ABSTRACT
In vitro somatic embryogenesis and regeneration are important techniques for crop improvement and mass propagation. This study was conducted to establish and optimize a regeneration system for agronomically important potato cultivars (‘Victoria’ and ‘Rutuku’) of Solanum tuberosum L. in Uganda. Completely randomized design experiments were set up at Bioscience East and Central Africa (BecA) Tissue Culture Laboratory for this purpose. Callus induction and plant regeneration were initiated on internodes and leaf segments of the two potato cultivars in vitro on Murashige and Skoog (MS) medium supplemented with different phytohormones that included auxins such as α naphthalene acetic acid (NAA), cytokinins like benzyl amino purine (BAP), gibberellic acid (GA3), 2,4-dichlorophenoxyacetic acid (2,4-D) and Zeatin at varying concentrations. Callus response depended on the genotype, the concentrations and composition of growth substances. Hormone combination MS + Sucrose 30% + NAA (2 mg/l) + 2,4-D (2 mg/l) + KN (2 mg/l) was found to have the highest callusing rate, that is, 95 and 53%, respectively for internodes and leaf explants. The calli formed from internodes and leaves were friable and soft. The callus colour in all cases ranged from light brown to light cream and in some cases to light green. Shoot bud initiation was observed in all regeneration culture media, with media combination MS + KN (2 mg/l) + Zeatin (2 mg/l) giving the highest shooting rate. The intervening callus phase led to less number of shoot buds for each callus leading to long incubation period. The study showed that it is possible to regenerate potato cultivars (‘Victoria’ and ‘Rutuku’) from cell suspension culture using Murashige and Skoog (MS) medium supplemented with different phytohormones. Combination of MS with NAA + 2,4-D + Kinetin is suitable for callus induction while MS + kinetin and zeatin was better for shoot induction on calli of potato cultivars ‘Victoria’ and ‘Rutuku’.
Key words: Regeneration, somatic embryogenesis, Solanum tuberosum.
Irish potato (Solanum tuberosum) is an important staple food crop as well as a cash crop in the highland areas of Uganda where it is grown by over 300,000 smallholder households. Potatoes play a major role in national food and nutritional food security by providing a cheap but nutritionally rich staple food required in the fast growing population of Uganda, contributing protein, vitamins, zinc and iron to the diet (Abong et al., 2009). However, the productivity has stagnated between 5 - 7.5 t/ha at farmers level for many years while on-station yields go as high as 20 t/ha (FAOSTAT, 2014). This yield gap is attributed to pests and diseases (mainly late blight and bacterial wilt), low yielding varieties, poor disease management practices (Olanya et al., 2001), inadequate soil fertility management (Lemaga et al., 2001) and use of low quality seed potatoes (Byarugaba et al., 2017; Aheisibwe et al., 2015). With the rapid development of cell engineering, studies using suspension cells as starting plant material have gained momentum over the past several years. A fine cell suspension line is a good target tissue for gene transfer and somatic embryogenesis. Therefore, the establishment of in vitro reproducible regeneration protocols for cell suspension cultures in economically important potato cultivars provide an opportunity to make Biotechnology applications such as genetic engineering techniques more successful (Hussain et al., 2005).
Use of in vitro techniques such as somatic embryogenesis and regeneration combined with genetic engineering thus hold great prospect in addressing some of the production challenges for this crop especially diseases and seed quality (JayaSree et al., 2001). In vitro regeneration of plants have contributed to mass propagation of many plant species including potatoes and has emerged as an alternative for reducing cost of production (Vasil, 2012; Abbott and Belcher, 1986; Brar and Jain, 1998) as well as induction of fast crop improvements. Callus induction and subsequent plant regeneration using phytohormones accelerate the multiplication of young and strong plantlets. In potatoes (Solanum tuberosum L.), different approaches have been tried to obtain efficient in vitro regeneration system from petioles with intact leaflets (Yee et al., 2001), leaves (Sarker and Mustafa, 2002; Andersson et al., 2003), leaf discs (Osusky et al., 2005), tuber discs (Vasquez and Clarence, 2002), and from stem (Chang et al., 2002) through callus induction. However, somaclonal variation in plants coupled with long regeneration period have been reported from potato plants derived from leaf discs (Fleming et al., 1992; Trujillo et al., 2001), stem segments (Cardi et al., 1993), anthers (Véronneau et al., 1992) and protoplasts (Sree-Ramulu et al., 1983; Coleman et al., 1990) and these have been reported to be genotype dependent. The development of somatic embryo has been done successfully using several explants tissues of potato such as tuber discs, nodes and leaf tissues on solid media (JayaSree et al., 2001).
