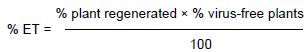ABSTRACT
Cassava production in eastern and Central Africa is severely threatened by the current epidemic of cassava brown streak disease (CBSD). The disease is caused by cassava brown streak virus (CBSV) and Ugandan cassava brown streak virus (UCBSV) of the genus Ipomovirus, family Potyviridae. Using virus-free planting material is one effective way to reduce CBSD yield losses in farmer fields. The effects of chemical and heat treatment on tissue cultured plants was investigated to eliminate CBSV and UCBSV infections in three cassava varieties with differing levels of resistance to CBSD. Cassava node buds taken from young shoots of the varieties Kaleso (CBSD resistant), Kiroba (tolerant) and Albert (susceptible) infected with CBSV and UCBSV were grown in vitro in standard Murashige and Skoog tissue culture media, which alone eliminated viruses from up to 30% of the plants. To increase the number of virus-free plants, two-week old virus-infected in vitro plants were exposed to constant temperature regimes of 30, 35, 40 or 45°C for three weeks. Heat treatment at 40°C had the optimum effect as up to 50% virus-free plants were obtained for Kiroba; however, incubation at 45°C was lethal to all three varieties while the lower temperatures produced fewer virus-free plants. Chemical treatment of in vitro plants was performed using the antiviral chemical ribavirin at various concentrations. Ribavirin at 0.10 mM generated up to 40% virus-free plants in all three varieties but concentrations of 0.21 mM were lethal. These findings indicate important methods to produce virus-free planting material; their use by breeders and in the field, will help develop effective control strategies for CBSD management.
Key words: Africa, cassava, chemical and heat treatment, viruses, tissue culture, disease resistance.
Cassava productivity in sub-Saharan Africa (SSA) was estimated at around 9.2 tons per hectare compared to the world average of 12.3 tons for 2014 (FAOSTAT, 2015). The low African cassava yields are attributed to pests and diseases, among which cassava mosaic disease (CMD) and cassava brown streak disease (CBSD) are the most prominent (Hillocks et al., 2015; Legg et al., 2011, 2015; Mohammed et al., 2016; Patil et al., 2015). Epidemics of one or both these diseases have occurred over the last two decades and have severely affected cassava production and threatened livelihoods and food security in eastern and central African countries (Legg et al., 2015). Susceptible plants infected as cuttings are severely stunted and produce poor yields.
Since the 1930s, CBSD has been endemic in areas along the Indian Ocean coast of eastern Africa, from the north-eastern border of Kenya across the Tanzanian border down as far as the Zambezi River in Mozambique; it was also widespread along the shores of Lake Malawi. In 2004, however, an outbreak of CBSD was reported in Uganda which has now spread into neighbouring countries in the Great Lakes region of East and central Africa, including Burundi, Rwanda and the eastern part of the Democratic Republic of Congo (Hillocks and Jennings, 2003; Alicai et al., 2007; Legg et al., 2011; Mulimbi et al., 2012). This is of great concern because incidence of CBSD up to 100% in cropping areas has been recorded, and in sensitive varieties the disease causes rotting of the tuberous roots, reducing both the quality and quantity. Recent estimates indicate that CBSD causes economic losses of up to US$726 million annually for African farmers (Maruthi et al., unpublished data). The disease is the most important cause of food insecurity in the coastal and lake zone areas of eastern Africa, and has been a serious threat to the entire cassava-growing belt of SSA (Legg et al., 2014). CBSD is caused by two RNA viral species; cassava brown streak virus (CBSV) and Ugandan cassava brown streak virus (UCBSV) belonging to the family Potyviridae of the genus Ipomovirus (Mbanzibwa et al., 2009; Monger et al., 2010; Winter et al., 2010), and are collectively referred to as cassava brown streak ipomoviruses (CBSIs).
CMD has been managed through the development and dissemination of virus-resistant varieties. Progress in managing CBSD, however, has been slower. A small number of varieties expressing a range of resistance levels are currently available in CBSD affected countries but they can still be infected with CBSIs. To-date, this represents the best solution available for CBSD control. Recent findings about CBSD epidemiology, however, have provided new opportunities for control. These include the finding that CBSIs are not retained for periods of more than 48 h in whitefly vectors and that an isolation distance of about 100 m is sufficient to notably minimize the spread of CBSIs between infected and disease-free plots (Maruthi et al., unpublished data). Furthermore, it has been found that tolerant cassava varieties support low viral titres and cuttings from them have a higher recovery from CBSD compared to susceptible cultivars (Maruthi et al., 2014; Mohammed et al., 2016). These results provide a strong indication that phytosanitary measures, involving the use of virus-free planting material coupled with isolation from surrounding potential sources of infection, offer excellent potential for CBSD control. Meristem culture and heat treatment have been used with considerable success to eliminate CBSD in the past (Kaiser and Teemba, 1979; Wasswa et al., 2010). The study therefore aimed to use tissue culture and chemical and thermal treatments to eliminate CBSIs infections from cassava varieties with different levels of field resistance, with the aim of identifying optimum conditions for effective treatment. The three therapies were compared for their efficiency on plant regeneration as well as for the elimination of viruses.
