Vegetatively propagated crops are exposed to pests every growing season, providing them with the opportunity to feed on the plants and transmit diseases. Meristem culture is an important method to clean for viruses to restore high yields. Media optimizations with different concentrations and combinations of 6-benzylamino purine (BAP) and Naphthalene Acetic Acid (NAA) were tested for their effects on shoot initiation from meristems. This was followed by regeneration and shoot multiplication from nodal cuttings in five elite varieties of sweet potato. There was a highly significant genotype (variety) x environment (media) interaction for all experiments. Concentrations and combinations of BAP and NAA significantly affected the percentage of meristem survival/regeneration and subsequent shoot multiplication. The best combination of the plant growth regulators was NAA (0.1 mg/L) and BAP (1.0 mg/L). For further propagation, shoot numbers per single nodal cuttings were significantly affected by BAP concentrations (p < 0.05) and genotypes. The medium which gave maximum numbers of shoots was ½ MS media supplemented with BAP (2.5 mg/L) and GA3 (0.5 mg/L). The tallest shoots, with the highest number of internodes, suitable for transfer to soil, was obtained from ½ MS media without any growth regulators for all of the cultivars.
Sweet potato (Ipomoea batatas L. Lam) ranks as the fifth most important food for most developing countries (FAO, 2017). Africa stands second in total production of sweet potato and this production is increasing at a fastest global rate (FAO, 2017). Ethiopia is the sixth largest sweet potato producer in Africa. The crop is critical at both household and national levels for food security (FAO, 2017). However, the production of sweet potato suffers from virus infections which results in low national average yields (8.2 tonnes), far below the world average of 12.3 tonnes (FAO, 2017).
Tissue culture techniques have been used as a biotechnological tool for virus elimination and mass multiplication of vegetatively propagated crops, including sweet potatoes (International Atomic Energy Agency, 2004). Uniform and disease-free planting materials can be multiplied using tissue culture. Tissue culture may improve agriculture by developing quality-planting materials to meet the ever-increasing global demand for food and feeds. In addition, plant tissue culture techniques have a great potential for mass multiplication, conserving elite cultivars and rare cultivars treated to extinction using artificial media (Torres, 1989). Tissue-cultured plants often out-perform those propagated conventionally in productivity and also in the quality of produce.
Initiation and regeneration of an explant cultured on artificial nutrient media is influenced by the type and concentration of nutrients, plant growth regulators (PGRs) and their appropriate ratios, genotype and type of explants (Varshney and Anis, 2014) in addition to mother plant treatments prior to propagation in vitro. For the initiation of growth and development of an explant in any culture, in addition to the basic MS medium (Murashige and Skoog, 1962), the explant requires additional inputs and is highly affected by concerted and cooperative activities of PGRs, particularly auxins and cytokinins (Gaspar et al., 1996). The most important and most commonly used synthetic auxins in tissue culture are 2, 4-dichlorophenoxyacetic acid (2, 4-D) and naphthalene acetic acid (NAA) whereas that of cytokinins are kinetin and BAP (Gaspar et al., 1996). The concentrations and the combination between PGRs determine the morphogenetic responses towards shoot initiation.
Varieties of the same species often respond differently to artificial media (Abe and Futsuhara, 1986; Jan et al., 2001), through genotype x environment interactions. The success of sweet potato shoot initiation and multiplication in tissue culture may vary depending on the genotype used and the type and concentrations of PGRs supplemented (Dugassa and Feyissa, 2011; Wondimu et al., 2012; Abubakar et al., 2018). These previous studies show that media optimized for sweet potato cultivar are genotype-specific and if not optimized, may result in poor, or no, regeneration. In Ethiopia, several sweet potato varieties have been evaluated for traits of interest (yield, disease resistance, nutritional content, adaptability to locations) and released for use (Tofu et al., 2007; Gurmu et al., 2017). However, efficient and universal protocols for rapid multiplication of virus-free planting materials have not been optimized, except for a few of these cultivars (Dugassa and Feyissa, 2011; Wondimu et al., 2012). For this study, we have used five high yielding varieties of sweet potato released for different regions (southern, eastern and western) of Ethiopia. Three of them are white and the remaining two are orange-flesh sweet potato varieties (containing the valuable β-carotene, the precursor of vitamin A).
