ABSTRACT
The influence of boiling lima bean, using trona solution, on its phytochemical constituents and selected micronutrients was investigated. Lima bean was initially boiled for 3 h using 0.1, 0.2, 0.3, 0.4 and 0.5% trona solution, respectively at ratio 1:5 (bean/trona solution). The boiled lima bean was subsequently dried in an oven at 55°C for 12 h after which it was subjected to various analyses. Maximum reduction of phytochemicals such as trypsin inhibitor activity (74.1%), haemagglutinin (98%), tannin (68.6%), phytic acid (39.6%), and cyanogenic glycoside (73.8%) was obtained at 0.5% boiling with trona-solution. The reduction profile for the B-vitamins includes thiamine (0.58 to 0.13 mg/100 g), riboflavin (0.37 to 0.21 mg/100 g), niacin (0.97 to 0.28 mg/100 g) and pyridoxine (0.41 to 0.11 mg/100 g). Boiling lima bean in trona solution led to significant decrease (p<0.05) in the concentration of calcium, magnesium and phosphorus while mineral concentration enhancement was observed with potassium, sodium and iron. Maximum in vitro protein and starch digestibilities of 88.6% and 51.9 mg/g, respectively were obtained for 0.5% trona solution boiling of lima bean. Trona usage in the boiling of lima bean therefore served as a beneficial step in the utilization of the legume.
Key words: Lima bean, trona, boiling, phytochemicals, micronutrients.
Leguminous seeds such as soybean, faba bean, pea, mung bean, cowpea, kidney bean, pigeon bean and lima bean constitute an important and inexpensive dietary source for many people particularly in the developing countries (Bello-Perez et al., 2007; Al-Abdalall, 2010). They are regarded as principal sources of macronutrients, micronutrients and phytochemicals containing protein, carbohydrate, vitamins, minerals and polyphenols among others (Zhao et al., 2014). The leguminous seeds are largely utilized as valuable ingredients of diverse food products for human consumption as well as for animal feed (Du et al., 2014).
Lima bean (Phaseolus lunatus) is regarded as one of the under-utilized legumes which can be grown in many parts of the world including Latin America countries, the United States, Canada, African and Asian countries (Bello-Perez et al., 2007). Lima bean contains a class of chemicals called phytochemicals, the term generally used to describe chemicals from plants that may affect health, but are not essential nutrients (El-Gharras, 2009). Phytochemicals are essentially plant secondary metabolites presents in all plant tissues and their primary role is to protect plants from insects, ultraviolet radiation, and microbial infections and to attract pollinators (Del-Rio et al., 2013). Examples of these phytochemicals are trypsin and chymotrypsin inhibitors, haemagglutinins, cyanogenic glycosides, tannin and phytic acid, which are collectively known as anti-nutrients (Adeniran et al., 2013). These anti-nutritional factors, through their metabolism, interfere with nutrient intake, absorption, utilization and availability in the body systems thereby affecting the health of consumers (Ezeagu and Ibegbu, 2010).
The utilization of lima bean essentially lies in culinary and medicinal uses. These include its uses in soup preparation, making of sandwich filling and salad preparation among others (Sarkar, 2012). Lima bean has been used as an alternative raw material for the production of ‘daddawa’, a Nigerian condiment (Adeniran et al., 2013). This grain legume has also been recognized to reduce low-density lipoprotein cholesterol thereby leading to the reduction of risk of ischemic heart disease and diabetes (Messina, 2014). Most of the utilization of lima bean fundamentally involves cooking of the grain legume for it to get softened. However, the long cooking duration with concomitant high energy usage is a major drawback and discouragement to lima bean usage. The hard-to-cook characteristic of the grain legume is due to its hard cotyledon which naturally predisposes it to such cooking time elongation (Mamiro et al., 2011).
A lot of attempts have been made by researchers to increase the utilization of lima bean using a wide range of appropriate processing techniques. The elimination methods of the anti-nutritional factors in lima bean had been suggested and these include soaking, autoclaving and toasting (Adeparusi, 2001), boiling (Aremu et al., 2016), heat treatment and fermentation (Adeniran et al., 2013) among others. In dealing with hard-to-cook phenomenon peculiar to lima bean, alkaline cooking with calcium bicarbonate [Ca(HCO3)2] and a crude rock salt of carbonates (trona), chemically called sodium sesquicarbonate (Na2CO3.NaHCO3.2H2O) are the traditional methods of alleviating this problem in most African households (Mamiro et al., 2011; Olapade and Umeonuorah, 2014). The use of ‘trona’ for alkaline thermal treatment brings about softness of the bean and reduces the time of boiling which may also have effect on the chemical components of the bean. The process of softening of leguminous seed, during boiling, has been attributed to the disintegration of the cotyledonous tissue in individual cells. According to Belitz et al. (2009), the disintegration of the cotyledonous tissue is usually caused by the conversion of native protopectin to pectin, which quickly depolymerizes on heating. The middle lamella of the cell walls, which consists of pectin and strengthens the tissues, disintegrates in this process.
The objective of this study, therefore, was to investigate the effect of trona-aided boiling on the phytochemical composition and beneficial micronutrients of lima bean (P. lunatus).
Raw lima bean of the red variety was purchased from Odo-Oba market while trona (Na2CO3.NaHCO3.2H2O; sodium sesquicarbonate) was purchased from Sabo market in Ogbomoso, Oyo State, Nigeria.