Since regeneration systems for most potato cultivars are genotype-specific, this limits their wide applicability to all genotypes (Ritchie and Hodges, 1993). Available evidence suggests that populations of regenerated plants may contain tremendous somaclonal variants which can be used to complement existing breeding programs. Studies have also shown that there is a strong link between the somatic embryos and the original explants tissue hence, production of somatic embryos from cell suspension cultures are more desirable. Due to differences in regeneration abilities of cultivars that are recalcitrant to various biotechnological advances (Sharma et al., 2008; Kumar and Kumar, 1996), identification and screening of useful cultivars for embryogenic callus formation and subsequent plant regeneration using in vitro cell culture, forms the key steps in potato genetic improvement program (Hoque et al., 2007). Therefore, genotype and nutrient media composition are the most important factors which affect callus induction and subsequent plant regeneration. Therefore, this study was conducted to establish and optimize a regeneration system for two agronomically important potato cultivars (‘Victoria’ and ‘Rutuku’).
Potato explant materials and experimental design
Explants of two (2) potato cultivars of Victoria and Rutuku were obtained from Kachwekano Zonal Agricultural Research and Development Institute and taken to Bioscences for east and central Africa (BecA) Tissue Culture Laboratory. They were surface sterilized using sodium hypochorite (20%) and ethanol 70% for 5 min and rinsed 3 times with distilled water before cutting them into different segments of internodes, leaf disc and stems. The experiment was set up as a 3 factor factorial (media composition, Variety and explant type) and laid out in Completely Randomized Design (CRD) with 4 replications and 8 explants in each replicate.
Media used
MS basal medium (Table 1) was used and chemicals for preparation of the MS media were acquired from Duchefa. It contained the full macro and micro elements. The media was supplemented with vitamins (thiamine (Vit B1) 0.1 mg/l, Niacine (0.5 mg/l), Glycine (2.0 mg/l), pyridoxine (HCl) Vit B6 (0.5 mg/l) and Sucrose (25%), obtained from Sigma Aldrich inc. Plant growth regulators (phytohormones) were also obtained from Sigma Aldrich Inc and included mainly gibberellic acid (GA3), α-naphthalene acid (NAA), N-6-benzylaminopurine(BAP), indoleacetic acid (IAA), kinetin, 2,4-dichlorophenoxyacetic acid (2,4-D) and zeatin riboside. For callus induction, leaf and internodal segments were put on callus induction media and maintained in the growth room at 16/8 light dark photoperiod at 18°C. The experiment was carried out with 4 replications with 5-8 explants in each replicate. The cultures were observed periodically for the development of the callus. Well-developed calli were excised and put on regeneration media containing different concentration levels of phytohormones (Table 1) for shooting and rooting and incubated at 25±2°C with a 16-h photoperiod. For production of cell suspension, calli were put into liquid callus production media with constant shaking at 150 rpm at 25°C. After two weeks of culture, the aggregated calli cells were transferred into 50 – 100 ml of fresh liquid MS media supplemented with NAA (2 mg/l) + 2-4,D (2 mg) and kinetin (2 mg/l). The cultures were maintained in complete darkness for a period of one month with sub-culturing every two weeks on fresh media. After removing the liquid, the aggregated cells in the flasks were removed and transferred to solid plant regeneration media supplemented with phytohormones as indicated in Table 1, followed by incubation in the darkness for 3 weeks at 18°C.
Data collection
Data were collected on callus induction frequency, days to callus initiation, callus texture, color and percentage of callus formation. Data were also recorded on the following parameters, days to shoot regeneration, number of shoots per explant, days to root induction, number of roots per explant.
Data analysis
Three factor factorial analysis of variance were performed using Genstat statistical software and mean separated by LSD at 5% level of significance.
