Full Length Research Paper
ABSTRACT
The objective of this work was to analyze the growth of Scenedesmus acutus, Nannochloropsis oculata and Chlorella vulgaris at different temperatures (25, 30 and 38°C) in order to identify changes in lipid content, accumulation of neutral lipids and fatty acids profile. According to the results obtained, the temperature of 30°C does not affect the growth of the microalgae S. acutus, N. oculata and C. vulgaris; however, there is a greater amount of total lipids in S. acutus at 38°C, while for N. oculata and C. vulgaris, temperature variation does not affect the accumulation of total lipids. From the data obtained from the fatty acid profile, we observed a greater accumulation of palmitic acid followed by oleic acid. According to the cetane number, any temperature condition of any evaluated microalgae culture can be used for biodiesel production. The results suggest that a change in temperature during the growth of microalgae could be applied to enhance lipid production and to obtain fatty acids suitable for biodiesel production.
Key words: Chlorella vulgaris, Nannochloropsis oculata, Scenedesmus acutus, fatty acids, lipids, temperature.
INTRODUCTION
Humanity confronts two problems in the energy area: The decrease in oil reserves and pollution caused by the burning of fossil fuels. Due to this, viable energy alternatives have been pursued to replace the use of oil, which must be renewable, sustainable, and come from environmentally friendly sources (Banerjee et al., 2018; Zhang et al., 2022). It has been estimated that the production of biodiesel is the only technology capable of substituting the consumption of fuels derived from petroleum (Chisti and Yan, 2011). In addition, it offers several environmental advantages such as the reduction of greenhouse gas emissions up to 70 to 90% compared to conventional diesel (Ajikumar et al., 2008; Timilsina and Mevel, 2013).
Biodiesel is a liquid biofuel composed of alkyl esters of short-chain alcohols, such as ethanol and methanol, with long-chain fatty acids obtained from renewable biomass, like vegetable oils, animal fats and microalgae oils (Robles-Medina et al., 2009). Among the main advantages of biodiesel is that during its combustion it produces fewer harmful emissions of sulfides, aromatic hydrocarbons and soot particles (Balat and Balat, 2010), it also has lubricating properties that reduce engine wear, and it is a safe product for transport and handling due to its high flash point (150°C) and low volatility (Demirbas, 2009).
Microalgae-based biofuels can be used as a substitute for petroleum fuels. The biofuels derived microalgae can be processed by using thermochemical and biochemical conversion. After the oil extraction from algal cells, the lipids can be converted by a chemically transesterified into biodiesel. Other microalgae biofuels such as bioethanol and biomethane can be produced by fermentation of the biomass under anaerobic conditions. Microalgae also have potential in the production of gaseous biofuel such as biohydrogen (Zhang et al., 2022; Banerjee et al., 2018).
The use of microalgae for biodiesel production is an advantageous alternative due to their lipid content, ability to assimilate CO2 as a carbon source and their rapid biomass generation compared to plants (Sharma et al., 2012). Lipids in microalgae are composed of polar and neutral lipids. Neutral lipids are composed of triacylglycerides (TAGs), which, once extracted, can be easily converted into biodiesel through transesterification reactions (Sharma et al., 2012; Sato et al., 2014). Lipid production and accumulation in microalgae depends mainly on the species and its genetic constitution, but it is also affected by various physical and chemical conditions of the culture, such as: Growth phase, nutrient availability, salinity, periods of light, intensity, temperature, and pH. Under these abiotic stresses, the microalgae constantly adjust their cellular mechanisms to cope with them. The accumulation of stress metabolites, such as lipids, is directly related to the changes occurring in their metabolic pathways; under these conditions, the yields are higher because microalgae accumulate more neutral lipids as a mechanism to protect cells against oxidative damage (Paliwal et al., 2017; Poh et al., 2020).