Cooking of lima bean
Cooking in distilled water
Five hundred grams of lima bean seeds were added to boiling distilled water at ratio 1:5 (bean/distilled water, weight/volume) at 100°C and cooked on a kerosene stove for 3 h. Water was drained off and the seeds were dried in an oven at 55°C for 12 h. The dried seeds were later milled using a hammer mill and sieved to pass through a sieve with a 250 µm mesh size. The sieved flour samples were stored in air tight plastic container for subsequent use.
Cooking in trona solution
Trona was ground into a fine powder and dried at 70°C overnight in an oven. The dried powder was then cooled in a desiccator and kept in a stoppered glass bottle at room temperature (30±2°C). Five hundred grams of lima bean seeds were then boiled in trona solutions at different concentrations of 0.1, 0.2, 0.3, 0.4 and 0.5%, respectively at ratio 1:5 (bean/trona solution; weight/volume) at 100°C for 3 h using a kerosene stove. Thereafter, the boiled seeds were dried, milled and stored as described when boiled in distilled water. The concentrations of trona were so chosen to simulate what is obtainable with traditional processing procedures where very low trona concentration (usually <1%; trona/food material) is commonly used to avoid darkening of cooked product.
Determination of trypsin inhibitor activity
The method of Kakade et al. (1974) was used to determine the trypsin inhibitor activity (TIA) of lima bean flour sample using benzoyl-DL-arginine-p-nitroanilide (BAPNA) as substrate. A 4.0 g sample was treated with 40 ml of 0.05 M sodium phosphate buffer (pH 7.5) and 40 ml of distilled water. After series of chemical manipulations, the absorbance of end-point solution was read at 410 nm wavelength in a spectrophotometer (UV-160A, Shimadzu, Osaka, Japan). Blank sample was treated similarly through the entire determination. Results were expressed as trypsin inhibitor units (TIU). One TIU was defined as an increase of 0.01 in absorbance units under conditions of assay. Trypsin inhibitory activity was defined as the number of TIU.
Determination of tannin content
Tannin was determined according to the method of Price and Butler (1977). Sixty milligrams of ground sample was shaken manually for 1 min in 3.0 ml methanol. The mixture was filtered followed by mixing the filtrate with 50 ml distilled water and analyzed within an hour. About 3 ml of 0.1 M FeCl3 in 0.1 M HCl was added to 1 ml filtrate, followed immediately by the addition of 3 ml freshly prepared K3Fe(CN)6. The absorbance was read on a spectrophotometer (Shimadzu UV-1700, Tokyo, Japan) at 720 nm after 10 min from the addition of 3 ml of 0.1 M FeCl3 and 3 ml of 0.008 M K3Fe(CN)6. Similar treatments were also carried on the blank. Results were expressed as tannic acid equivalent (mg/100 g sample), calculated from a calibration curve using tannic acid.
Determination of haemagglutinin concentration
Haemagglutinin level of the samples was determined by the method of Arntified et al. (1985). Two grams of each sample was weighed and 50 ml of the solvent of mixture of isobutyl alcohol and trichloroacetic acid was added and allowed to shake on a shaker for 6 h to extract the haemagglutinin. The mixture was then filtered through a double layer filter paper and maintained in a water bath for 2 h at 80°C and the filtrate was allowed to cool. A set of standard solutions of haemagglutinin ranging from 0 to 10 ppm was prepared from the haemagglutinin stock solution. The absorbance of the standard solution as well as that of the filtrate was read at 220 nm on a spectrophotometer (UV-160A, Shimadzu, Osaka, Japan). The result was expressed as haemagglutinin unit (HU)/g sample.
Determination of cyanogenic glycoside content
Determination of cyanogenic glycoside content of the samples was done by the alkaline titration method of the AOAC (1990). Two hundred millilitres of distilled water was added to 1 g of the sample in an 800 cm3 capacity distillation flask. The flask was fitted for distillation and allowed to stand for 2 h for autolysis to take place. An antifoaming agent (silicon oil) was then added. Steam distillation was carried out and 150 cm3 of the distillate collected into 250 cm3 capacity conical flask containing 20 cm3 of 2.5% sodium hydroxide, then diluted to mark with distilled water. Thereafter, 8 cm3 of 6 N NH4OH solution and 2 cm3 of 5% potassium iodide was added to 100 cm3 of diluted distillate. This was titrated against 0.02 N silver nitrate (AgNO3) solution using a 10 cm3 microburette. The end-point was noted as a permanent turbidity against a black background. Titre values were obtained and used to calculate cyanide content (mg HCN/Kg), using the formula:
CG = (TV × 1.08 × EV / SM × AL) × 100 (1)
Where, CG is cyanogenic glycoside; TV is titre value (cm3); EV is extract volume (cm3); SM is sample mass (g); AL is aliquot (cm3) used; and 1 cm3 of 0.02 N AgNO3 = 1.8 mg HCN.
Determination of phytic acid content
The method of Wheeler and Ferrel (1971) was used to determine the phytic acid content of each sample. A 2 g sample was used for the extraction. A standard curve was prepared expressing the results as Fe(NO3)3 equivalent. Phytate phosphorus was calculated from the standard curve assuming a 4:6 iron to phosphorus molar ratio. The phytic acid content was also calculated by multiplying the amount of phytate phosphorous by the factor 3.55 based on the empirical formula C6P6O2H18 and result was expressed as mg/100 g sample.