Effects of growth regulators on callus formation of two potato varieties
Regeneration was done using internodes and leaf segments as explants from two different potato cultivars namely: ‘Victoria’ and ‘Rutuku’. The different phyto-hormones used in the preparation of media for regeneration were 2,4-D and naphthalene acetic acid (NAA) for callus induction while benzyl amino purine (BAP), zeatin and kinetin were used for shoot induction. The response to callus induction of internodes, and leaf segments of Victoria and Rutuku varied with genotype, explants type and the media composition. Initiation of callus was seen from internodal stem segments and leaf segments of Rutuku and Victoria in the media between 17 - 20 days after inoculation. All the explants formed callus (100%) but varied in the degree of callus development. A combination of MS+ 2-4,D (3 mg/l) + NAA (3 mg/l) + KN (3 mg/l) were found to be most effective auxin concentration level for callus induction in all the explant types for Victoria and Rutuku with greater than 60% of the explant forming friable callus within 40 days from the time of initiation (Table 2). These findings are in agreement with work done by Elaleem et al. (2009); Shirin et al. (2007); Castillo et al. (1998) who reported that 2,4-D and NAA can be used to enhance callus induction and maintenance at 3 mg/l as a growth regulator in MS media. It was also observed that media combination without Zeatin turned brown after prolonged incubation period.
The media combination with Zeatin, turned green but took longer time (> 60 days) to initiate shoots from the callus. For Rutuku, the best callusing rate was obtained from internodes (85 - 100%) compared to leaf segments (9 - 73.3%) while for Victoria internode callus rate ranged from 13 - 30% and for leaves 3 - 55%. In terms of texture, all the calli formed from leaf and internodes were friable and soft with a light to cream colour (Table 2). Comparing the two types of explants, internodes of Rutuku gave a very good response to callus formation with callusing rate of 100% in M2 compared to other media compositions (M3, M4 and M5) (Table 2). Victoria also formed callus but the degree of callus formation was poor compared to that of Rutuku for both explants and in all media compositions (M1 - M5). The callus colour was gradually modified to light brown as it stayed on the media while on transfer to shooting media the colour (50%) of the callus changed to light green or light brown (Figure 1). The result of the study shows that hormone combination of auxins [2, 4-D (2 mg/l) + NAA (2 mg/l) + KN (2 mg/l) is the most effective growth regulator for callus initiation in potato explants on Rutuku and Victoria (95/80%) compared to media combinations under M3, M4 and M5.

Effects of different phytohormones on shoot initiation of two potato varieties
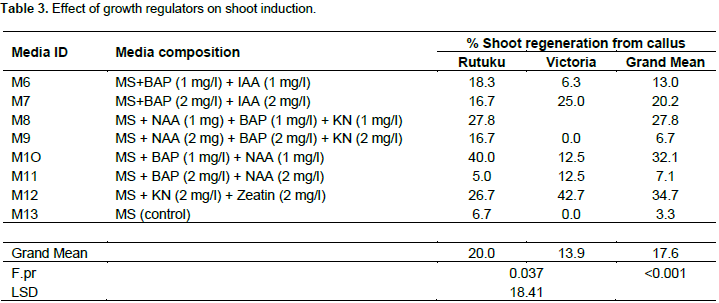
Seven (7) weeks old calli of Rutuku and Victoria developed from the best callus media (Table 1) were transferred to regeneration media for development of the shoots. The two potato cultivars showed varying responses in shoot induction on different media composition. BAP + NAA at 3 and 5 mg/l caused calli from both varieties to grow in size with some calli developing shoots (Figure 2) while in some cases, no regeneration was observed even after 50 days on culture as shown in Figure 1. In all these cases, intervening callus phase resulted in initiation of very few numbers of shoots from the callus and where shoot induction occurred, there was a prolonged period of incubation. Based on this study, it was found out that use of NAA in combination with BAP is good for maintenance of the callus and callus growth at 3 mg/l. The results of the study shows that the callus from Victoria grew much bigger in size compared to Rutuku (Figure 2) and this was attributed to difference in the genotype of the two varieties. Application of MS + BAP (2 mg/l) + IAA (2 mg/l) at 2 mg/l and 1 mg/l each resulted in the calli developing roots instead of shoot for both Rutuku and Victoria.
The calli became light brown in colour and the developed roots became hairy and elongated with size over 3 cm due to the action of these hormones.This implies that combination of Zeatin and BAP should be applied after the shoots have developed and not before shoot development as its action suppresses shoot development when applied at callus stage. In all these cases, intervening callus phase resulted in initiation of very few number of shoots from the callus. Where shoot induction occurred from callus, there was a prolonged period of incubation and this could result in undesirable somaclonal variations (Table 3). The best media combination was noted as M12 with 34% shoot regeneration. This media component contained Zeatin riboside that is good for controlling the development of highly organogenic micro calli and supports both organogenesis and somatic embryogeneis. This result is supported by the report of Beaujean et al. (1998) and Ghosh et al. (2015) that noted that Zeatin when applied at 0.8 mg/l stimulates shoot development. However, in this case, the optimum rate of application was observed at 2 mg/l in combination with Kinetin at 2 mg/l.