Our hypothesis is that these elite genotypes will vary in their responses to the PGRs. It is important to optimize the best media for each genotype. Moreover, we intend to clean the elite varieties from the infecting virus(es) and undertake further mass propagation of these clean genotypes for distribution to the farmers. The present study was carried out to (1) optimize the best combinations of PGRs to obtain a high rate of shoot initiation from meristem explants of four local varieties selected for their strong agronomic performances and (2) to optimize the appropriate concentrations of BAP for the shoot multiplication of five Ethiopian sweet potato varieties using single nodal cuttings. These two aims will, potentially, secure enough clean plant material for Ethiopian famers of these genotypes, since Ethiopia already has commercial tissue culture facilities in place for propagation of sugar cane with the capacity to take on disease-free sweet potato propagation as well. This piece of work contributes to the undergoing effort of cleaning the virus from elite variety and multiplying the virus-free sweet potato plants.
Plant materials and experimental sites
Five high yielding Ethiopian sweet potato varieties, three of which (‘Hwassa-83’, ‘Guntute’ and ‘Kulfo’) were obtained from the Hawassa Agricultural Research Center, and two from Bako Agricultural Research Center (‘Tola’) and Haramaya University (‘Berkume’) were used for this study. The vines of the varieties were imported into Norway with a permit from the Norwegian Food Authority (Mattilsynet) for virus indexing and virus-cleaning. Plants were grown in quarantine rooms and used as stock to provide shoot tips for excising meristems. The studies on PGRs in the two media were conducted in the Plant Cell Laboratory at the Norwegian University of Life Sciences (NMBU), Norway. Meristem tip culture and single nodal cuttings were used as explant for shoot initiation and shoot multiplication experiments, respectively. The nodal cuttings used as explants for the multiplication experiments were from plants in tissue cultures grown on MS media without PGRs, as the last medium before the transfer to soil.
The virus-cleaned and virus-tested cultures were exported back to Ethiopia as tissue cultures where the acclimations were performed at Hawassa University.
Experimental design and treatment set-up
Medium preparation
The basal medium used throughout was Murashige and Skoog (MS) (1962) from Duchefa Biochemie (M0222.0001) containing 4.4 g/L (macronutrients, micronutrients, and vitamins). The MS medium was used in either full (initiation) or half concentration (multiplication). The media were all supplemented with 30 g/L sucrose and 6 g/L agar (Sigma-Aldrich, Spain). Plant growth regulators (PGRs) were supplied according to the two experimental set-ups described below.
Initiation of meristems
The experiments were set up as complete factorial experiments (4x4x4) with 16 media combinations of two PGRs (NAA and BAP) (Table 1A, media 1-16) for each of the four varieties (‘Berkume’, ‘Guntute’, ‘Kulfo’ and ‘Tola’).
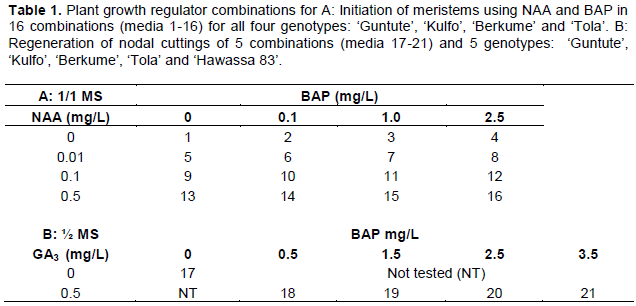
Regeneration of nodal cuttings
MS medium (1/2 strength) supplemented with five concentrations of BAP (0, 0.5, 1.5, 2.5, and 3.5) mg/L was combined with 0.5 mg/L of the natural gibberellin, GA3 (Table 1B, media 17-21) and were tested to assess the response differences of the five sweet potato varieties (‘Berkume’, ‘Guntute Kulfo’, ‘Tola’ and ‘Hawassa-83’). No PGR was included as a control. Shoot multiplication from each single nodal in vitro cutting was recorded for each combination.
