Full Length Research Paper
ABSTRACT
The aim of this study was to investigate the effect of substrates on the growth, yield, nutritional, and medicinal value of Pleurotus ostreatus supplemented with four medicinal plants. A completely randomized block design was laid out with 4 treatments replicated 4 times with and without medicinal plants. T1 (sawdust), T2 (sawdust + corncobs), T3 (palm cones), and T4 (elephant stalks) were the treatments used. Croton macrastarchus, Harungana madagascariensis, Tithonia diversifolia, and Rauwolfia vomitoria were the medicinal plants used. Nutritional and phytochemical analysis was carried out. Sawdust + corn cobs indicated the highest effect on growth as it had the highest mean height (19.5 ± 3.3 cm), diameter (29.0 ± 4.3 cm) and mean weight of individual fruiting bodies (175.8 ± 84.3 cm). Biological efficiency was highest in palm cones (77.1%), second by sawdust + corn cobs (61.1%), saw dust (53.0%) and elephant stalk (6.3%). The protein content was highest in sawdust + corn cobs (12.4 g), lipid concentration highest in sawdust only (1.51 g), total carbohydrate highest in palm cones (82.98 g), and total ash highest in sawdust only (7.32 g) per 100 g. The supplementation of sawdust + corn cobs with R. vomitoria had the highest phytochemical components.
Key words: Medicinal properties, mushroom cultivation, growth and yield, nutritional content, Pleurotus ostreatus, medicinal plants substrates.
INTRODUCTION
Mushrooms are fleshy saprophytic fungi, and it can be found growing on damp rotten log of wood trunk of trees, decaying organic matter and in damp soil rich in organic substances. Edible mushrooms are highly nutritious and can be compared with eggs, milk and meat (Mata et al., 2005). Pleurotus ostreatus (Basidiomycota), is of the Pleurotaceae family. Pleurotus originated from China; however, nowadays it is distributed all over the world, except for the north-west Pacific because of the arctic climate (Wojewoda, 2003). Cultivation methods were developed in Germany during World War I and then successfully applied on a large scale. This was the result of the search for new food sources, due to the problem of hunger in Germany. In Poland, P. ostreatus is a common species (Wojewoda, 2003). P. ostreatus (white-rot fungus), also known as oyster mushroom, is commercially important in the world mushroom market.
Oyster mushroom is an edible mushroom having an excellent flavour and taste. P. ostreatus has received increased attention for applications in bio-bleaching and the catalysis of difficult chemical conversions in the paper industry, textile dye decolorization, and detoxification of environmental pollutants (Park et al., 2014). Oyster mushrooms are prized for their exclusive flavour and deliciousness. They are rich in proteins, contain less fat, less carbohydrates, salt and rich in fibres and have high vitamin B12 and folic acid which are uncommon in vegetables. High availability of lysine and tryptophan and other amino acids usually absent in cereals make them ideal for food for patients suffering from hypertension, diabetes and obesity (Carel et al., 2013). Mushrooms require carbon, nitrogen and inorganic compounds as their nutritional sources and the main nutrients are carbon sources such as cellulose, hemicellulose and lignin. The use of various wastes is recommended for the growth of P. ostreatus (Vendrusco et al., 2008)
The waste obtained from fruit processing industry is extremely diverse due to the use of wide variety of fruits and vegetables, the broad range of processes and the multiplicity of the product. Full utilization of horticultural produce is a requirement and a demand that needs to be met by countries wishing to implement low-waste technology in their agribusiness. Food processing industries generate high amount of waste and direct disposal of such residues poses a serious threat to the environment and represents an important loss of biomass (Vendrusco et al., 2008). Apple pomace is an important fruit industrial waste that remain unutilized and creating an environmental pollution. Therefore, it can be utilized along with the wheat straw as a substrate for P. ostreatus production. Several million tons of Apple pomace is generated because of its high carbohydrate content which is used for microbial processes for single cell protein, enzymes, ethanol, low alcoholic drinks and pigments (Bhushan et al., 2008). Therefore, this horticultural waste can be utilized and replaced with wheat straw for mushroom cultivation under mushroom house.