Mineral content determination
The mineral content was determined according to AOAC (1995). Two grams of lima bean flour was ashed in a muffle furnace at 550°C. The resultant ash was dissolved in 5 ml of HNO3/HCl/H2O (1:2:3, v/v/v) followed by heating on a hot plate at the boiling temperature of the solution until brown fumes disappeared. Five millilitres of deionized water was later added to the remaining content in the crucible, and the mixture was heated until a colourless solution was obtained. The colourless solution was then filtered into a 100 ml volumetric flask using a Whatman No. 42 filter paper, and diluted to volume with deionized water. The concentration of the following elements: Ca, Mg and Fe was then determined from the filtered solution using atomic absorption spectrophotometer (Model SP9 Pye Unicam, UK), having initially prepared a standard curve for each element under investigation. The concentration of each element was calculated as mg/100 g of sample.
The analysis of sodium and potassium concentrations of the sample was carried out using flame photometry while the phosphorous content of the filtered solution was determined colorimetrically using vanadate-molybdate reagent according to the method described by Egan et al. (1981). The content of phosphorus in the filtered solution was determined using a standard curve obtained for potassium dihydrogen phosphate (KH2PO4) and expressed as mg phosphorus per 100 g of sample.
Thiamine determination
The method of AOAC (1995) was used for the determination. Five grams of lima bean flour was weighed into a 250 ml conical flask and 100 ml 0.1 N H2SO4 was slowly added without shaking and left overnight. The mixture was thereafter filtered through Whatman No. 2 filter paper and the first 10 to 15 ml of filtrate was discarded. Ten millilitres of the extract was then pipetted into 100 ml separating funnel. Three millilitres of 15% NaOH was then added into the separating funnel immediately followed by four drops of ferricyanide solution and then shaken gently for exactly 30 s. Fifteen millilitres of isobutanol was rapidly added, followed by vigorous shaken for 60 s to allow the layers to separate and the bottom layer drained off and discarded. One spatulaful of sodium sulphate was directly added into the separating funnel and then swirled gently to clarify the extract. The clear extract was then collected using a Pasteur pipette into a clean dry test tube. The blank sample was similarly prepared by pipetting 10 ml of the extract following the procedures above but with the omission of ferricyanide solution. The thiamine content was estimated using a fluorimeter and results are expressed as mg/100 g sample.
Riboflavin determination
The method of AOAC (1995) was used for the determination. Five grams of lima bean flour was weighed into a 250 ml conical flask and 75 ml 0.1 N H2SO4 was added with the flask immersed in boiling water for 30 min and the flask shaken every 5 min. This was allowed to cool at room temperature. Five millilitres of 2.5 M sodium acetate solution was added and allowed to stand for at least 1 h. The solution was then transferred to a 100 ml volumetric flask, made up to the volume with distilled water and filtered through Whatman No. 2 while the first 10 to 15 ml of filtrate was discarded. The filtrate was then oxidized with potassium permanganate with elimination of its excess with peroxide. The riboflavin content was estimated by interpreting the spectrofluorometry and results were expressed as mg/100 g sample.
Niacin determination
An approximately 0.1 ml of the standard niacin solution was pipetted into two test tubes, respectively. Each of the test tubes was made up to 6 ml with distilled water. Thereafter, 3 ml of cyanogen bromide was added and the content shaken. After 10 min, 1 ml of 4% aniline was added to each tube and the yellow colour developed and after 5 min was read at 420 nm (Spectrophotometer; UV-160A, Shimadzu, Osaka, Japan) against a reagent blank (AOAC, 1995).
Pyridoxine determination
The reversed-phase HPLC method described by Ekinci and Kadakal (2005) was used. The sample treatment consisted of solid phase extraction (SPE) with Sep-Pak C18 (500 mg) cartridges that enabled separation of pyridoxine and removed most of the interfering components. Twenty millilitres of distilled water was added to 5 g of the sample. The mixture was homogenized using a homogenizer at 2000 rpm for 1 min. The homogenized sample was centrifuged for 10 min at 14000 × g with a centrifuge (model H–2000C, Japan). The stationary phase was prepared by flushing the Sep-Pak C18 (500 mg) cartridge with 10 ml methanol and 10 ml acidified water (pH 4.2) to activate the column. The homogenized and centrifuged sample was then loaded on the Sep-PaK C18 (500 mg) cartridge at a flow rate of 1 ml min-1 using a syringe. The eluent was collected in a bottle and evaporated to dryness in a vacuum evaporator (model Rotavapor R-3000, Bucht, Switzerland). The residue was dissolved in mobile phase (0.1 mol/L KH2PO4 (pH 7.0): methanol; 90: 10) and then filtered through 0.45 μm pore size filters. Twenty microlitres of the sample was injected into the HPLC column. The column elute was monitored with a photodiode-array detector at 324 nm for pyridoxine. Identification of compound was achieved by comparing their retention times and UV spectra with those of standards. Five different concentrations of each standard were used to prepare calibration plots for pyridoxine. This was done by plotting concentration (μg/ml) against peak area (mAU). Their correlation coefficients were greater than 0.997. Concentration of pyridoxine was calculated from integrated areas of the sample and the corresponding standards.
