Exeter, UK, University of Exeter, MSc Thesis.
|
|
|
|
|
Mkandawire AB, Mabagala RB, Guzmán P, Gepts P, Gilbertson RL. (2004). Genetic diversity and pathogenic variation of common blight bacteria (Xanthomonas campestris pv. phaseoli and X. campestris pv. phaseoli var. fuscans) suggests pathogen coevolution with the common bean. Phytopathology 94:593-603.
Crossref
|
|
|
|
|
Mwangi M, Mwebaze M, Bandyopadhyay R. (2007). Development of a semi-selective medium for the isolation of Xanthomonas campestris pv. musacearum from insect vectors, infected plant material and soil. Plant Pathol. 56:383-390.
Crossref
|
|
|
|
|
Mwangi M, Pillay M, Bandyopadhyay R, Tushemereirwe W, Ragama P. (2006). Progress in understanding mechanisms of host plant tolerance to banana bacterial wilt. Proceedings of the 4th International Bacterial wilt Symposium, 17-20 July 2006. York, UK, Central Science Laboratory. P 65.
|
|
|
|
|
Mwebaze JM, Tusiime G, Tushemerweire WK, Maina M (2006). Development of a semi-selective medium for Xanthomonas campestris pv. musacearum. Afr. Crop Sci. J. 14:129-135.
|
|
|
|
|
Quimio JA (1992). Annual report of the plant pathologist 16-17 July 1991. Enset Team Support Project Sidama Gamo Gofa. Peasants Agricultural Development Program PADEPIII. Awasa Research Center (IAR). Awasa, Ethiopia.
|
|
|
|
|
SAS Institute (2003). SAS User's Guide, Version 9. Cary, N.C. USA.
|
|
|
|
|
Schaad NW (1988). Initial identification of common genera. In. Schaad NW (ed) Laboratory guide for identification of plant pathogenic bacteria 2nd ed. St Paul, MN, USA, APS Press. pp. 81-84.
|
|
|
|
|
Spring A, Hiebsch C, Tabogie E, Weldmichael G (1996). Enset needs assessment project phase I Report. Awasa, Ethiopia.
|
|
|
|
|
Ssekiwoko F, Taligoola HK, Tushemereirwe W. (2006). Xanthomonas campestris pv. musacearum host range. Afr. Crop Sci. J. 14(2):111-120.
|
|
|
|
|
Thwaites R, Eden-Green S, Black R (2000). Diseases caused by bacteria. In. Jones DR (ed) Diseases of banana, Abacá and enset, Wallingford, UK, CABI Publishing. pp. 213-239.
|
|
|
|
|
Todorović B, Milijašević S, Rekanović E, PotoÄnik I, Stepanović M (2008). Susceptibility of bean genotypes to Xanthomonas campestris pv. phaseoli in greenhouse conditions. Pest. Phytomed. 23:167-173.
Crossref
|
|
|
|
|
Tripathi L, Mwangi M, Abele S, Aritua V, Tushemereirwe WK, Bandyopadhyay R (2009). Bacterial Wilt: A Threat to Banana Production in East and Central Africa. Plant Dis. 93:440-451.
Crossref
|
|
|
|
|
Wasukira A, Kubiriba J, Tusiime G, Grant MR (2012). Potential application of natural variability of Arabidopsis thaliana accessions in genomics of Xanthomonas campestris pv musacearum. Third RUFORUM Biennial Meeting 24 - 28 September 2012, Entebbe, Uganda. pp. 331-335.
|
|
|
|
|
Weldemichael G, Bobosha K, Addis T, Blomme G., Mekonnen S, Mengesha T (2008a). Evaluation of Enset Clones against Enset bacterial wilt. Afr. Crop Sci. J. 16(1):89-95.
|
|
|
|
|
Weldemichael G, Bobosha K, Addis T, Blomme G, Mekonnen S, Mengesha T (2008b). Mechanical transmission and survival of bacterial wilt on enset. Afr. Crop Sci. J. 16(1):97-102.
|
|
|
|
|
Weldemichael G (2000). Variation in isolates of enset pathogen (Xanthomonas campestris pv. musacearum) and reaction of enset clones (Ensete ventricosum Cheesman) to the disease. Alemaya, Ethiopia, Alemaya University, MSc Thesis.
|
|
|
|
|
Winstead N, Kelman A (1952). Inoculation techniques for evaluating resistant to Pseudomonas solanacearum. Phytopathology 42:628-634.
|
|
|
|
|
Wondimagagne E (1981). The role of Poecilocarda nigrineruis, Pentanolia nigronervousa and Planonococcus ficus in the transmission of enset wilt pathogen Xanthomonas musacearum sp. In. Wolyita, Ethiopia. Addis Ababa, Ethiopia, Addis Ababa University, MSc. Thesis.
|
|
|
|
|
Yirgou D, Bradbury JF (1968). Bacterial wilt of Enset (Ensete ventricosum) incited by Xanthomonas musacearum sp. Phytopathology 58:111-112.
|
|
|
|
|
Yirgou D, Bradbury JF. (1974). A note on wilt of banana caused by the Enset wilt organism Xanthomonas musacearum. E. Afr. Agric. For. J. 40:111-114.
|
|