Cassava varieties and virus isolates
Healthy cassava plants of the varieties Kaleso (CBSD resistant), Kiroba (tolerant) and Albert (susceptible) were graft-inoculated with either CBSV or UCBSV (Mohammed et al., 2012) and infection was confirmed by reverse transcription polymerase chain reaction (RT-PCR; Abarshi et al., 2010, 2012; Otti et al., 2016). The two viral isolates were collected as stem cuttings from farm fields in Uganda and Mozambique, respectively (Mohammed et al., 2012; Maruthi et al., 2014).
Media preparation for cassava tissue culturing
The tissue culture method of Frison (1994) was optimised and used in this study. Basal medium, 2.2 g Murashige and Skoog (1962) (MS; Sigma, UK), 20 g of sucrose and 2 ml of plant preservative mixture (PPM) were dissolved in about 800 ml of single distilled water. PPM is a broad-based effective pesticide against bacteria and fungi. Fifty micro litres of the growth regulator 1-Naptherlenacetic acid (NAA) was added to enhance rooting. 2 g of phytagel (Sigma, UK) was then added to the solution and the volume adjusted to 1 litre and pH 5.8. The medium was boiled, and 10 ml was dispensed into 25 ml glass tubes (Sterlin, UK). Tubes were closed with plastic caps and sterilized by autoclaving.
Surface sterilization and inoculation of nodal buds into the media
Single node cuttings from young stems were excised from each of the three cassava varieties. The nodes were washed with tap water and pre-sterilized with 70% ethanol for 3 to 5 s. The nodes were then transferred into 10% (v/v) bleach solution of sodium hypochlorite containing 2-drops of Tween-20, and sterilized by vigorous shaking for 30 min in glass jars. The nodes were subsequently washed in sterile distilled water (SDW) 3 to 4 times until no foam was left in the jar. Using sterile conditions, nodes were transferred into sterile tubes containing MS basal medium. The tubes were covered with sterile plastic lids, labeled and incubated in a tissue culture control growth room for 4 to 6 weeks at 25 ± 3°C, RH 60% and 14:10 h of light and darkness (L14:D10).
Transplanting and hardening of in vitro cassava plants
Following the growth period above, in vitro plants were gently removed from glass tubes and residual medium was washed away with running tap water. Older and middle leaves were then removed from the plants to prevent excessive moisture loss through transpiration but, the upper 3 to 4 leaves were retained. Plantlets were rinsed in a fungicide solution of dithane (6 g/l) (Mancozeb 80% a.i.) for 10 min to prevent fungal infection. They were then planted in small pots (10 cm diameter) with 1:1 compost and soil, and grown at 28 ± 5°C, 50 to 60% relative humidity (RH), 14:10 light: dark hours under propagator lids. Pots were soaked with a Bacillus thuringiensis (Bt)-based biological insecticide Gnat-Off (Hydro gardens, UK) at a dilution rate of 1 ml/l of water following manufacturer’s instructions for the control of fungus gnat insect (black flies). High humidity was created by spraying water inside the lids and the vents remained closed for two weeks. The vents were then opened for one week to acclimatize the plants to ambient humidity, and the lids were gradually lifted to further harden the plants. Lids were taken off completely by the end of four weeks and 1 g/L of NPK fertilizer with Mg 30 + 10 + 10 + 2 was applied (Sinclair Ltd., UK). Plants were hardened-off by gradually reducing the amount of water provided over the next four weeks.
Effect of stem position and varieties on virus elimination
Nodes from each plant were numbered 1 to 10 from top to bottom and classified into two categories; top (node numbers 1 to 5) and bottom (node numbers 6 to 10), and grown in vitro as described previously. Fifteen nodes generated from each stem position from the three different cassava varieties, Albert, Kiroba and Kaleso were planted in MS media in three replications. Regenerated plants were grown and scored for CBSD symptoms. Plants that remained symptomless for six months were tested for the presence or absence of CBSV and UCBSV by RT-PCR using virus-specific primers (Abarshi et al., 2010, 2012).
Effect of thermal treatment on virus elimination
Ten node cuttings each from UCBSV-or CBSV-infected plants of the three varieties were excised (~0.4 mm size), and grown in tubes containing MS media. The plantlets were then kept in incubators (Leec, UK) under different temperature regimes of 30, 35, 40 and 45°C for three weeks at L14:D10 h. Plantlet survival was recorded from each temperature regime and for each variety-virus combination. The plantlets were then transferred into a tissue culture growth room for stabilization for 1 week at 25 ± 3°C, RH 60% and L14:D10 h. They were then grown in soil and compost as described above. Presence or absence of CBSD symptoms was recorded monthly by visual observation and tested for viruses using RT-PCR. The experiment was repeated three times using 10 nodal cuttings for each variety-virus combination and the four temperature regimes. Twenty healthy nodal cuttings from each variety for each treatment served as the control.