It has been established that an increase in temperature has a positive effect on photosynthesis, cell division, lipid production, and fatty acids formation. High temperature promotes the uptake and fixation of CO2 by microalgae; however, extremely high temperature inhibits the respiratory metabolism of microalgae (Tripathi et al., 2002; Barten et al., 2021). Due to heat stress, highly thermal sensitive enzymes which control the synthesis and accumulation of lipids may be affected; in addition, proteins involved in photosynthetic processes may be modified (Converti et al., 2009; Xin et al., 2011; Ras et al., 2013).
To the researchers’ knowledge, there have been no studies reported about the comparison of these 3 species of microalgae (S. acutus, N. oculata and C. vulgaris) at the temperatures of 25, 30 and 38°C and it effects in the cell growth, total and neutral lipid production, including the prediction of cetane number based on its fatty acid composition. Therefore, in the present study, the association between the growth of 3 species of microalgae at different temperatures and the accumulation of lipids and its effect on the fatty acid profile was probed. The cetane number prediction was also assessed.
MATERIALS AND METHODS
Microorganisms, culture media and aeration system
The microalgae Scenedesmus acutus UTEX 72, Nannochloropsis oculata CCAP 849/7 and Chlorella vulgaris OW-01 were obtained from the Autonomous University of Aguascalientes. The identification of these strains was performed via 18S rRNA gene amplification followed by sequencing. Cells were maintained in Bold Basal Medium (BBM) agar plates at 25°C under a photoperiod of 16/8 h light-dark. A single colony was picked-up to the sterilized test tube containing 5 mL of growth medium, the scaling up was made in BBM on a rotary shaker at 120 rpm and used as pre-inoculum for each microalga specie. Carbon supply, in the form of CO2, was provided by an aeration system (air diffusion) that was designed based on transparent 2 mm radius flexible PVC hoses connected to an air pump of 2.2 L.min-1. The air flow in the nine experiments was controlled by flow regulating valves of 1⁄4 in.
Strains growth at different temperatures and growth kinetics
The pre-inoculum was maintained at a temperature of 25°C with continuous stirring and photoperiods of 16 h light / 8 h dark until an optical density (OD) of 0.2 was reached at a wavelength of 750 nm. The readings were recorded using the GloMax®-Multi Microplate Reader spectrometer (Promega Corporation, Madison, WI, USA).
Once the desired OD was reached, nine 1000 mL flasks containing 500 mL each of BBM were inoculated. The cultures (by triplicate) were maintained with photoperiod and aeration, the latter to supply CO2 as a carbon source and maintain the system homogeneous. Cultures were grown at three different temperatures: 25, 30 and 38 °C. Every 24 h, 2 samples of 1 mL were taken until the cultures reached stationary phase, which were used for neutral lipid analysis, optical density recording to monitor growth and to perform cell counting. Upon reaching stationary phase, the biomasses were recovered by centrifugation at 2,000 x g for 20 min and stored at -20°C for use in lipid quantification. Each experiment consisted of triplicate flasks.
Cell counting in Neubauer chamber
A 0.1 mm deep hematocytometer with Neubauer's ruler was used for cell counting as described by Galarza et al. (2019).
Quantitative identification of total and neutral lipids
The final samples obtained at the end of the kinetics were processed based on previously described protocols (Folch et al., 1957; Bligh and Dyer, 1959) to quantify total lipids. For the analysis of neutral lipid content, the samples obtained during the growth kinetics were analyzed by triplicate as described before (Bertozzini et al., 2011).
Identification of the fatty acid profile
The free fatty acid profile was carried out using a methodology described by Flores Ruedas et al. (2020) using an Agilent 7890 gas chromatograph (Agilent Technologies, Sta. Clara Cal., USA), which is equipped with a flame ionization detector (GC-FID). The analysis was made in duplicate and the mean and standard deviation were reported. An analysis of variance (ANOVA, p < 0.05), using Fisher's test, was made to detect differences between the fatty acids obtained by the different strains.
Cetane number prediction
The cetane number (CN) was evaluate with the model as described by Piloto-Rodríguez et al. (2013) using the fatty acid profiles described previously.