A reliable regeneration system has been established for two potato cultivars ‘Victoria and ‘Rutuku’ This regeneration system can be applied in the genetic improvement targeting the most important diseases mainly late blight and bacterial wilt that are heavily ravaging the potatoes in Uganda. This study also shows that other potato varieties can be regenerated by optimization of the media components especially phytohormone concentration to enable regeneration and transformation.
The authors have not declared any conflict of interests.
REFERENCES
|
Abbott AJ, Belcher AR (1986). Potato tuber formation in vitro. In: Withers LA & Alderson PG (eds) Plant Tissue Culture and its Agricultural Applications. London: Butterworths. pp. 113-121.
Crossref
|
|
|
|
Abong GO, Okoth MW, Karuri EG, Kabira, JN, Mathooko FM (2009). Nutrient contents of raw and processed products from Kenyan potato cultivars. J. Appl. Biosci. 16:877-886.
|
|
|
|
|
Aheisibwe, AR, Barekye A, Namugga P, Byarugaba AA (2015). Challenges and opportunities for quality seed potato availability and production in Uganda. Uganda J. Agric. Sci. 16(2):149-159.
|
|
|
|
|
Andersson MA, Trifonova AB, Andersson M, Johansson LB, Hofvander P (2003). A novel selection system for potato transformation using a mutated AHAS gene. Plant Cell Rep. 22:261-267
Crossref
|
|
|
|
|
Beaujean A, Sangwan RS, Lecardonnel A Sangwan-Norreel BS (1998). Agrobacterium-mediated transformation of three economically important potato cultivars using sliced internodal explants: an efficient protocol of transformation. J. Exp. Bot. 49(326):1589-1595.
Crossref
|
|
|
|
|
Brar DS, Jain SM (1998). Somaclonal variation: mechanism and applications in crop improvement. In. Somaclonal variation and induced mutations in crop improvement. Springer Netherlands. pp. 15-37
Crossref
|
|
|
|
|
Byarugaba, AA, John K, Aheisibwe RA, Deo T, Barekye A. (2017). Bridging the gap in quality and quantity of seed potatoes through farmer managed screen houses in Uganda. Afr. J. Plant Sci. 11(2):30-37.
Crossref
|
|
|
|
|
Cardi T, Iannamico V, D'Ambrosio F Filippone E, Lurquin PF (1993). In vitro regeneration and cytological characterization of shoots from leaf explants of three accessions of Solanum commersonii. Plant Cell, Tissue Organ Cult. 34(1):107-114.
Crossref
|
|
|
|
|
Castillo AM, Egana B, Sanz JM, Cistue L (1998). Somatic embryogenesis and plant regeneration from barley cultivars grown in Spain. Plant Cell Rep. 17(11):902-906.
Crossref
|
|
|
|
|
Chang MM, David CJ, Lee HA (2002). Agrobacterium mediated co transformation of pea P-1,3-glucanase and chitinase genes in potato (Solanurn tuberosum L,c.v. Russet Burbank) using a single selectable marker. Plant Sci. 163(1):83-89.
Crossref
|
|
|
|
|
Coleman M, Waugh R, Powell W (1990). Genetical analysis of in vitro cell and tissue culture response in potato. Plant Cell Tissue Organ Cult. 23(3):181-186.
|
|
|
|
|
Elaleem KGA, Modawi RS, Khalafalla MM (2009). Effect of plant growth regulators on callus induction and plant regeneration in tuber segment culture of potato (Solanum tuberosum L.) cultivar Diamant. Afr. J. Biotechnol. 8(11):2529-2534.
|
|
|
|
|
FAOSTAT-Food and Agriculture Organization of the United Nations (2014). http://www.fao.org/faostat/en/#data/QC
|
|
|
|
|
Fleming ML, DE Maine MJ, Powell W (1992). Ploidy doubling by callus culture of potato dihaploid leaf explants and the variation in regenerated plants. Ann. Appl. Biol. 121(1):183-188.
Crossref
|
|
|
|
|
Ghosh S, Majumdar S, Sarkar D, Datta K (2015). An efficient adventitious shoot regeneration system for potato (Solanum tuberosum. J. Plant Biochem. Biotechnol. 24(3):298-304.