Preparation of explants, surface sterilization and the establishment of a culture
Preparation of meristems
Shoots of about 2 - 3 cm were trimmed for leaves and parts of leaf petioles, the surface was then sterilized in 70% ethanol for about 30 s, and thereafter 10 min in a solution of 5% (v/v) sodium hypochlorite (VWR) and 0.02% v/v of Tween 20 (Sigma-Aldrich, St. Louis, USA). Finally, the shoot tips were rinsed three times with double distilled, autoclaved (sterile) water. The apical and the two adjacent axillary meristems from the top were excised under a dissecting microscope and placed on the various media under sterile conditions. Seven meristems were cultured on each of the 16 different combinations of BAP and NAA levels. Treatments were arranged in a completely randomized design (CRD) and each experiment was replicated three times. In total, 336 meristems per variety were cultured on the different combinations of BAP and NAA.
Regeneration of nodal cuttings for multiplication
In vitro cultures of five sweet potato varieties, which were first kept on hormone-free ½ conc MS medium for 6 weeks, were used as the starting material for this experiment. They were grown under the same growth room conditions as described below and transferred to ½ MS medium, with PGR combinations 17-21 (Table 1B) to evaluate shoot multiplication using single nodal cuttings. Four single nodal cuttings were cultured in each jam jar as a single experimental unit and each combination was replicated three times, that is, 12 single nodal cuttings of each of the varieties represented each treatment.
Culture conditions
All the in vitro cultured explants were grown under the same growth room conditions: temperature of 25 ± 1°C, a light intensity of 28 μmol m-2 s-1 and 16/8 h (light/dark) photoperiod provided by white fluorescent lamps (Osram L 58W/840 Lumilux), and a relative humidity of 70%.
Acclimatization
Cleaned, virus tested tissue culture plantlets were returned to Ethiopia from Norway with an issued Health Certificate according to the Convention of Biological Diversity (1993). The cultures were transferred to an insect-proof screen house at Hawassa University, Ethiopia into polyethylene plastic tubes filled with solarized top soil: compost: sand in a ratio of 3:2:1. Plants were covered with a plastic sheet for the first two weeks to maintain plant turgidity. One month after planting, the surviving plants were counted and a percentage of survival was calculated.
Data collections and subculturing
Shoot initiation
Cultures with infection symptoms (if any) were, immediately recorded and discarded. Meristems with a green color after two weeks of culture were counted as survived; whereas those that failed to turn green were considered to be dead. The percentage of meristem survival was determined by dividing the green meristems that survived by total cultured. After 6 weeks of incubation, the survived meristems were transferred into test tubes, which contained the same fresh media combination, and to allow for shoot regeneration. Twelve weeks later, the number of meristems that induced a shoot size of more than 1 cm, the number of meristems that produced callus, and the callus weight (mg) induced for each meristem combination were recorded.
For regeneration of nodal cuttings experiment, culture survival, the number of shoots induced per nodal explant, the length of shoots, the callus weight per nodal explant, and the number of nodes were counted after four weeks.
Data analysis
All of the data were subjected to ANOVA (General Linear Model) using Minitab Statistical Software version 18 (Minitab Inc., Pennsylvania, U.S.A). Means that were significantly different were separated using a Tukey LSD test at the probability level of 5% (p ≤ 0.05).
Genotype and PGR effects on meristem survival and further shoot development
Different concentrations and combinations of NAA and BAP had no significant effects on the survival of the meristems after two weeks; they greened in a similar way for all PGR combinations (data not shown). As time went by (a further 16 weeks), the different concentration and combinations of PGRs gave significantly different survival responses amongst the four varieties tested (Table 2). Many meristems did not develop further into shoots and eventually died, particularly when no NAA was added (Figure 1). We can safely conclude that auxin is needed for meristem survival of sweet potato. This is in accordance with previous findings by Dugassa and Feyissa (2011).