Effect of chemical treatment on virus elimination
The antiviral chemical ribavirin (1,ß-D-Ribofuranosyl-1,2,4-triazole-3-carboxamide; Sigma R9644), supplied in powder form, was tested for its efficiency in the elimination of UCBSV and CBSV from the three cassava varieties. Extreme care was taken when handling ribavirin due to its toxicity and broad spectrum of anti-viral activities (Dawson, 1984; Fletcher et al., 1998). Ribavirin treatment was carried out at three concentrations: 15 mg/l (0.06 mM), 25 mg/l (0.1 mM) and 50 mg/l (0.21 mM) in MS medium on nodes from five plants of the three cassava varieties infected with UCBSV or CBSV. The experiment was repeated three times using 50 nodes for each variety-virus combination and for three ribavirin concentrations. Twenty healthy nodal cuttings from each variety for each treatment served as the control.
Combined application of the therapies
Combinations of chemical and heat treatments were carried out on tissue-cultured plants of the three cassava varieties for UCBSV and CBSV elimination. Thirty nodes per variety were transferred into glass tubes containing the medium supplemented with ribavirin at 0.1 mM concentration. The plantlets were kept in the incubator (Leec, UK) at 40°C for three weeks with L14:D10 h. Plantlet survival was recorded from each variety-virus combination. After three weeks, the plantlets were removed from the incubator and transferred into a tissue culture growth room for one week and then planted in pots in a quarantine glasshouse. Plants were grown under propagator lids for four weeks. Presence or absence of CBSD symptoms was recorded monthly by visual observation of treated plants. After six months, leaf samples were tested for UCBSV and CBSV by RT-PCR. The experiment was repeated three times. Twenty healthy nodal cuttings from each variety exposed to identical treatments served as the control.
Assessment of treatment efficacy and statistical analysis
The tissue culture plantlets resulting from the trials outlined above were tested for the presence or absence of UCBSV and CBSV six months after growing in soil. The efficiency of the treatment (ET) was determined using the formula described previously (Hormozi-Nejad et al., 2010; Meybodi et al., 2011):
The ET of tissue culture, heat, chemical- and co-treatments were compared for their efficacy in eliminating UCBSV and CBSV from in vitro cassava plants. Experimental data were analysed using the statistical platform R (R Development Core Team, 2011). The proportions of virus-free plants after the treatments were analysed using a generalised linear model with binomial errors with logit link function. Statistical inference was based on resulting analysis of deviance and estimated standard errors.
Cassava nodal bud culture for virus elimination
Elongation of auxiliary buds and the emergence of new leaves were observed two weeks after seeding of the explants into the MS medium, while root formation took three weeks. The node position, cassava variety and virus significantly affected the number of virus-free plants generated (Table 1). Nodes from lower stem positions produced a lower number of virus-free plants for both CBSV and UCBSV (5.1 and 25.3%, respectively) than from upper stem positions (34.3 and 51.4%, respectively). A greater number of virus-free plants were recorded from UCBSV-infected plants than CBSV (Figure 1). Kaleso (18.3 and 51.7%) and Kiroba (26.3 and 44.3%) produced a significantly higher number of virus free plants than Albert (14.6 and 19.0%) from CBSV- and UCBSV-infected plants, respectively (Figure 1). All plants that remained symptom-free after six months were also shown to be virus-free by RT-PCR.
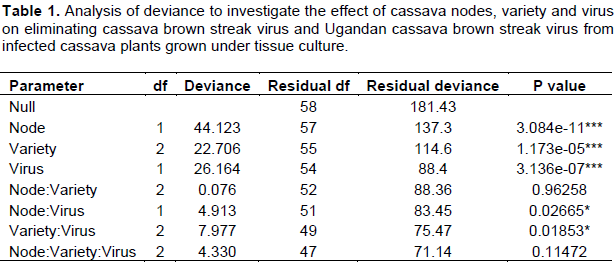
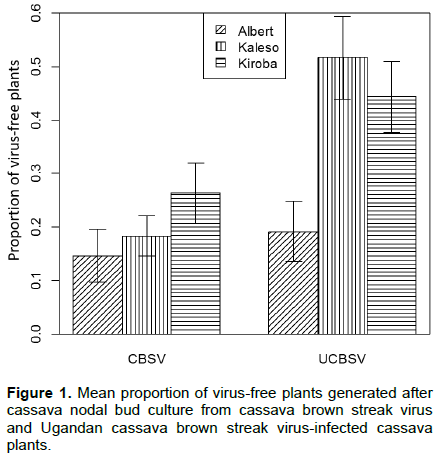
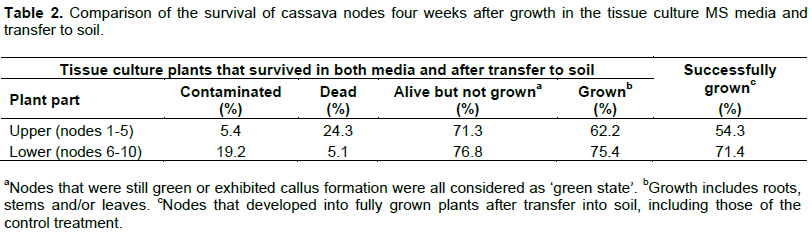
More node buds developed roots and leaves from the lower part (75.4%) compared to the upper part (62.2%) of the plant (Table 2). About 62.6% of the in vitro plants grown from nodes taken from the lower plant section (positions 6 to 10) survived after being transferred into soil. This number was reduced to 57.2% for nodes (positions 1 to 5) from the upper plant section. Contamination from bacteria and fungi was also higher for nodes from the lower (19.2%) than upper (5.4%) sections (Table 2).