Determination of in vitro starch digestibility
The enzymatic method of Kumar and Venkataraman (1976) was adopted by using enzymatic glucose oxidase peroxidase kit. Glucose was used as a standard and the degree of hydrolysis was expressed as mg of glucose liberated from the samples after correction for blank values and percent in vitro starch digestibility was calculated on the basis of total starch content using the following equation:
IVSD = (Glucose released (mg) × 0.9 / g of total starch) × 100 (2)
Determination of in vitro protein digestibility
The in vitro protein digestibility (IVPD) of lima bean flour samples was measured according to the multienzyme technique (Hsu et al., 1977) and was calculated by using the following equation:
y = 210.464 – 18.103x (3)
Where, y = the percentage of protein digestibility and x = the pH of the protein suspension after 10 min digestion with a four-enzyme solution.
Statistical analysis
All determinations in this study were done in triplicates. In each case, a mean value and standard deviation were determined. Analysis of variance (ANOVA) was also performed and separation of the mean values was done by Duncan’s Multiple Range Test at p<0.05 using Statistical Package for Social Scientists (SPSS) software, version 16.0.
Effect of trona-aided boiling on some phytochemicals of lima bean
Table 1 shows the results of the effect of trona-aided boiling on some phytochemicals of lima bean. It was generally observed that boiling (with or without trona) caused reduction in the concentration of phytochemicals in lima bean. However, there were variations in the reduction capacity of different trona concentrations used for boiling. The reduction profile of the phytochemicals from raw lima bean to 0.5% trona-boiling was 29.3 to 7.6 TIU/g (trypsin inhibitor), 61.4 to 1.3 HU/g (haemagglutinin), 22.8 to 7.2 mg/Kg (tannin), 860.4 to 519.8 mg/100 g (phytic acid) and 46.1 to 12.1 mg HCN/kg (cyanogenic glycoside). The reduction in trypsin inhibitor activity can be attributed to the destruction of disulphide bonds which normally guarantee the heat stability of the inhibitor. Therefore, the application of thermal treatment might have destroyed these bonds while boiling in trona solution facilitated further destruction of the bonds and hence the denaturation of the inhibitor (Kalpanadevi and Mohan, 2013). Greater destruction of the disulphide bonds seemed to occur with higher concentration of the trona solution involved in the boiling.
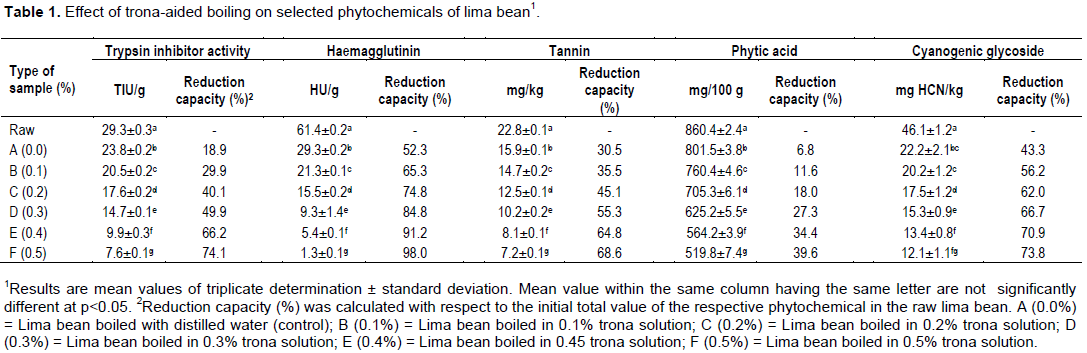
The destruction of haemagglutinin in lima bean was as high as 98% in 0.5% trona-solution boiling. An earlier observation had stated that haemagglutinin could be inactivated at temperature exceeding 50°C (Damang et al., 2017), while it readily dissociates by a change of pH or ionic strength (Belitz et al., 2009). The reduction in tannin concentration by boiling in ordinary distilled water was 30.5%, while that in 0.5% trona-solution boiling was 68.6%. This reduction could be attributed to the solubility property of tannin in water (Ezeocha et al., 2012) while boiling at high temperature could lead to degradation of the phytochemical (Rakic et al., 2007; Udensi et al., 2007). The involvement of trona in the boiling essentially facilitated further destruction of the tannin.
The role of trona boiling on the phytic acid content was relatively low giving a maximum reduction at 39.6 by0.5% trona-solution boiling. The factors that pre-disposed phytic acid in lima bean to destruction include its leaching tendency into the surrounding solution (Onwuka, 2006) coupled with its heat-labile nature at elevated temperature (Udensi et al., 2007). The reduction in the cyanogenic glycoside concentration in lima bean boiling was also relatively high. Boiling in ordinary distilled water gave 43.3% reduction level while that in 0.5% trona-solution boiling gave about 73.8%. The use of trona solution might have acted the role of complementarity to the heat treatment in the reduction of the cyanogens. Heat treatment had earlier been observed to contribute substantially to the elimination of cyanogenic glycosides in cassava processing for such products as ‘akyeke’ (Obilie et al., 2004), ‘garri’ (Agbor-Egbe and Lape-Mbome, 2006) and cassava leaves (Ngudi et al., 2003).
The reduction of phytochemicals as a function of the concentration of trona may be explained as greater impacts (through destruction, dissociation, etc.) being inflicted on the compounds at higher trona concentrations.