‘Kulfo’ obtained the highest shoot initiation rate (89%) after a further 16 weeks on the MS media supplemented with 0.01 mg/L NAA and 1.0 mg/L BAP (medium 7) (Figure 1). This is the same optimal medium as ‘Berkume’, while ‘Tola’ preferred a medium with only NAA (0.5 mg/L) and ‘Guntute’ performed best on 0.1 mg/L NAA + 1 mg/L BAP. On average across all of the media, the highest survival rate of 83% was recorded for ‘Kulfo’, while the lowest (of the media with growth) was for ‘Guntute’ (71%) (Table 2). It is reported that two or more hormones (BAP and NAA in this case) can interact synergistically or antagonistically in many circumstances (George et al., 2008).
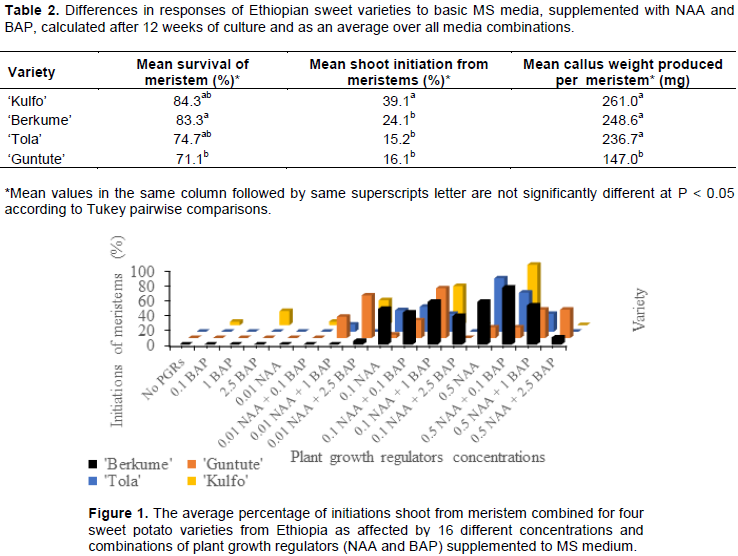
When comparing our study with that of Dugassa and Feyissa (2011), we have included a wider variety of genotypes from several regions of Ethiopia. Our aim was to include the best varieties from different sweet potato producing regions in Ethiopia to be able to clean elite Ethiopian varieties from viruses. Our results are in agreement with the results of Dugassa and Feyissa (2011) for the two varieties that they tested. We have demonstrated the optimal PGR combinations for improved initiation of shoots from shoot tip meristems of three more varieties. In descending order of response: ‘Kulfo’, ‘Berkume’, Guntute’ (same as Dugassa and Feyissa, 2011) and ‘Tola’ as the least responsive on these media. Dugassa and Feyissa (2011) noted that ‘Guntute’ had the highest response rate of the three varieties tested, on their media combinations. However, we have tested a much higher level of NAA, combined with the same levels of BAP, and found that their optimal NAA (0.01 mg/L) had not reached an optimum for any of the varieties that we tested, and ‘Guntute’ was tested in both studies. Our overall optimal NAA level was x10 higher (0.1 mg/L), combined with 1.0 mg/L BAP. The BAP optimal for all varieties combined (Figure 1), on the other hand, is in accordance with Dugassa and Feyissa’s findings (2011). We can conclude that the optimal medium for a new variety would be to try out this combination (NAA 0.1 mg/L + BAP 1.0 mg/L).
The picture is different when looking at each variety separately, since we have a clear genotype x environment interaction (Figure 1). This shows that each variety has their own requirements, as previous reports have also concluded for both Ethiopian (Dugassa and Feyissa, 2011) and West African varieties (Addae-Frimpomaah et al., 2014). This kind of factorial experiment, to reveal the optimal concentrations of PGRs on meristem initiation and regeneration has been confirmed by Su et al. (2011). The number of weeks to induce shoots varied for the varieties; ‘Kulfo’, ‘Hawassa 83’ and ‘Berkume’ took 5 to 7 weeks, while ‘Tola’ and ‘Guntute’ took 3 weeks longer to respond.
The statistical analysis shows that the interaction in the response of meristems, between the varieties and PGR combinations, was highly significant (p=0.001), while the response was only significant, at a level of 5%, when testing the varieties and media independently (Table 3).