RESULTS AND DISCUSSION
Strains growth kinetics and the specific growth rate
To determine the effect of temperature on the growth of the microalgae S. acutus, N. oculata and C. vulgaris growth kinetics were obtained. As can be seen in Figure 1a and b, the microalga N. oculata exhibited apparently higher growth at 2 temperature conditions (25 and 30°C), followed by C. vulgaris and S. acutus, while for the third condition (38°C) the microalga C. vulgaris showed the highest growth (Figure 1c).

The specific growth rate m [d-1] was calculated and a comparison was made between the three strains. According to Table 1, there is no significant difference between the three microalgae subjected to the three temperature conditions, and when the comparison was made by species at different temperatures, the same result was found, indicating that this temperature range does not affect the growth of these microalgae.
Staehr and Birkeland (2006) reported for S. acutus at 25°C a value of µ=0.57 d-1, this difference may be due to the growth conditions used by these authors, who included the use of a modified O2 medium in a final volume of 2 L, and an evaluation period of growth of 14 days. The photoperiod evaluated by them was the same as the used in this work (16 h of light and 8 h of darkness). Wei et al. (2015) reported for N. oculata at 25, 30 and 35°C, similar values of µ; the conditions under which they obtained these values included the use of 60 L photobioreactors with f/2 medium for a growth period of 10 days, after which they make the total lipids extraction.
In the same way, Converti et al. (2009) reported for C. vulgaris at 25 and 30°C similar values of µ as the ones found in this work, while for N. oculata at 25°C the value of µ=0.07 was two times lower than that reported by us. The growth conditions reported involved the use of BBM for the culture of C. vulgaris and f/2 medium for N. oculata during a 14-day culture period, followed by ultrasonication and Folch's method for lipid extraction.
Extraction of total lipids
According to Table 2, S. acutus accumulates the highest amount of lipids (21.72%) at the temperature of 38°C. At the same temperature, N. oculata accumulate 15.68%, although statistically there is no significant difference (p<0.05) with respect to the other two temperature conditions. On the other hand, C. vulgaris at 30°C accumulates 11.21% of lipid, however, there is no significant difference statistically with the data obtained at the other two temperatures.
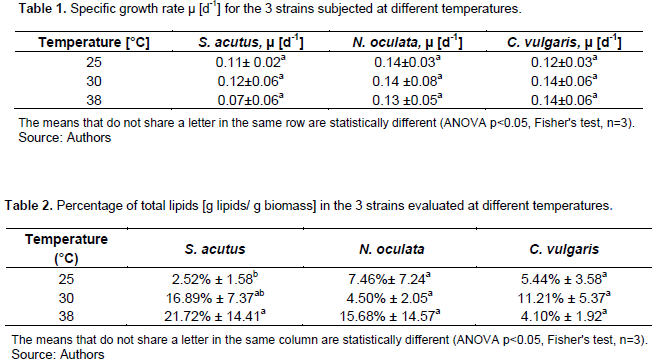
El-sheekh et al. (2017) reported for S. acutus, at temperature of 25°C, a percentage of total lipids 3.82 times higher than that reported in this work and at 30°C only 1.78 times lower. Different factors affect the lipid accumulation, one of the main is the culture media, and other variable is the duration of the photoperiod. El-sheekh et al. (2017) conducted their experiments for 14 h light and 10 h dark, this change can cause a greater accumulation of lipids at 25°C since it increases the time in which the microalgae are photosynthetically active. Likewise, there are differences in the lipid extraction procedure which can cause differences in the content of total lipids of the microalgae. Their experiments also included the use of KC culture medium for 22 days.