Effect of trona-aided boiling on selected vitamin contents of lima bean
Figure 1 shows the selected vitamin contents of lima bean as influenced by trona-aided boiling. The vitamin concentrations were generally decreased with an increase in the trona concentration of the boiling solution. The initial contents of thiamine, riboflavin, niacin and pyridoxine in the raw lima bean were 0.58, 0.37, 0.97 and 0.41 mg/100 g, respectively while at 0.5% trona-boiling, these contents were reduced to 0.13, 0.21, 0.28 and 0.11 mg/100 g, respectively. The reduction in thiamine content may be attributed to its instability in trona solution, which is naturally alkaline. An earlier observation had stated that thiamine stability in alkaline solution is relatively low and is usually influenced by pH, temperature, ionic strength and metallic ions (Gregory, 2008). In the case of riboflavin, its reduction may be attributed to its water solubility property, but relatively stable under normal high temperature food processing conditions (Golbach et al., 2014). The leaching tendency of niacin into the boiling water could be responsible for its reduction level as observed in lima bean processing with trona solution. The involvement of trona in the boiling at high temperature might have contributed substantially to the leaching of niacin. Belitz et al. (2009) had observed that niacin normally occurs in food as nicotinic acid which is quite stable with moderate losses in food processing such as vegetable blanching.
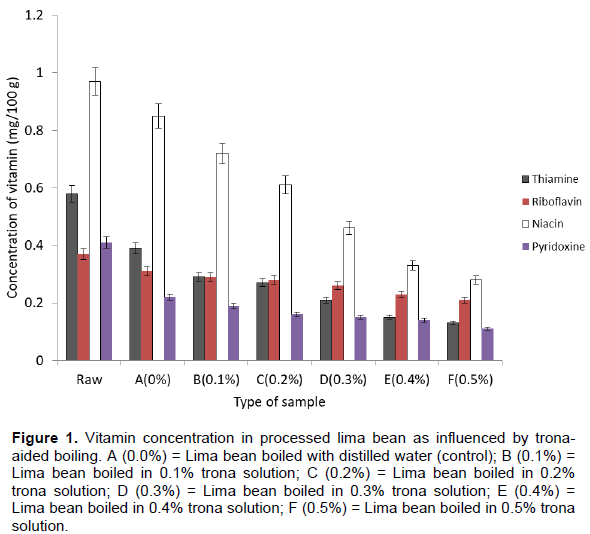
The reduction in the pyridoxine content of food during processing is usually regarded as a complex phenomenon through such factors as elevated temperature and its possible reaction with an amino acid, cysteine (Bui and Small, 2012). The presence of trona during boiling might have contributed to the liberation of the amino acid during the softening process of lima bean seed, thereby facilitating such pyridoxine-cysteine interaction.
Influence of trona-aided boiling on selected mineral contents of lima bean
The effect of trona-aided boiling on selected mineral contents of lima bean is shown in Table 2. The use of ordinary distilled water in the boiling of lima bean generally led to the decrease in the mineral concentration. However, the involvement of trona solution in the boiling caused further decrease in calcium, magnesium and phosphorus while it caused an increase in the concentration of potassium, sodium and iron. The reduction profile of calcium, magnesium and phosphorus was 331.7 to 312.3, 105.1 to 91.7, and 530.1 to 502.3 mg/100 g, respectively while the increment profile of potassium, sodium and iron was 1620.7 to 1642.9, 8.5 to 15.5 and 4.3 to 13.2 mg/100 g, respectively. Minerals generally had been observed to be heat-stable under normal processing conditions (Rickman et al., 2007), but considerable losses could occur through leaching into the cooking water (Mugendi et al., 2010). The increase in the concentration of potassium, sodium and iron could be attributed to their uptake from the trona solution due to possible ionic dissociation at elevated temperature of boiling (Goyal, 2000).
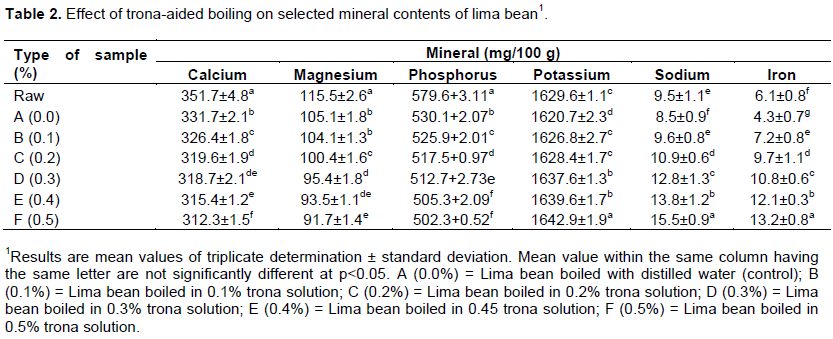
From nutritional standpoint, certain minerals have been recognized to play significant roles in the maintenance of optimal health conditions in humans (Lachance, 1998). Calcium is considered as an essential mineral for human health participating in the biological functions of several tissues such as musculoskeletal, nervous and cardiac systems, bones and teeth, and parathyroid glands (Morgan, 2008; Williams, 2008). Magnesium has been linked to energy metabolism, release of neurotransmitters and endothelial cell functions (Bo and Pisu, 2008) among others. Phosphorus is related to bone and teeth formation and the majority of the metabolic actions in the body including kidney functioning, cell growth and the contraction of the heart muscle (Renkema et al., 2008). Potassium has been implicated for its role in the transmittance of nerve impulses coupled with its relation to heart muscle contraction activity (Lambert et al., 2008). The role of sodium in human physiology is essentially related to the maintenance of the balance of physiological fluids such as in blood pressure, kidney function, nerve and muscle functions (Sobotka et al., 2008). The principal function of iron is connected with the synthesis of haemoglobin and myoglobin coupled with its complementary role of energy production (Huskisson et al., 2007; Shenkin, 2008). Therefore, the loss or gain of some minerals during trona-aided boiling of lima bean might have implications on their beneficial effects when the cooked legume is consumed.