Figure 1 illustrates the best concentration and combinations of BAP and NAA for shoot initiation media. The details of the statistics behind this figure are presented in Online Resource 1. No, or low, concentration of PGRs did not result in shoot regeneration, as with the initiation meristems. Both ‘Kulfo’ (81%) and ‘Berkume’ (76%) had the highest percentage of meristems giving shoots on the same PGR combination: MS with 0.5 mg/L NAA and 0.1 mg/L BAP (medium 14). ‘Guntute’ performed best on MS medium supplemented with 1 mg/L BAP and 0.1 mg/L NAA (medium 7) with (67%) of the meristems producing shoots. This is the same result that Dugassa and Feyissa (2011) obtained for ‘Guntute’, except that they also added 1 mg/L GA3 in their medium, in addition to BAP and NAA. We can conclude that adding gibberellin was not necessary in our case. The maximum regeneration from meristems in ‘Tola’ was 72%, on yet another medium: 0.5 mg/L NAA without any added BAP (medium 13). These three genotypes were not part of the study by Dugassa and Feyissa (2011), so this is the first report of their optimal combinations. Preliminary studies (data not shown) with ‘Hawassa-83’ was also consistent with Dugassa and Feyissa (2011), so we decided to omit this genotype from the large factorial media experiment, but kept ‘Guntute’ for reference. Other studies, using other genotypes, have also reported on the effect of variety, the concentrations and combinations of PGRs on the regeneration capacity of sweet potato plants from meristems (González et al., 2008; Sivparsad and Gubba, 2012; Alam et al., 2013; Mbinda et al., 2016). So, it is not surprising that the regeneration of sweet potato plants from meristem cells varies depending on the tissue culture media components (PGRs, nutrients, vitamins, sugar, agar), genotypes and the culture conditions (Dugassa and Feyissa, 2011; Addae-Frimpomaah et al., 2014). Already in 1957, Miller and Skoog revealed the importance of auxin and cytokinin concentrations and their combinations for cell proliferation and new organ regeneration in tobacco. Since then, this kind of factorial experiment, to reveal the optimal concentrations of PGRs, has been repeated by numerous authors for other plants. When optimizing for a species with genotype x media interactions, we believe it is important to perform such an exercise. It is necessary to be sure that we have the best possibility of obtaining surviving meristems when the purpose is to clean a particular genotype for viruses, to increase the chance of obtaining a clean plant. From our results for the initiation of shoots from meristems for all varieties, it averaged between a low of 1.2% on 1 mg/L BAP to a high of 57.7% on 1 mg/L BAP and 0.1 NAA (Figure 2). Moreover, this media combination induced an average low callus weight of 98 mg/meristem, which is desired to reduce the risk of somaclonal variation. Therefore, this combination of 1 mg/L BAP and 0.1 NAA could be the one to use for new cultivars, if a factorial PGR experiment is not feasible.
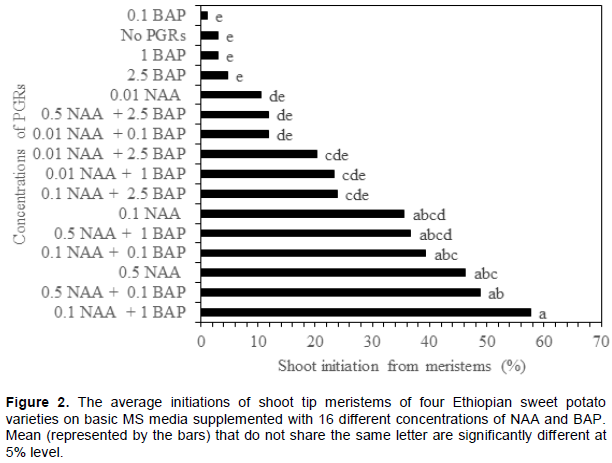
PGRs combinations and the varieties, when analyzed separately, both had statistically significant effects (p<0.05) on the initiations of shoot from meristems (Table 2). When considering overall performances for all tested genotypes, 0.1 mg/L NAA and 1 mg/L BAP (medium 11) was the best concentration and combination tested that regenerated shoots in 58% of the cultures (Figure 2). Figure 2 reveals that media 9, 15, 13, 14 and 11 are not significantly different at a level of 5% but do show an increasing level of regeneration when averaging over all genotypes. Significant differences, in respect of days for shoot induction, were also observed. ‘Kulfo’ and ‘Berkume’ took 5 to 7 weeks to regenerate shoots of about 1 cm or more, whereas ‘Tola’ and ‘Guntute’ took between 8 to 10 weeks (data not shown).