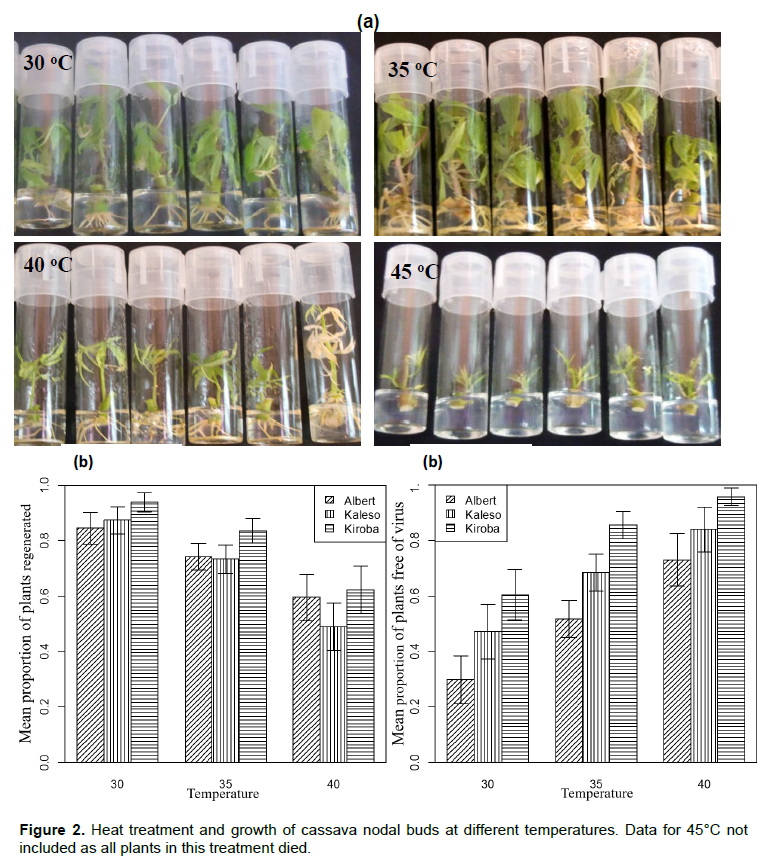
Heat treatment
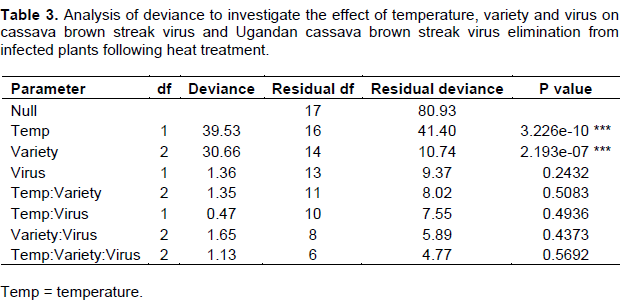
Heat treatment significantly affected the number of plants regenerated (P < 0.001). Heat stress caused a reduction in the size of leaves and shoots tips at 40°C, and caused plantlet mortality at the highest temperature of 45°C (Figure 2 a and b). Maximum plant growth was achieved at 30°C (88.6%) and 35°C (77.1%) as compared to 40°C (56.9%; Figure 2c).Temperature and cassava varieties had a significant effect on virus elimination (Table 3). The maximum number of virus-free plants was obtained at 35 (68.3%) and 40°C (84.2%) as opposed to 30°C (46.5%). A greater number of virus-free plants were obtained from tolerant Kiroba and Kaleso varieties than the susceptible Albert (Figure 2c).
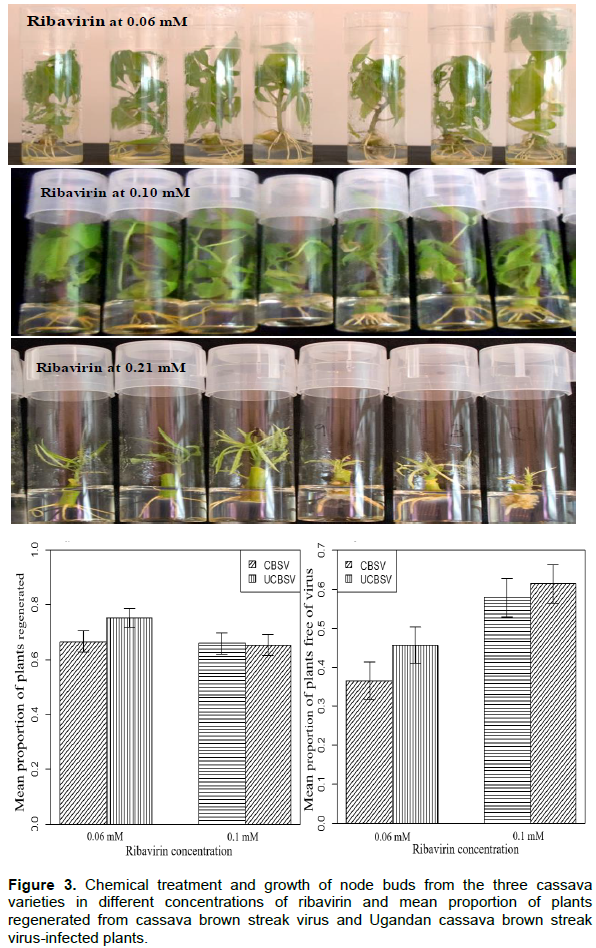
Chemical treatment
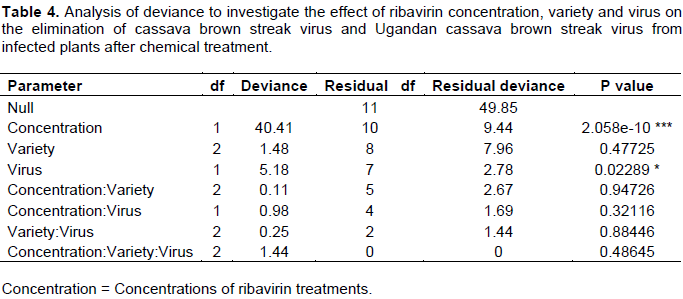
The phytotoxic effects of ribavirin were observed at 0.21 mM which resulted in severe stunting of plantlets, thin stems, stunted leaflets, sluggish root development (Figure 3a) and finally death of all plantlets of all three cassava varieties. The number of plants generated at 0.1mMribavirin (65.6%) was like that of 0.06 mM (71.0%). However, the development of roots was sluggish (at 0.1 mM) while plantlets regenerated at 0.06 mM were morphologically identical to those regenerated fromthe non-treated control. Ribavirin concentrations and the virus type had a significant effect on the number of virus-free plants generated (Table 4). Ribavirin at 0.1 mM generated a significantly higher (59.6%, P≤ 0.01) number of virus-free plants than 0.06mM (41.1%; Figure 3b). Number of virus-free plants obtained from UCBSV-affected plants were also significantly higher (53.6%; P ≤ 0.05) compared to CBSV (47.2%; Figure 3c).