In vitro protein and starch digestibility of lima bean as influenced by trona-aided boiling
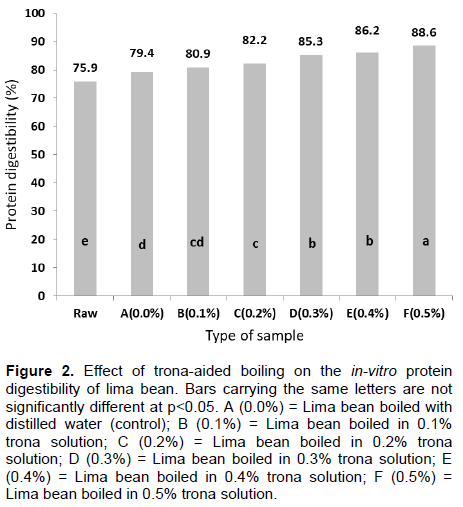
The in vitro protein digestibility of lima bean boiled in trona solution is shown in Figure 2. Boiling of lima beans in distilled water was observed to increase its digestibility from 75.9 to 79.4% while the involvement with trona caused further increase in digestibility to 88.6% in 0.5% trona-solution boiling. The increase in protein digestibility could generally be attributed to the reduction in inhibitory activity of enzymes and protein-complexing reactions (Embaby, 2010; Pushparaj and Urooj, 2011). The implication of this occurrence is that the reduction in the level of trypsin inhibitor activity (Kalpanadevi and Mohan, 2013) and tannin (Ezeocha et al., 2012) could enhance protein digestibility. Similarly, reduction in the level of phytate, which is capable of forming phytate-protein complex,could also contribute to improved protein digestibility (Selle et al., 2012).
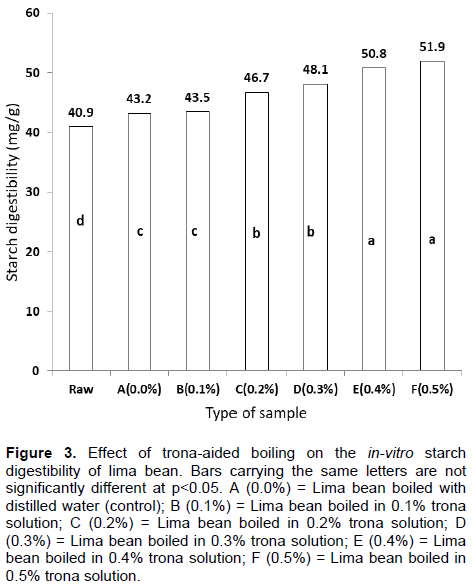
The in vitro starch digestibility of lima bean as influenced by trona-aided boiling is as shown in Figure 3. Boiling of lima bean in ordinary distilled water increased starch digestibility from 40.9 to 43.2 mg/g. However, the involvement of trona in the boiling increased starch digestibility to 51.9 mg/g in 0.5% trona-solution boiling. The role of trona in the softening of lima bean had been attributed to the disintegration of the cotyledonous tissue in individual cells. This is caused by the conversion of native protopectin to pectin, which depolymerizes quickly at elevated temperature (Belitz et al., 2009). Therefore, the disintegration of the cotyledonous tissue had, most probably, made starch substrate more accessible to enzymes thereby leading to higher starch digestibility (Ezeogu et al., 2005). The reduction in the phytic acid level could also play a role in enhancing starch digestibility. Singh et al. (2010) had earlier observed that calcium could catalyze amylase activity while phytic acid could form phytic acid-calcium complex. Thus, the reduction in phytic acid level, as a result of trona boiling, will minimize complex formation and hence availability of calcium for effective amylase activity and by extension, an enhanced starch digestibility.
The use of trona in the boiling of hard-to-cook lima bean was highly beneficial. The beneficial effects include the reduction in the phytochemicals traditionally known to interfere in the utilization of protein and minerals, the enhancement of both protein and starch digestibility, and the uptake of certain minerals (potassium, sodium and iron) in the course of boiling. However, marginal losses of certain minerals (calcium, magnesium and phosphorus) were observed while substantial losses of B-vitamins could occur at higher trona concentration of boiling.
The authors have not declared any conflict of interests.
REFERENCES
|
Adeniran HA, Farinde EO, Obatolu VA (2013). Effect of heat treatment and fermentation on anti-nutrients content of lima bean (Phaseolus lunatus) during production of daddawa analogue. Annu. Rev. Res. Biol. 3(3):256-266.
|
|
|
|
Adeparusi EO (2001). Effect of processing on the nutrients and anti-nutrients of lima bean (Phaseolus lunatus L.) flour. Mol. Nutr. Food Res. 45(2):94-96.
Crossref
|
|
|
|
|
Agbor-Egbe T, Lape-Mbome I (2006). The effects of processing techniques in reducing cyanogen levels during the production of some Cameroonian cassava foods. J. Food Comp. Anal. 19:354-363.