Converti et al. (2009) described for N. oculata a percentage of total lipids of 13.89% at 25°C which is 1.86 times higher than that reported in this work; at the same temperature, for C. vulgaris, they reported a 14.71% of total lipids while at 30°C it was only 5.9%, which is 1.90 times lower than that reported by us. They also quantified the lipids at 38°C, obtaining a 11.3% which is 2.76 times higher in relation to this work
Neutral lipids content
The neutral lipids present in the microalgae cultures were quantified by a fluorometric method described as described before (Bertozzini et al., 2011) and the results are presented in Table 3. With the data obtained, we identified the day in which the highest content of neutral lipids was accumulated. At 25°C, among the three microalgae, S. acutus presents the highest accumulation of neutral lipids on day 19 (3.02 µg/mL), however, it does not show significant difference with respect to the lipids accumulated on days 23 and 21. At 30°C, S. acutus was the strain that accumulated the most neutral lipids, with 2.84 µg/mL on day 21, being the highest amount recorded among the three microalgae. For a temperature of 38°C, N. oculata reached a concentration of 2.44 µg/mL on day nine, which was the highest among the three microalgae studied.

Fatty acid profiles
Fatty acid profiles were made to identify the fatty acids (FA) produced under each temperature condition for each microalga strain. It has been reported that the FA composition affects the quantity and quality of the synthesized biodiesel since the longer and more saturated the fatty acid carbon chains are, the higher the number of cetanes (CN), which is related to the ignition delay time and combustion quality (Piloto-Rodríguez et al., 2013). The FA were classified into four categories: saturated fatty acids (SFA), mono-unsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA) and Others (which included the fatty acids that weren’t identified) (Figure 2).
SFA are the most abundant in the 3 microalgae evaluated at different temperatures, followed by MUFA and finally PUFA. Figure 3a shows that C16:0, C18:0 and C18:1 are the fatty acids that accumulate in greater amount in the 3 strains of microalgae incubated at 25°C; in particular, for N. oculata, the fatty acid with the highest percentage was C18:1, followed by C18:0 and C16:1. On the other hand, for C. vulgaris the following distribution was found: C16:0, C18:0 and C14:0, this indicates a different fatty acid distribution depending on the microalgae strain even when grown at the same temperature.
At 30°C, is observed that C16:0 is still the highest percentage found, followed by C18:0, and is observed that C16:1 begins to accumulate (Figure 3b), a decrease in the percentage of C18:1, C18:2 and C18:3 was also observed. Figure 3c shows, at 38°C, a decrease in C16:0 and C18:1 for N. oculata and C. vulgaris with respect to the temperature of 25°C. At this temperature, the main FAs present in a greater proportion in the 3 microalgae are C16:0, C18:0 and C16:1.
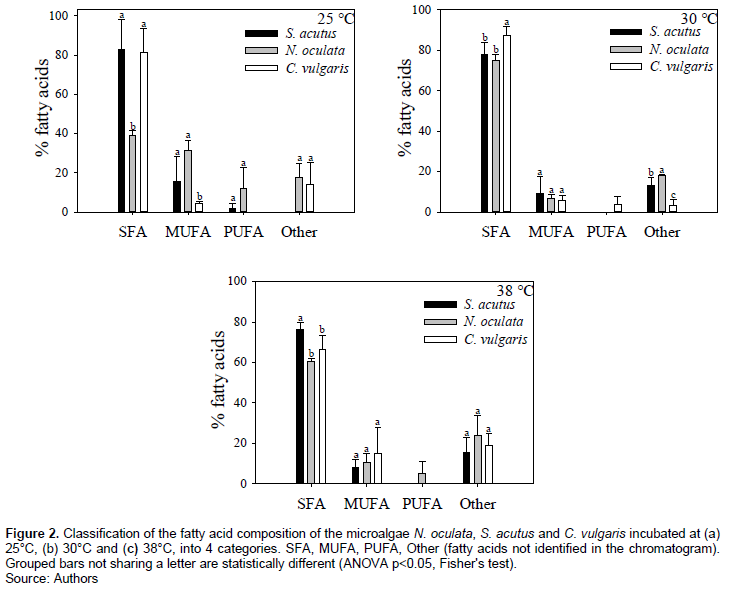
El-sheekh et al. (2017) reported for S. acutus much lower levels of SFA compared to those obtained in this work at temperatures of 25, 30 and 38°C. A similar study was carried out with N. oculata (Wei et al., 2015), in which the authors reported higher values of SFA compared to those obtained in this study at 25, 30 and 35°C.