Combined application of the therapies
Of the 50 nodes inoculated in the tissue culture media supplemented with ribavirin at 0.10 mM and simultaneously exposed to heat treatment at 40°C, a high percentage of plantlets were regenerated from node cuttings and this varied between therapies and varieties (Table 5). For instance, 55.4% Kaleso, 65.6% Kiroba and 58.6% Albert plantlets were regenerated from nodal buds infected with UCBSV/CBSV after tissue culture. Similarly, heat treatment of in vitro plants at 35°C resulted in good regeneration of plants (77.1%) and a high generation of virus-free plants. Simultaneous application of the three treatments resulted in decreased regeneration of plantlets in Kiroba (46.6%), Kaleso (41.6%) and Albert (51.6%) for both viruses.
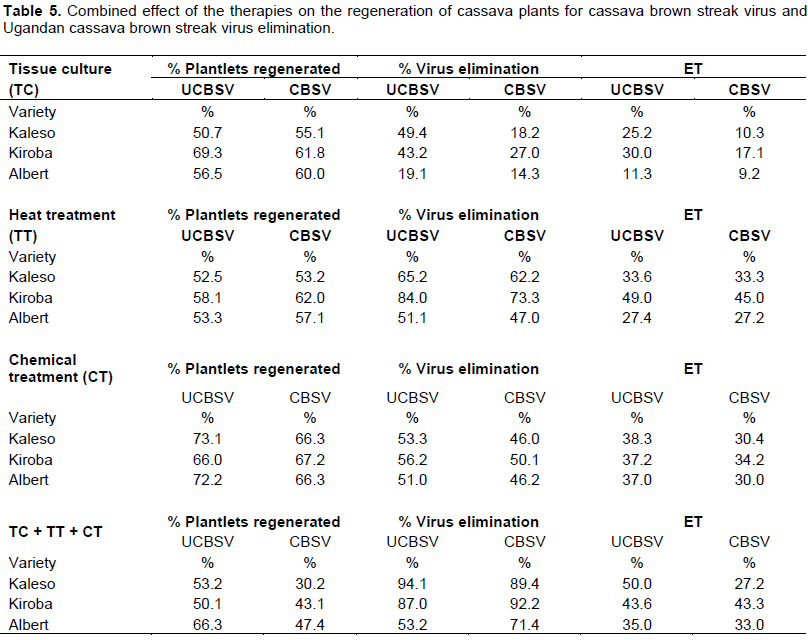
Similarly, dual effects of heat and chemical treatments, applied to in vitro cassava plants, were more efficient in eliminating UCBSV and CBSV from the three cassava varieties than either of the single methods. The highest number of virus-free plants was found from UCBSV-infected plants compared to CBSV (Table 5). Tissue culture alone eliminated UCBSV and CBSV from Kaleso (25.2 and 10.3%, respectively), Kiroba (30.0 and 17.1%), and Albert (11.3 and 9.2%). Simultaneous application of the three treatments resulted in an increased elimination of both UCBSV and CBSV. Combining the three treatments resulted in the lowest ET (27.2%) from CBSV and highest (50.0%) from UCBSV on Kaleso (Table 5).