Crossref
|
|
|
|
|
Al-Abdalall AHA (2010). Pathogenicity of fungi associated with leguminous seeds in the Eastern kingdom of Saudi Arabia. Afr. J. Agric. Res. 5(10):1117-1126.
|
|
|
|
|
Association of Official Analytical Chemists (AOAC) (1990). Official Methods of Analysis 15th edn. Association of Official Analytical Chemists Arlington VA.
|
|
|
|
|
Association of Official Analytical Chemists (AOAC) (1995). Official Methods of Analysis 16th edn. Association of Official Analytical Chemists Arlington VA.
|
|
|
|
|
Aremu MO, Ibrahim H, Ekanem BE (2016). Effect of processing on in–vitro protein digestibility and anti–nutritional properties of three underutilized legumes grown in Nigeria. Br. Biotechnol. J. 14(1):1-10.
Crossref
|
|
|
|
|
Arntified SD, Isomond MA, Muvary ED (1985). Fate of anti-nutritional factors during micellization technique. Food Sci. Technol. J. 19:137-147.
|
|
|
|
|
Belitz HD, Grosch W, Schieberle P (2009). Food Chemistry, 4th ed. Springer-Verlag Berlin Heidelberg Germany.
|
|
|
|
|
Bello-Perez LA, Sayago-Ayerdi SG, Chavez-Murillo CE, Agama-Acevedo E, Tovar J (2007). Proximal composition and in vitro digestibility of starch in lima bean (Phaseolus lunatus) varieties. J. Sci. Food Agric. 87:2570-2575.
Crossref
|
|
|
|
|
Bo S, Pisu E (2008). Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr. Opin. Lipidol. 19:50-56.
Crossref
|
|
|
|
|
Bui LTT, Small DM (2012). The stability of pyridoxine hydrochloride used as a fortificant in Asian wheat flour noodles. Food Chem. 130(4):841-846.
Crossref
|
|
|
|
|
Damang PJ, Tuleun CD, Oluremi OIA, Carew SN (2017). Biochemical evaluation of raw and processed kidney bean (Phaseolus vulgaris L.) seeds for monogastric animal feed production. J. Anim. Prod. Res. 29(1):301-311.
|
|
|
|
|
Del-Rio D, Rodriguez-Mateos A, Spencer JPE, Tognolini M, Borges G, Crozier A (2013). Dietary (Poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 18(14):1818-1892.
Crossref
|
|
|
|
|
Du S, Jiang H, Yu X, Jane J (2014). Physicochemical and functional properties of whole legume flour. Food Sci. Technol. 55:308-313.
Crossref
|
|
|
|
|
Egan H, Kirk RS, Sawyer R (1981). Pearson's Chemical Analysis of Foods. Churchill Livingstone: New York.
|
|
|
|
|
Ekinci R, Kadakal C (2005). Determination of seven water–soluble vitamins in Tarhana, a traditional Turkish cereal food, by high performance liquid chromatography. Acta Chromatogr. 15:78-79.
|
|
|
|
|
El-Gharras H (2009). Polyphenols: Food sources properties and applications-a review. Int. J Food Sci. Technol. 44:2512–2518.
Crossref
|
|
|
|
|
Embaby HE (2010). Effect of soaking, dehulling, and cooking methods on certain antinutrients and in vitro protein digestibility of bitter and sweet lupin seeds. Food Sci. Biotechnol. 19(4):1055-1062.
Crossref
|
|
|
|
|
Ezeagu, IE, Ibegbu MD (2010). Biochemical composition and nutritional potential of ukpa: a variety of tropical lima beans (Phaseolus lunatus) from Nigeria – a short report. Pol. J. Food Nutr. Sci. 60:231-235.
|
|
|
|
|
Ezeocha VC, Ojimelukwe PC, Onwuka GI (2012). Effect of cooking on the nutritional and phytochemical components of trifoliate yam (Dioscorea dumetorum). Glob. Adv. Res. J. Biochem. Bioinform. 1(2):26-30.
|
|
|
|
|
Ezeogu LI, Duodu KG, Taylor JRN (2005). Effects of endosperm texture and cooking conditions on the in vitro starch digestibility of sorghum and maize flours. J. Cereal Sci. 42:33-44.
Crossref
|
|
|
|
|
Golbach JL, Ricke SC, O'Bryan CA, Crandall PG (2014). Riboflavin in nutrition, food processing, and analysis-a review. J. Food Res. 3(6):23-35.
Crossref
|
|
|
|
|
Goyal RK (2000). Nutritive value of fruits vegetables and their products. In. Postharvest Technology of Fruits and Vegetables (Verma LR, Joshi VK, eds). Indus New Delhi, pp. 337-389.
|
|
|
|
|
Gregory JF (2008). Vitamins. In. Fennema' Food Chemistry. 4th edn. (Damodaran S, Parkin KL, Fennema OR, eds). CRC Press, Boca Ratoon, USA, pp. 439-521.
|
|
|
|
|
Hsu HW, Vavak DL, Satterlee LD, Miller GA (1977). A multi-enzyme technique for estimating protein digestibility. J. Food Sci. 42:1269- 1273.
Crossref
|
|
|
|
|
Huskisson E, Maggini S, Ruf M (2007). The role of vitamins and minerals in energy metabolism and well-being. J. Int. Med. Res. 35:277-289.
Crossref
|
|
|
|
|
Kakade ML, Rackis JJ, McGhee JE, Puski G (1974). Determination of trypsin inhibitor activity of soy bean products: A collaborative analysis of an improved procedure. Cereal Chem. 51:376-382.
|
|
|
|
|
Kalpanadevi V, Mohan VR (2013). Effect of processing on antinutrients and in vitro protein digestibility of the underutilized legume, Vigna unguiculata (L.) Walp subsp. unguiculata. Food Sci. Technol. 51(2):455-461.