For C. vulgaris at 25°C, Converti et al. (2009) reported SFA values of 71.00 %, which is 1.15 times lower as reported in this work, but their values of MUFA and PUFA were higher. Similar behavior was observed for SFA at 38°C.
Cetane number
Cetane number, which indicates ignition delay characteristics, is considered the most significant fuel property of biodiesel (Lin and Wu, 2022). Using the model for the prediction of the cetane number (CN) and the fatty acid percentage data obtained from the fatty acid profile (Figure 3), the CN corresponding to each of the nine experiments was obtained. The results are reported in Table 4, where the data obtained were compared with the CN required by the ASTM D6751 standard of the United States of America and the EN 14214 of Europe, giving as a result that the three different temperatures evaluated in S. acutus, N. oculata and C. vulgaris are in premise candidates for obtain biodiesel because their CN is major that the minimum required in both international standards. Accordingly, it is proposed that efforts should be focused on evaluating the feasibility of using C. vulgaris at 30°C, S. acutus at 25°C, 30°C or 38°C, and C. vulgaris at 25°C as possible platforms for biodiesel production.

CONCLUSION
This study compares the effect of different temperatures (25, 30 and 38°C) on the growth, lipid accumulation and fatty acid profile in 3 species of microalgae. The data obtained for the specific rates indicate that the growth of microalgae is not affected by the temperatures of 30 and 38°C. S. acutus accumulates a higher amount of total lipids at 38°C. N. oculata and C. vulgaris show no significant differences in total lipid accumulation at the different temperatures analyzed in this study. Of the total lipids extracted, there is a greater accumulation of palmitic acid (C16:0) followed by oleic acid (C18:1) for the 3 microalgae, which are be suitable for biodiesel production. These results suggest that a change in temperature during the growth of microalgae could be applied to enhance lipid production and to obtain fatty acids suitable for biodiesel production.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
RESG acknowledges the Fondo Sectorial de Investigación para la Educación CONACyT, CB2017-2018 A1-S-8367.
REFERENCES
|
Ajikumar PK, Tyo K, Carlsen S, Mucha O, Phon TH, Stephanopoulos G (2008). Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Molecular Pharmaceutics 5(2):167-190. |
|
|
Balat M, Balat H (2010). Progress in biodiesel processing. Applied Energy 87(6):1815-1835. |
|
|
Banerjee A, Banerjee C, Negi S, Chang JS, Shukla P (2018). Improvements in algal lipid production: a systems biology and gene editing approach. Critical Reviews in Biotechnology 38(3):369-385. |
|
|
Barten R, Djohan Y, Evers W, Wijffels R, Barbosa M (2021). Towards industrial production of microalgae without temperature control: the effect of diel temperature fluctuations on microalgal physiology. Journal of Biotechnology 336:56-63. |
|
|
Bertozzini E, Galluzzi L, Penna A, Magnani M (2011). Application of the standard addition method for the absolute quantification of neutral lipids in microalgae using Nile red. Journal of Microbiological Methods 87(1):17-23. |
|
|
Bligh EG, Dyer WJ (1959). A rapid method of total extraction and purification of lipids. Canadian Journal of Biochemistry and Physiology 37(8):911-917. |
|
|
Chisti Y, Yan J (2011). Energy from algae: Current status and future trends. Algal biofuels - A status report. Applied Energy 88(10):3277-3279. |
|
|
Converti A, Casazza AA, Ortiz EY, Perego P, del Borghi M (2009). Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chemical Engineering and Processing: Process Intensification 48(6):1146-1151. |