Experiments to produce virus-free cassava plants were carried out on node cuttings of CBSD-infected cassava plants of three different cassava varieties by tissue culture, chemical and heat treatments. Regeneration of plants from the lower nodes, 6 to 10, was high compared to the upper node positions of 1 to 5 in all treatments applied including the controls. This was expected because the upper nodes were tender and fragile, and did not survive the rigors of sterilization (treatment with 10% bleach and alcohol) and subsequent treatments. However, despite the rigorous sterilization procedures, a higher proportion of plants from nodes 6 to 10 became contaminated with fungi and bacterial growth. This was likely due to the nature of the mature cassava stem that has a cracked surface, so complete removal of microbes from the cracks was not always possible. Where possible, a balance can be achieved in cassava node bud culture experiments by taking nodes from close to positions 3 to 7 to prevent high plant losses from the tender uppernodes and minimizing contamination from the lower nodes. The size of the node cuttings was also an important factor for initial plant growth, particularly in chemical and heat treatment experiments. The effect of the size of node bud on plant regeneration or virus elimination was not investigated; however, this work has been done previously in cassava by Kartha and Gamborg (1975). They observed a 60% virus elimination rate when explants were excised at a length of 0.4 mm; an increase in node size to 0.5 to 0.8 mm resulted in complete plant regeneration but 100% viral infection. Thus, anode size of ~0.4 mm or smaller would be ideal for virus elimination and regeneration of cassava plants.
The three cassava varieties subjected to various treatments responded differently to tissue culture. A comparatively high number of nodes were developed from Kiroba and Albert than from Kaleso. Plantlets from tissue culture and the ones that underwent chemical treatment developed at a slower rate than those exposed to temperature regimes of 30 and 35°C. High temperature treatments are known to stimulate plant growth as mentioned previously by Kartha and Gamborg (1975). Similar results were obtained in yams (Mantell et al., 1980; Chandler and Haque, 1984) and potatoes (Salazar and Fernandez, 1988), although the reasons behind such a growth spurt are not well understood. Differences in the rate of virus elimination through heat treatment were greater than other treatments in the three varieties and it was also more efficient in eliminating mild UCBSV than the severe CBSV (Mohammed et al., 2012). This is consistent with the elimination of potato viruses, in which heat treatment was more efficient in eliminating mild potato virus X (PVX) than the severe potato virus S (PVS) (Stace-Smith and Mellor, 1968). Thus, the elimination of virus by heat depended on the temperature regime used and the virus isolate as well as the host plant species. The highest number of plantlets was regenerated at 35°C compared to other treatments. Similarly, the largest number of plantlets was virus-free at 40°C when tested by RT-PCR, suggesting that the viruses were inhibited by higher temperature, and new shoots produced during the heat treatment could be virus-free (Kassanis, 1957). It has been suggested that the bonds between protein sub-units that protect the virus nucleic acid become weaker at high temperatures, resulting in temporal fissures and allowing attack by nucleases (Allam, 2000). The high rate of UCBSV and CBSV elimination at 40°C could therefore be attributed to the fact that increased temperatures destroy essential chemical processes in the virus life cycle.
The high percentage of virus-free plants obtained from heat treatment in this study (47 to 84%) was generally higher than the 49% achieved by Wasswa et al. (2010) at 40°C. Walkey (1976) further demonstrated that cucumber mosaic virus (CMV) did not multiply at 30°C in the experimental host Nicotiana rustica; the virus was inactivated at 32°C and was eliminated after 30 days. The highest temperature used in this study (45°C) resulted in the death of plants of all three cassava varieties, thus indicating the temperature threshold at which cassava nodes cannot survive. The use of ribavirin at different concentrations did not positively influence plant development. The maximum tolerance was reached at 0.21 mM ribavirin concentration, at which all node cuttings from the three cassava varieties died. Highest plant regeneration was obtained at 0.10 mM ribavirin although plant development was slow. Ribavirin has also been shown to slow the regeneration of potatoes (Klein and Livingston, 1982; Slack et al., 1987). These observations confirmed the toxic nature of ribavirin on the in vitro development of cassava and other plants above 0.10 mM. Developing virus-free plants by chemical treatment (Klein and Livingston, 1982) will take longer than heat treatment (Stace-Smith and Mellor, 1968) or tissue culture alone. Together with the toxic nature of ribavirin, these factors make it the least favourable choice of treatment for generating virus-free cassava plants.
Regeneration of plants was also slow in the co-treatments, although they were more efficient in eliminating the two viruses. Treatments that included the addition of ribavirin at 0.10 mM into the tissue culture media and exposure to 40°C resulted in increased virus-elimination compared to single treatments as was also achieved with PVY elimination in potato plants (Nascimento et al., 2003). The ET for UCBSV and CBSV elimination varied between the therapies and cassava varieties used. Heat treatment was most efficient for eliminating both viruses from Kaleso and Kiroba when compared to Albert, in which chemical treatment alone was more efficient than simultaneous application of the three therapies. Considering the toxic nature of ribavirin, maximum gains can be obtained without its inclusion with in vitro cultures and heat treatments (Klein and Livingston, 1982; Ng et al., 1992; James et al., 1997). Phytosanitary measures have been used to eliminate viruses from cassava by heat (Nyland and Goheen, 1969) and chemical treatments (Quak, 1961), while tissue culture alone has been found to be sufficient for eliminating DNA viruses (cassava mosaic begomoviruses) from cassava (Kartha, 1981; Roca et al., 1984). Wasswa et al. (2010) demonstrated CBSV elimination in cassava through a combination of tissue culture and heat treatment. The result of the study confirmed these findings in the three cassava varieties with different levels of resistance to CBSD, indicating that these effects were not variety specific. Tissue culture together with heat treatment is therefore the preferred methods for virus elimination in cassava due to high levels of plant regeneration, rapid plant growth and high success rates.