PGRs and genotypes highly affected shoot multiplication and height of nodal explants
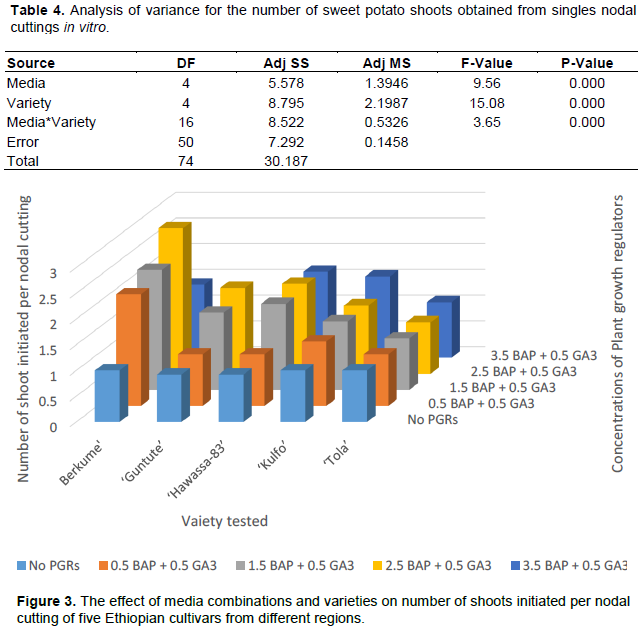
The factorial media experiment using nodal explants further emphasized the importance of such media experiments. Table 4 shows the highly significant effects of the five media combinations, the genotypes on shoot multiplication from nodal cuttings, as well as the interaction between them.
The sweet potato nodal cuttings responded well to transfer from in vitro cultures when propagated on to hormone-free ½ MS medium. As expected, the survival rate was between 95 to 100% in all cases.
Figure 4A shows that the highest mean number of shoots for all varieties were obtained when supplying either 1.5 or 2.5 mg/L of BAP combined with 0.5 mg/L GA3 (medium 19 or 20). Figure 3 provides more details for the optimal medium for each cultivar. ‘Berkume’, ‘Hawassa’ and ‘Guntute’ produced the maximum mean number of shoots of 2.8, 1.7 and 1.8 correspondingly on ½ basic MS (medium 20) supplemented with 2.5 mg/L of BAP and 0.5 mg/L GA3 (Figure 3). However, ‘Kulfo’ produced the highest mean shoot number of 1.6 at a concentration of 3.5 mg/L BAP and 0.5 mg/L GA3 (medium 21). ‘Berkume’ produced a significantly higher number of shoots, compared to the other four varieties, on average over all media tested (Figure 4B). ‘Tola’, essentially, did not multiply on any of these media, as it produced only, or at the very best, 1.1 shoot per nodal cutting (medium 21) in one month (Figure 3).
In this study, increasing the concentration of BAP significantly decreased the height of the shoots induced from the nodal cuttings in all of the studied varieties (Figures 4A and 5). The results agreed with the findings of the previous studies conducted that evaluated the effect of BAP concentration on nodal cuttings regenerated using sweet potato from Ghana and Ethiopia (George et al., 2008; Dugassa and Feyissa, 2011; Addae-Frimpomaah et al., 2014). Interestingly, the lowest mean number of shoots and the shortest mean shoot height was obtained from ‘Tola’ among the other varieties that were tested in response of the BAP concentrations. This could agree with the suggestion that the effect of hormones on any developmental process depends on the species (George et al., 2008). However, the highest (6.9 cm) and shortest (1.68 cm) mean shoot height were registered for ‘Tola’ and ‘Berkume’ cultured in basic ½ MS media, without PGRs (Table 4 and Figure 5). Furthermore, this study also showed that varieties responded differently to the same concentration of BAP and resulted in varying mean shoot number. This can be explained by the suggested theories that the effects of plant growth regulators vary depending on culture conditions, explant types and the genotype (Gaspar et al., 1996).