Crossref
|
|
|
|
|
Kumar GK, Venkataraman LV (1976). Studies on the in vitro digestibility of starch in some legumes before and after germination. Nutr. Rep. Int. 13:115-124.
|
|
|
|
|
Lachance PA (1998). International perspective: Basis, need, and application of recommended dietary allowances. Nutr. Rev. 56:S2-S4.
Crossref
|
|
|
|
|
Lambert IH, Hoffmann EK, Pedersen SF (2008). Cell volume regulation: Physiology and pathophysiology. Acta Physiol. 194:255-282.
Crossref
|
|
|
|
|
Mamiro P, Nyagaya M, Kimani P, Mamiro D, Jumbe T, Macha J, Chove B (2011). Similarities in functional attributes and nutritional effects of magadi soda and bean debris-ash used in cooking African traditional dishes. Afr. J. Biotechnol. 10(7):1181-1185.
|
|
|
|
|
Messina V (2014). Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 100(suppl):437S-42S.
Crossref
|
|
|
|
|
Morgan KT (2008). Nutritional determinants of bone health. J. Nutr. Elder. 27:3-27.
Crossref
|
|
|
|
|
Mugendi JB, Njagi ENM, Kuria EN, Mwasaru MA, Mureithi JG, Apostolides Z (2010). Effects of processing technique on the nutritional composition and anti-nutrient content of mucuna bean (Mucuna pruriens L.). Afr. J. Food Sci. 4(4):156-166.
|
|
|
|
|
Ngudi DD, Kuo YH, Lambein F (2003). Cassava cyanogens and free amino acids in raw and cooked leaves. Food Chem. Toxicol. 41:1193-1197.
Crossref
|
|
|
|
|
Obilie EM, Tano-Debrah K, Amoa-Awua WK (2004). Souring and breakdown of cyanogenic glucosides during the processing of cassava into 'akyeke'. Int. J. Food Microbiol. 93:115-121.
Crossref
|
|
|
|
|
Olapade AA, Umeonuorah UC (2014). Chemical and sensory evaluation of African breadfruit (Treculia africana) seeds processed with alum and trona. Nig. Food J. 32(1):80-88.
Crossref
|
|
|
|
|
Onwuka GI (2006). Soaking boiling and antinutritional factors in pigeon peas (Cajanus Cajan) and cowpeas (Vigna Unguiculata). J. Food Process. Preserv. 30:616-630.
Crossref
|
|
|
|
|
Price ML, Butler LG (1977). Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. J. Agric. Food Chem. 25:1268-1273.
Crossref
|
|
|
|
|
Pushparaj FS, Urooj A (2011). Influence of processing on dietary fiber, tannin and in vitro protein digestibility of pearl millet. Food Nutr. Sci. 2:895-900.
Crossref
|
|
|
|
|
Rakic S, Petrovic S, Kukic J, Jadranin M, Tesevic V, Povrenovic D, Silver-Marinkovic S (2007). Influence of thermal treatment on phenolic compounds and antioxidant properties of oak acorns from Serbia. Food Chem. 104:830-834.
Crossref
|
|
|
|
|
Renkema KY, Alexander RT, Bindels RJ, Hoenderop JG (2008). Calcium and phosphate homeostasis: Concerted interplay of new regulators. Ann. Med. 40:82-91.
Crossref
|
|
|
|
|
Rickman JC, Bruhn CM, Barrett DM (2007). Nutritional comparison of fresh frozen and canned fruits and vegetables. II. Vitamin A and carotenoids vitamin E minerals and fiber. J. Sci. Food Agric. 87:1185-1196.
Crossref
|
|
|
|
|
Sarkar J (2012). Lima Bean (Phaseolus lunatus). [Internet document].
View. Accessed on 12-07-2017.
|
|
|
|
|
Selle, PH, Cowieson AJ, Cowieson NP, Ravindran V (2012). Protein–phytate interactions in pig and poultry nutrition: A reappraisal. Nutr. Res. Rev. 25:1-17.
Crossref
|
|
|
|
|
Shenkin A (2008). Basics in clinical nutrition: Physiological function and deficiency states of trace elements. e-SPEN 3:255-258.
|
|
|
|
|
Singh J, Dartois A, Kaur L (2010). Starch digestibility in food matrix: a review. Trend Food Sci. Technol. 21:168-180.
Crossref
|
|
|
|
|
Sobotka L, Allison S, Stanga Z (2008). Basics in clinical nutrition: Water and electrolytes in health and disease. e-SPEN 3:259-266.
|
|
|
|
|
Udensi EA, Ekwu FC, Isinguzo JN (2007). Antinutrient factors of vegetable cowpea (Sesquipedalis) seeds during thermal processing. Pak. J. Nutr. 6:194-197.
Crossref
|
|
|
|
|
Wheeler EL, Ferrel RE (1971). A method for phytic acid determination in wheat and wheat fractions. Cereal Chem. 48:312-320.
|
|
|
|
|
Williams FH (2008). Neuromuscular complications of nutritional deficiencies. Phys. Med. Rehabil. Clin. N. Am. 19:125-148.
Crossref
|
|
|
|
|
Zhao Y, Du S, Wang H, Cai M (2014). In vitro antioxidant activity of extracts from common legumes. Food Chem. 152:462-466.
Crossref
|
|