There has been an increased demand for healthy and certified cassava planting material in recent years because of the recent CBSD outbreak in eastern and central Africa. Developing methods for efficient elimination of UCBSV and CBSV from infected cassava plants is critical in providing healthy planting materials as required by farmers. The methods will also aid in effective germplasm exchange between countries and thus facilitate CBSD control regionally. The availability of virus-free planting material will facilitate the implementation of new disease management strategies and greater effective control over CBSD in East Africa.
The authors have not declared any conflict of interests.
REFERENCES
|
Abarshi MM, Mohammed IU, Jeremiah SC, Legg JP, Lava Kumar P, Hillocks, RJ, Maruthi MN (2012). Multiplex RT-PCR assays for the simultaneous detection of both RNA and DNA viruses infecting cassava and the common occurrence of mixed infections by two cassava brown streak viruses in East Africa. J. Virol. Methods 18:176-179.
Crossref
|
|
|
|
Abarshi MM, Mohammed IU, Wasswa P, Hillocks RJ, Holt J, Legg JP., Seal SE., Maruthi MN (2010). Optimization of diagnostic RT-PCR protocols and sampling procedures for the reliable and cost-effective detection of Cassava brown streak virus. J. Virol. Methods 163:353-359.
Crossref
|
|
|
|
|
Allam EK (2000). Eradication of Banana bunchy top virus and Banana mosaic virus from diseased banana plants. Ann. Agric. Sci. 45:33-48.
|
|
|
|
|
Chandler FL, Haque SQ (1984).The use of tissue culture in the production of improved yam and sweet potato planting material. In: Micropropagation of selected root crops, palm, citrus and ornamental species. FAO plant production and protection paper, No. 59 Rome.
|
|
|
|
|
FAOSTAT (2015). Food and Agriculture Organisation of the United Nations. Production Statistics.
View [accessed 03/09/2016].
|
|
|
|
|
Fletcher PJ, Fletcher JD, Cross RJ (1998). Potato germplasm: in vitro storage and virus reduction. N. Z. J. Crop Hortic. Sci. 26:249-252.
Crossref
|
|
|
|
|
Frison EA (1994). Sanitation techniques for cassava. Trop. Sci. 34:146-153.
|
|
|
|
|
Hillocks R, Maruthi M, Kulembeka H, Jeremiah S, Alacho F, Masinde E, Ogendo J, Arama P, Mulwa R, Mkamilo G, Kimata B (2015). Disparity between leaf and root symptoms and crop losses associated with cassava brown streak disease in four countries in eastern. Afr. J. Phytopathol. 164(2):86-93.
Crossref
|
|
|
|
|
Hillocks RJ, Jennings DL (2003). Cassava brown streak disease: A review of present knowledge and research needs. Int. J. Pest Manag. 49:225-234.
Crossref
|
|
|
|
|
Hormozi-Nejad MH, Mozafari J, Rakhshanderoo F (2010). Production and certification of virus-free nucleus seeds incommon bean. J. Plant Dis. Prot. 117:201-205.
Crossref
|
|
|
|
|
James D, Trytten A, Mackenzie DJ, Towers GHN, French CJ (1997). Elimination of apple stem grooving virus by chemotherapy and development of an immunocapture RT-PCR for rapid sensitive screening. Ann. Appl. Biol. 131:459-470.
Crossref
|
|
|
|
|
Kaiser WJ, Teemba LR (1979). Use of tissue culture and thermotherapy to free East African cassava cultivars of African cassava mosaic and cassava brown streak diseases. Plant Dis. Rep. 63:780-784.
|
|
|
|
|
Kartha KK (1981). Meristem culture and cryopreservation methods and applications in Plant Tissue Culture: Methods and Applications in Agriculture. Thorpe, T.A. (ed.), Academic Press, New York. Pp. 181-211.
|
|
|
|
|
Kartha KK, Gamborg OL (1975). Elimination of cassava mosaic disease by meristem culture. J. Phytopathol. 65:826-828.
Crossref
|
|
|
|
|
Kassanis B (1957). The Use of Tissue Culture to Produce Virus-free Potato Varieties. Ann. Appl. Biol. 45:422-427.
Crossref
|
|
|
|
|
Klein RE, Livingston CH (1982). Eradication of potato virus X from potato by ribavirin treatment of culture potato shoot tips. Am. Potato J. 59:359-365.
Crossref
|
|
|
|
|
Legg JP, Jeremiah SC, Obiero HM, Maruthi MN, Ndyetabula I, Okao-Okuja G, Bouwmeester H, Bigirimana S, Tata-Hangy W, Gashaka G, Mkamilo G, Alicai T, Kumar PL (2011). Comparing the regional epidemiology of the cassava mosaic and cassava brown streak pandemics in Africa. Virus Res. 159:161-170.