|
|
Demirbas A (2009). Political, economic and environmental impacts of biofuels: A review. Applied Energy 86(1):S108-S117. |
|
|
El-sheekh M, Abomohra AE, El-azim MA, Abou-Shanab R (2017). Effect of temperature on growth and fatty acids profile of the biodiesel producing microalga Scenedesmus acutus. Biotechnologie, Agronomie, Société et Environnement 21(4):233-239. |
|
|
Flores Ruedas RJ, Dibildox-Alvarado E, Pérez Martínez JD, Murillo Hernández NI (2020). Enzymatically interesterified hybrid palm stearin as an alternative to conventional palm stearin. CyTA-Journal of Food 18(1):1-10. |
|
|
Folch J, Lees M, Sloane Stanley GH (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry 226(1):497-509. |
|
|
Galarza JI, Arredondo Vega BO, Villón J, Henríquez V (2019). Deesterification of astaxanthin and intermediate esters from Haematococcus pluvialis subjected to stress. Biotechnology Reports 23:e00351. |
|
|
Lin CY, Wu XE (2022). Determination of cetane number from fatty acid compositions and structures of Biodiesel. Processes 10(8):1502. |
|
|
Paliwal C, Mitra M, Bhayani K, Bharadwaj SVV, Ghosh T, Dubey S, Mishra S (2017). Abiotic stresses as tools for metabolites in microalgae. Bioresource Technology 244:1216-1226. |
|
|
Piloto-Rodríguez R, Sánchez-Borroto Y, Lapuerta M, Goyos-Pérez L, Verhelst S (2013). Prediction of the cetane number of biodiesel using artificial neural networks and multiple linear regression. Energy Conversion and Management 65:255-261. |
|
|
Poh ZL, Wan NA, Man KL, Yoshimitsu U, Uganeeswary S, Jun-Wei L, Pau LS, Keat TL (2020). The effect of stress environment towards lipid accumulation in microalgae after harvesting. Renewable Energy 154:1083-1091. |
|
|
Ras M, Steyer JP, Bernard O (2013). Temperature effect on microalgae: a crucial factor for outdoor production. Reviews in Environmental Science and Bio/Technology 12(2):153-164. |
|
|
Robles-Medina A, González-Moreno PA, Esteban-Cerdán L, Molina-Grima E (2009). Biocatalysis: Towards ever greener biodiesel production. Biotechnology Advances 27(4):398-408. |
|
|
Sato A, Matsumura R, Hoshino N, Tsuzuki M, Sato N (2014). Responsibility of regulatory gene expression and repressed protein synthesis for triacylglycerol accumulation on sulfur-starvation in Chlamydomonas reinhardtii. Frontiers in Plant Science 5:444. |
|
|
Sharma KK, Schuhmann H, Schenk PM (2012). High lipid induction in microalgae for biodiesel production. Energies 5(5):1532-1553. |
|
|
Staehr PA, Birkeland MJ (2006). Temperature acclimation of growth, photosynthesis and respiration in two mesophilic phytoplankton species. Phycologia 45(6):648-656. |
|
|
Timilsina GR, Mevel S (2013). Biofuels and climate change mitigation: A CGE analysis incorporating land-use change. Environ. Environmental and Resource Economics 55(1):1-19. |
|
|
Tripathi U, Sarada R, Ravishankar GA (2002). Effect of culture conditions on growth of green alga Haematococcus pluvialis and astaxanthin production. Acta Physiologiae Plantarum 24(3):323-329. |
|
|
Wei L, Huang X, Huang Z (2015). Temperature effects on lipid properties of microalgae Tetraselmis subcordiformis and Nannochloropsis oculata as biofuel resources. Chinese Journal of Oceanology and Limnology 33(1):99-106. |
|
|
Xin L, Hong-ying H, Yu-ping Z (2011). Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresource Technology 102(3):3098-3102. |
|
|
Zhang S, Zhang L, Xu G, Li F, Li X (2022). A review on biodiesel production from microalgae: Influencing parameters and recent advanced technologies. Frontiers in Microbiology 13:970028. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0