Nodal cuttings planted on MS media without BAP generated the highest shoots compared to any other levels of BAP concentration we tested. It appears that nodal cuttings cultured on different BAP levels induced shoots of varying number and length (Dugassa and Feyissa, 2011). The higher shoot length can provide more cuttings per plant and can compensate for the number of shoot induced by adding BAP. We suggest that using ½ MS media without PGRs could be good for shoot multiplication of sweet potato varieties as it avoids costs of PGRs and gives longer shoots that can provide more nodal explant for multiplication.
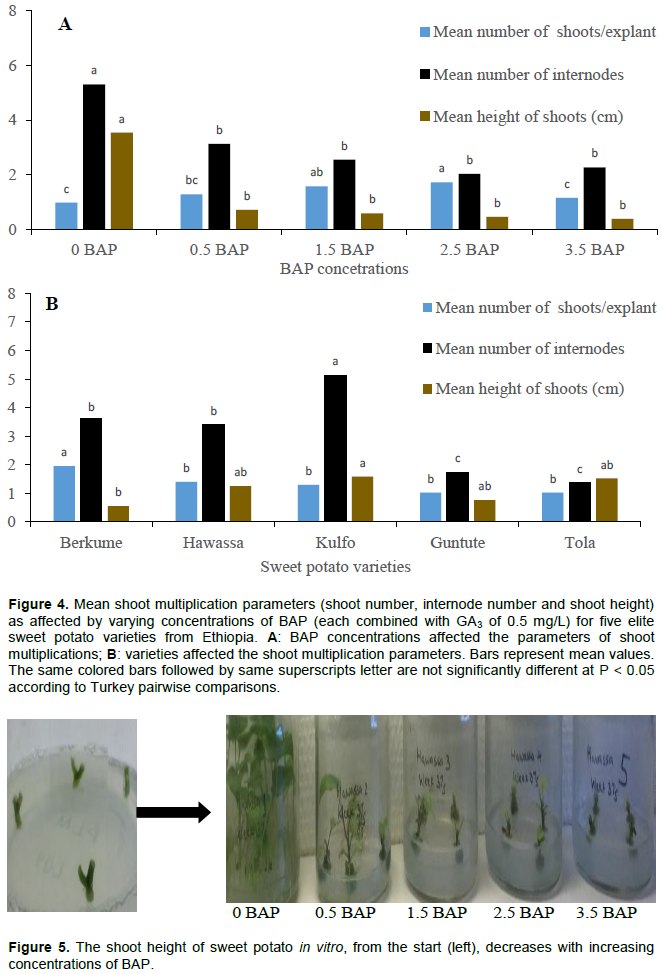
The tallest shoots and highest number of internodes were obtained on ½ basic MS media without BAP or GA3 (hormone-free medium 17). Shoot length declined with increasing concentration of BAP for most varieties (Figures 4A and 6). ‘Kulfo’ obtained the tallest shoots of all varieties (Figure 4B) on average over all media tested. ‘Tola’, however, had the tallest shoots of all, 6.90 cm on the hormone-free medium 17 (Online Resource 4). Taller shoots secure a better transfer to soil and are, therefore, beneficial.
PGR and genotype affect internode number
The BAP concentrations (Figure 4A) as well as varieties (genotypes) tested (Figure 4B) significantly affected the number of internodes per single initial nodal explant. The number of internodes gives an indication of the potential of mass propagation since there will normally be at least one axillary bud for each node. Basic ½ MS media without BAP nor GA3 (medium 17) gave the highest average (over all genotypes) internode number of 5.3, whereas the minimum (2.0) was obtained with a concentration of 2.5 mg/L BAP and 0.5 mg/L GA3 (Figure 4A). The highest average internode number obtained from the various genotypes was 5.1 from ‘Kulfo’, while the lowest was ‘Tola’ with 1.4 internodes from each initial nodal cutting (Figure 4B).