Crossref
|
|
|
|
|
Legg JP, Kumar PL, Makeshkumar T, Tripathi L, Ferguson M, Kanju E, Ntawuruhunga P, Cuellar W (2015) Cassava virus diseases: biology, epidemiology, and management. Adv. Virus Res. 91:85-142.
Crossref
|
|
|
|
|
Legg J, Somado EA, Barker I, Beach L, Ceballos H, Cuellar W, Elkhoury W, Gerling D, Helsen J, Hershey C, Jarvis A, Kulakow P, Kumar L, Lorenzen J, Lynam J (2014). The importance of cassava as a food security crop in Africa and the world. Food security, 6(2) 231–248.
Crossref
|
|
|
|
|
Mantell SH, Haque SQ, Whitehall AP (1980). Apical meristem tip culture for eradication of flexous rod viruses in yams (Dioscorea alata). Trop. Pest Manage. 26:170-179.
Crossref
|
|
|
|
|
Maruthi MN, Bouvaine S, Tufan HA, Mohammed IU, Hillocks RJ (2014). Transcriptional response of virus-infected cassava and identification of putative sources of resistance for cassava brown streak disease. PLoS ONE 9: e96642.
Crossref
|
|
|
|
|
Maruthi MN, Jeremaih SC, Mohammed IU, Legg JP (unpublished data). The role of the whitefly, Bemisiatabaci (Gennadius), and farmer practices in the spread of cassava brown streak ipomoviruses. Phytopathol. (Submitted).
|
|
|
|
|
Meybodi DE, Mozafari J, Babaeiyan N, Rahimian H (2011). Application of electrotherapy for the elimination of potato potyviruses. J. Agric. Scie. Technol. 13:921-927.
|
|
|
|
|
Mohammed IU, Abarshi MM, Muli B, Hillocks RJ, Maruthi MN (2012). The symptoms and genetic diversity of cassava brown streak viruses infecting cassava in East Africa. Adv. Virol. Volume 2012 (2012), Article ID 795697, 10 pages.
Crossref
|
|
|
|
|
Mohammed IU, Ghosh S, Maruthi MN (2016). Host and virus effects on reversion in cassava affected by cassava brown streak disease. Plant Pathol. 65:593-600.
Crossref
|
|
|
|
|
Mohammed IU, Ghosh S, Maruthi MN (2016). Host and virus effects on reversion in cassava affected by cassava brown streak disease. Plant Pathol. 65: 593–600.
Crossref
|
|
|
|
|
Murashige T, Skoog F (1962). A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 15:473-497.
Crossref
|
|
|
|
|
Nascimento LC, Pio-Ribeiro G, Willadino L, Andrade GP (2003). Stock indexing and Potato virus Y elimination from potato plants cultivated in vitro. Sci. Agric. 60:525-530.
Crossref
|
|
|
|
|
Ng KC, Handley CJ, Preston BN, Robinson HC (1992). The extracellular processing and catabolism of hyaluronan in cultured adult articular cartilage explants. Arch. Biochem. Biophys. 298:70-79.
Crossref
|
|
|
|
|
Nyland G, Goheen AC (1969). Heat therapy of virus diseases of perennial plants. Annu. Rev. Phytopathol. 7:331-354.
Crossref
|
|
|
|
|
Otti G, Bouvaine S, Kimata B, Mkamillo G, Kumar PL, Tomlins K, Maruthi MN (2016). High throughput multiplex real time PCR assay for the simultaneous quantification of DNA and RNA viruses infecting cassava plants. J. Appl. Microbiol. 120:1346-1356.
Crossref
|
|
|
|
|
Patil BL, Legg JP, Kanju E, Fauquet CM, (2015). Cassava brown streak disease: a threat to food security in Africa. J. Gen. Virol. 96:956-968.
Crossref
|
|
|
|
|
Quak F (1961). Heat treatment and substances inhibiting virus multiplication in meristem culture to obtain virus free plants. Adv. Hort. Sci. Appl. 1:144-148.
|
|
|
|
|
R Development Core Team (2011). R: A Language and Environment for Statistical Computing. R Found. Stat. Comput., R Foundation for Statistical Computing.
|
|
|
|
|
Roca WM, Rodrígues JA, Mafla G, Roa J (1984). Procedures for recovering cassava clones distributed in vitro. CIAT, Colombia. 21p.
|
|
|
|
|
Slack JMW, Darlington BG, Heath JK, Godsave SF (1987). Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature 326:197-200.
Crossref
|
|
|
|
|
Stace-Smith R, Mellor FC (1968). Eradication of potato viruses X and S by thermotherapy and axillary bud culture. Phytopathology 58:199-203.
|
|
|
|
|
Walkey DGA (1976). High temperature inactivation of Cucumber and Alfalfa mosaic viruses in Nicotianarustica cultures. Ann. Appl. Biol. 84:183-192.
Crossref
|
|
|
|
|
Wasswa P, Alicai T, Mukasa SB (2010). Optimisation of in vitro techniques for cassava brown streak virus elimination from infected cassava clones. Afr. Crop Sci. J. 18:235-241.
|
|