Extracts from wild species of Pleurotus have been reported to be used in treating some ailments (Osemwegie et al., 2007). Wild species are collected for consumption because they are a good source of carbohydrates, digestible proteins, fibres and vitamins (Barros et al., 2008). Structurally, polysaccharides and proteins comprise the main components of dry matter of Pleurotus, while lipid content is low. Chitin, glycogen, mannitol and trehalose are typical carbohydrate constituents. Pleurotus occupy the third position in the production of edible mushrooms, behind the species of the genus Agaricus and Lentinula (Cardoso et al., 2013). Pleurotus spp. are found in tropical and subtropical rainforests around the world, and can be artificially cultivated (Bonatti, 2004) due to their ability to colonize and degrade a wide variety of substrates containing cellulose, hemicellulose and lignin, using them in their own development (Pokhrel et al., 2013). Furthermore, these species have a quick mycelium growth, fruiting and a low cost of culture, being slightly affected by diseases, and requiring minimal monitoring of the cultivation environment due to an easy adaptation and maintenance (Pokhrel et al., 2013). Therefore, due to nutritional and functional characteristics, Pleurotus spp. is considered increasingly popular in a commercial point of view.
In general, the production of mushrooms may be divided into several stages: composting and filling, sterilization, inoculation, incubation, fruiting, and harvesting (Loss, 2009). Contrary to others, Pleurotus genus does not require a composting substrate (Fan et al., 2000), due to the presence of a powerful enzyme complex (with cellulases, hemicelulases, ligninases, peroxidases, laccases, proteases, among other enzymes) (Hyde et al., 2019). Pleurotus spp. only requires crushed materials to acquire the desired texture for a good mycelial colonization. The production of Pleurotus spp. has been tested using different substrates e.g., cotton waste textile (Chang et al., 1981), rice straw (Mehta et al., 1990) by products of corn (Loss, 2009), bark of coffee (Dias et al., 2003), wheat straw (Ramos et al., 2011) and sugarcane (Cardoso et al., 2013). The adaptation of this genus to new wastes represents one of the main methods for bioconversion of agro-industrial waste into edible products with high nutritional value (Cohen et al., 2002). The recycle of different materials is one of the most important contributions of fungi in nature (Sanchez et al., 2002).
P. ostreatus is often attacked by various fungal, bacterial, viral pathogens and this leads to a great loss of yield. So, these pathogens must be controlled to save crop production (Dawoud et al., 2005). Hot water extract of Curcuma aromatic inhibited the mycelia growth of the causative agent of root rot of cotton and wilt of tomatoes (Raja and Kurcheve, 1999). The incorporation of medicinal plant products with P. ostreatus is for the phytochemical control of pathogenic microbes during mushroom cultivation. Some medicinal plants used during mushroom cultivation include Eucalyptus, Neem cake, Citrus lemon and Lemon grass. The fine powder of these medicinal plant’s substances is mixed with mushroom substrates for yield improvement of various strains of oyster mushrooms (Nazir et al., 2010). The aim of this research was to investigate the effect of substrates on the growth, yield, nutritional and medicinal value of P. ostreatus when supplemented with four medicinal plants.
MATERIALS AND METHODS
Description of cultivation site
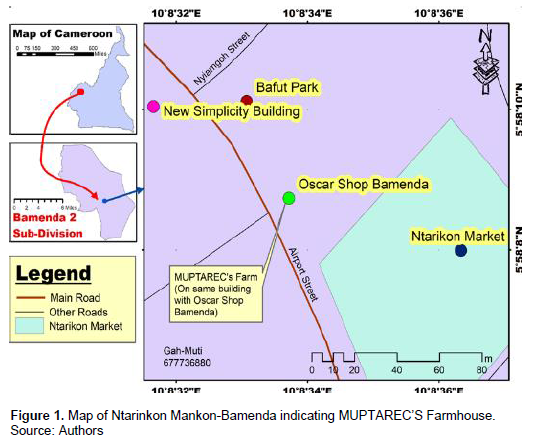
This research was conducted at the Mushroom Programme Training and Research Center (MUPTAREC) farmhouse at Ntarinkon Mankon-Bamenda. The farmhouse is situated in Mezam division in the Northwest Region of Cameroon. Bamenda also known as Abakwa town and Mankon town is a city in the Northwest Region and has a population of about 2 million inhabitants located at 366 km Northwest of the Cameroonian capital Yaounde. Bamenda is known for its cool climate. Mankon has coordinates 5°561N 10E and 5.933N 10.167°E (Figure 1). The area has a tropical savanna climate, bordering on a tropical monsoon climate with long wet season and short dry season. The soil type in Mankon is sandy loam (Alakeh et al., 2020).