No significant differences between varieties were recorded on the establishment rate (survival) when the tissue cultures of sweet potato plants were planted in screen house. In all of the varieties, more than 90% of the planted plants survived the acclimation and successfully established into mature plants within one month after planting (Figure 6).
PGR x genotype interactions for callus growth
This research did not set out to produce callus but it was recorded as a way of describing the responses to the media combinations. Also, callus has a potential use for the development of other regeneration methods, such as somatic embryogenesis protocols. In our case, this is an undesired feature, as we aim for shoots, not callus. Regeneration of shoots from callus is more prone to somaclonal variation than direct shoot organogenesis (Krishna et al., 2016), while the differentiation into somatic embryos probably requires an intact genetic constitution to facilitate the complex differentiation into somatic embryos. This hypothesis is supported by the work in poinsettia (Geier et al., 1992) who documented a higher variation in callus than in the subsequent somatic embryos.
The mean weight of callus induced per meristem was significantly affected by the concentrations and the combination of NAA and BAP in the varieties studied. The interaction of PGRs and varieties was significant at p < 0.05 for callus weight. Overall, on average across all varieties, the highest average callus weight (654 mg) was obtained from meristems cultured on basic MS medium (medium 16) supplemented with combinations of 0.5 mg/L NAA and 2.5 mg/L BAP (Figure 4). Medium 16 produced the highest callus weight for all varieties; ‘Berkume’ obtained a maximum mean weight (864 mg), of callus per culture, while ‘Kulfo’ obtained 643 mg, ‘Tola’ 642 mg, and ‘Guntute’ had only 465 mg (Figure 7, with statistics in Online Resource 2).
Meristems cultured on a basic MS media, supplemented with all concentrations of BAP but without NAA did not induce callus in any of the varieties (Figures 7 and 8, Online Resource 2). ‘Berkume’ was the culture with the highest callus mass that quickly outgrew the shoot bud.
The differences in weight of the callus measured between the varieties that we tested on the specific media concentrations and combinations could be explained by the differences in the amount of endogenous auxins in each variety (Onwubiko et al., 2015). Therefore, in order to improve the growth of the cultures, these authors suggested PGRs that supplemented the basic MS media and should be in line with the concentration of endogenously PGRs in tissue. While this may be the explanation for the genotype variation, we would recommend testing a variety of PGR combinations systematically, rather than the lengthy process of determining the endogenous hormone content.
This study demonstrated that different varieties of sweet potato respond very differently to the shoot initiation and shoot multiplication media, with respect to PGRs. For initiation of meristem, sweet potato have a clear requirement for auxin (NAA) without this, the addition of cytokinin (BAP) at any concentration has no effect. Then, the combinations of auxin and cytokinin are vital for meristem initiation and development into shoots. For the establishment of a novel genotype that did not form part of these experiments, the best chance of success would be to use MS medium supplemented with 0.1-0.5 mg/L NAA and 1.0 mg/L BAP.
For further multiplication in vitro of virus-free material, the best medium for ‘Berkume’, ‘Guntute’ and ‘Hawassa-83’, would be 2.5 mg/L BAP combined with 0.5 mg/L GA3 and no auxin, would be the medium to try for new genotypes. However, ‘Kulfo’ preferred higher amounts of BAP (3.5 mg/L and 0.5 mg/L GA3) to reach its highest potential. Since this is the highest amount of BAP used in this study, the optimum may be even higher. The same is probably true for ‘Tola’, which failed to multiply under our conditions, but still had the highest score on the same medium.
For all five of the tested varieties, the longest shoots with a higher number of internodes was obtained when single nodal cuttings were cultured on basic ½ MS media without any PGRs. These shoots are suitable for transfer to soil. We, therefore, recommend using hormone-free medium as the last medium prior to transfer to soil, to produce longer shoots with a greater chance of survival.
With this study, we have provided initiation medium, multiplication medium and elongation medium for elite sweet potato genotypes from Ethiopia, which can be used for multiplication of elite virus-free sweet potato varieties, and then distributed to the farmers or private multipliers.