Crossref
|
|
|
|
|
Hoque ME, Ali MS, Karim NH (2007). Embryogenic callus induction and regeneration of elite Bangladeshi Indica rice cultivars. Plant Tissue Cult. Biotechnol. 17(1):65-70.
|
|
|
|
|
Hussain IQ, Muhammad BAL, Chaudhry AI, Naqvi Z, Rashid H (2005). Morphogenic potential of three potato (Solanum tuberosum) cultivars from diverse explants, a prerequisite in genetic manipulation. Pak. J. Bot. 37(4):889.
|
|
|
|
|
JayaSree T, Pavan U, Ramesh M, Rao AV, Jagan MK, Sadanandam A (2001). Somatic embryogenesis from leaf cultures of potato. Plant Cell Tissue Organ Cult. 64(1):13-17.
Crossref
|
|
|
|
|
Kumar A, Kumar VA. (1996). Plant Biotechnology and Tissue Culture Principles and Perspectives, International Book Distributing Co, Lucknow.
|
|
|
|
|
Lemaga B, Kanzikwera R, Kakuhenzire R, Hakiza JJ, Manzi G (2001). The effect of crop rotation on bacterial wilt incidence and potato tuber yield. Afr. Crop Sci. J. 9(1):257-266.
Crossref
|
|
|
|
|
Olanya OM, Adipala E, Hakiza JJ, Kedera JC, Ojiambo P, Mukalazi JM, Nelson R (2001). Epidemiology and population dynamics of Phytophthora infestans in Sub-Saharan Africa: progress and constraints. Afr. Crop Sci. J. 9(1):185-193.
Crossref
|
|
|
|
|
Osusky M, Osuska L, William K, Misra S (2005). Genetic modificalion of potato against microbial diseases: In vitro and in planta activity of a dermaseptin B1 derivative, MsrA2. Theor. Appl. Genet. 111:711-722.
Crossref
|
|
|
|
|
Ritchie SW, Hodges TK (1993). Cell culture and regeneration of transgenic plants. Transgenic plants 1:147-178.
|
|
|
|
|
Sarker RH, Mustafa BM (2002). Regeneration and Agrobacterium-mediated genetic transformation of two indigenous potato varieties of Bangladesh. Plant Tissue Cult. 12(1):69-77.
|
|
|
|
|
Sharma SK, Millam S, Hein I, Bryan GJ (2008). Cloning and molecular characterisation of a potato SERK gene transcriptionally induced during initiation of somatic embryogenesis. Planta 228(2):319.
Crossref
|
|
|
|
|
Shirin F, Hossain, M, Kabir MF, Roy M Sarker SR (2007). Callus induction and plant regeneration from internodal and leaf explants of four potato (Solanum tuberosum L.) cultivars. World J. Agric. Sci. 3(1):1-6.
|
|
|
|
|
Sree-Ramulu K, Dijkhuis P, Roest S (1983). Phenotypic variation and ploidy level of plants regenerated from protoplasts of tetraploid potato (Solanum tuberosum L. cv.'Bintje'). Theor. Appl. Genet. 65(4):329-338.
Crossref
|
|
|
|
|
Trujillo C, Rodriguez-Arango E, Jaramillo S, Hoyos R, Orduz S, Arango R (2001). One-step transformation of two Andean potato cultivars (Solanum tuberosum L. subsp. andigena). Plant Cell Rep. 20(7):637-641.
|
|
|
|
|
Vasil I (2012). Plant regeneration and genetic variability (Vol. 3). Elsevier.
|
|
|
|
|
Vasquez JN, Clarence AR (2002). 'The systemin precursor gene regulates both defensive and developmental genes in Solarmm tuberosum Proc. Natl. Acad. Sci. USA. 99(24):15818-15821.
Crossref
|
|
|
|
|
Véronneau H, Lavoie G, Cappadocia M (1992). Genetic analysis of anther and leaf disc culture in two clones of Solanum chacoense Bitt and their reciprocal hybrids. Plant Cell, Tissue Organ Cult. 30(3):199-209.
Crossref
|
|
|
|
|
Yee S, Stevens B, Coleman S, Seabrook JE, Li XQ (2001). High-efficiency regneration in vitro from potato petioles with intact leaflets. Am. J. Potato Res. 78(2):151-157.
Crossref
|
|