Sterilization procedure
All apparatus, equipment, metallic instruments, glass wares and culture media were sterilized locally in a drum. The culture room was cleaned by washing with detergents followed by 70% ethyl alcohol.
Mother spawn production
An exotic strain of P. ostreatus was obtained from Belgium and multiplied using Potato Dextrose Agar. 100 kg bag of sawdust, 25 kg bag of rice bran, 50 kg bag of rice husk, and 500 g of slake lime (Calcium Carbonate) were mixed using clean spades and 30 L of water added to obtain 65% moisture content. Mixing was done properly to obtain a homogenous mixture. This mixture was put in bottles and closed with bottle lids that have been perforated at the center of the lid using a nail and cotton fitted on the perforated area to permit the supply of small amount of oxygen to the mycelium after planting. These bottles were sterilized in a sterilization drum for 4 h. Cooling was allowed to take place for an hour followed by inoculation of the bottles in an inoculator for 90 min. Planting of substrate in bottles took place with seeds obtain from mother spawn that was already prepared three weeks before. The bottles were then placed in an already prepared spawn room for colonization for 3 weeks after which they were suitable for planting substrates.
Substrate preparation
Sawdust, sawdust mixed with corn cobs, palm cones and elephant grass were mixed with equal quantities (1:1) of rice bran and CaCO3. Water was added to the mixture and mixed to homogeneity. The substrates used; sawdust, sawdust mixed with corn cobs, palm cones and elephant grass, were filled in plastic bags. Four (4) replications were made from each substrate, a kilogram in each bag. Tags were made on the bags for clear identification and differentiation. The substrate bags were tied and sterilized in a 25 ml metallic drum for 4 h and allowed in the drums for continuous gradual sterilization overnight. Bags were removed the next day and allowed to cool then spawning was done.
Substrates, composition and replications
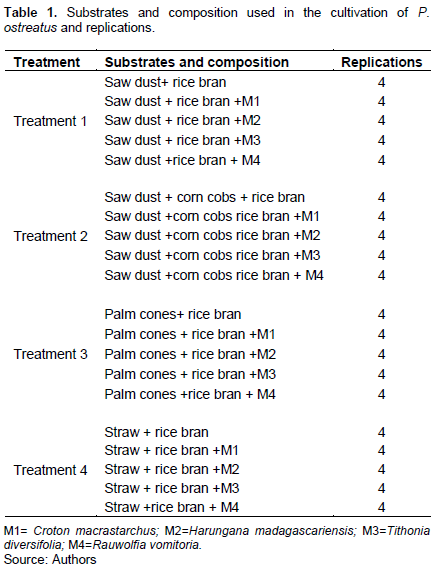
The cultivation of P. ostreatus on different substrates was done using the procedure of Anagho (2008). This experiment was laid out in a completely randomized design with 4 treatments replicated 4 times. The treatments were supplemented with and without the 4 medicinal plants and each had 4 replications. Table 1 shows the various substrates (treatments) and composition used in the cultivation of P. ostreatus with the various replications.
Substrate preparation with medicinal plants
Substrates were mixed as stated earlier and equal amount of the different medicinal plants; 35 g each were added to the substrates and mixed thoroughly for homogeneity. The four (4) medicinal plants used were Croton macrastarchus, Harungana madasgariensis, Tithonia diversifolia and Rauwolfia vomitoria. These medicinal plants were collected, chopped into pieces, dried in a bakery oven and ground into powder in a grinding mill. 20 replications were made from the substrates with different medicinal plants (4 replications per medicinal plant). 1 kg of compost was filled in each substrate bag. Tags were made on each substrate bag for clear identification. Bags were tied and sterilized in a 250 ml drum together with the control experiment for 4 h. Bags were removed the next day, and allowed to cool.
Spawning
The bags were removed from the drum and carefully placed in a spawning room. Cooling was allowed to take place for an hour before spawning. Washing and sterilization was done and spawning was carried out by use of a spoon. Hands and spoons were sterilized using medical alcohol. Planting spoons was used to prevent contamination and to mix spawn in the bags without damaging it. Bags were then untied and bottles of “sub mother” spawn weighing 760 g were used to plant substrates. 50 seeds were used to plant 100 substrates, that is planted at the ratio of 1 bottle of seed for two bags of substrate (1:2). The planted bags were properly tied to avoid the entering of microorganisms and to enhance proper colonization of the mycelium network. The substrates were then moved to the mushroom cultivation house with dark room with shelves where it was placed for colonization. After 21 days when the mycelium was fully colonized, the windows were open for ventilation to enhance and provide necessary conditions for fruiting. The temperature and moisture content of the room was maintained for healthy fruiting to take place. Watering of substrate to improve on the moisture content was done only after the first fruiting.
Morphological data collection
The trial was carried out according to the complete randomized block design with factorial arrangement using four replications for each treatment. The 100% saw dust substrate (treatment 1) was selected as the control. The pertinent data on growth and yield parameters such as time to spawn running, to pinhead formation, to first harvest; height of pileus, diameter of pileus, total yield, biological efficiency was collected during the experimental period according to Kinge et al. (2016).
Nutritional analysis
A laboratory analysis was done according to Association of Official Analytical Chemists (AOAC, 1984) in the National Polytechnic Bambui Nutritional Laboratory Bamenda to compare composition of nutrient in P. ostreatus cultivated on five substrates. The data was recorded on crude protein, crude fat, crude fiber ash, organic matter, and dry matter according to Raghuramulu et al. (2003).
Phytochemical analysis
The phytochemical analysis was done using standard procedures (AOAC, 1984). The phytochemical test was analysed after extraction in 1:1 methanol and methylene chloride. The concentrated residues were used to detect the secondary fungi metabolites such as alkaloids, flavonoids, steroids, saponins, phenolics and tannins using standard methods with some modifications (MacNee, 2005).
Data analysis
The data for growth and yield parameters were analyzed by descriptive analysis using M.S Excel version 2010 where the results on growth and yield were compared using bar charts. Also, statistical evaluations on the growth and yield parameters were done by one way ANOVA in SPSS version 20 and the comparisons of the means realized by the Turkey HSD multiple comparison at p<0.05.
RESULTS
Spawn running time
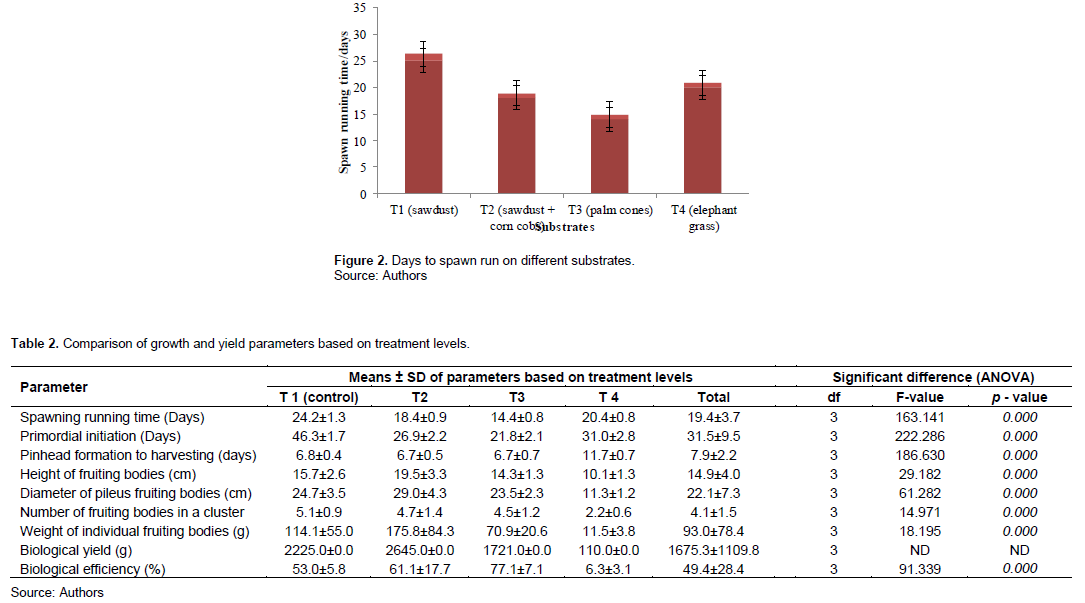
Spawn running time tested on the 4 different substrates took 14 to 25 days. Palm cones took 14.4 ± 0.8 days; corn cobs+ saw dust 18.4 ± 0.9 days, elephant straw 20.4 ± 0.8 days and saw dust 24.2 ± 1.3 days. Figure 2 and Table 2 show spawn running time measured by counting number of days of the different substrates.
Time required for primordial initiation (pinhead formation)
Two replications from treatment 1 and one replication
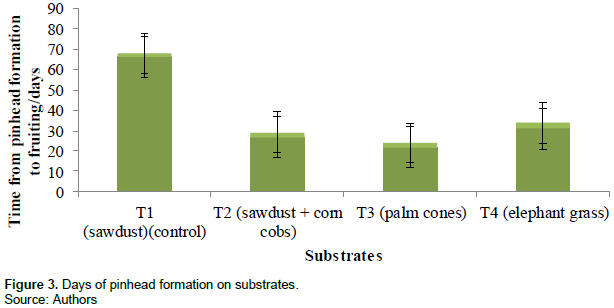
from treatment 2 were destroyed by green mould fungus. The time required for the formation of pinhead was least in treatment 3 (21.8 ± 2.1 days), treatment 2 (26.9 ± 2.2 days), treatment 4 (31.0 ± 2.8 days) and treatment 1(46.3 ± 1.7 days). Figure 3 and Table 2 show time required for pinhead formation measured by counting number of days of the different substrates.
Days from pinhead formation to harvesting
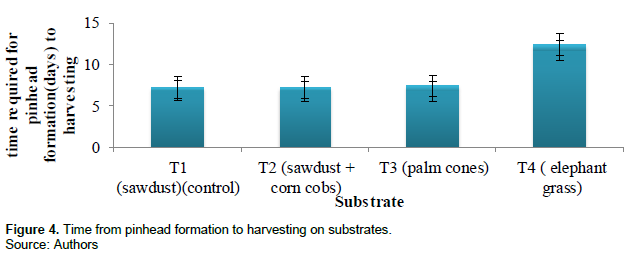
The number of days it took from fruiting to harvesting was equal in treatment 1, 2 and 3 (6.7 ± 0.7 days) and highest in treatment 4 (11.7 ± 0.7 days). Figure 4 and Table 2 show the time required for pinhead formation to harvesting measured by counting number of days of the different substrates.
Height of fruiting bodies
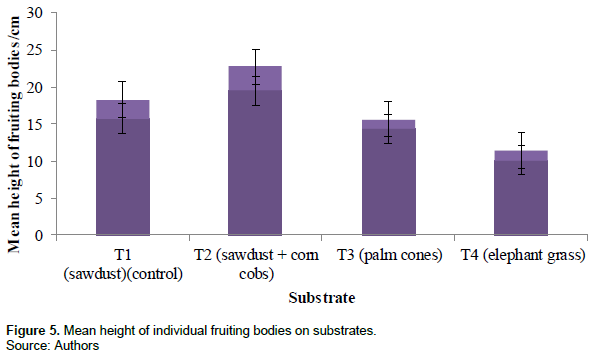
The mean height of individual fruiting bodies per treatment was measured in centimeters using a tailor’s tape beginning from where the stalk gets attached to the substrate to the pileus. The highest mean height was recorded in treatment 2 (19.5 ± 3.3 cm), followed by treatment 1 (15.7 ± 2.6 cm), treatment 3 (14.3 ± 1.3 cm) and treatment 4 (10.1 ± 1.3 cm). Figure 5 shows the mean height of fruiting bodies of the different substrates.
Diameter of the pileus of individual fruiting bodies
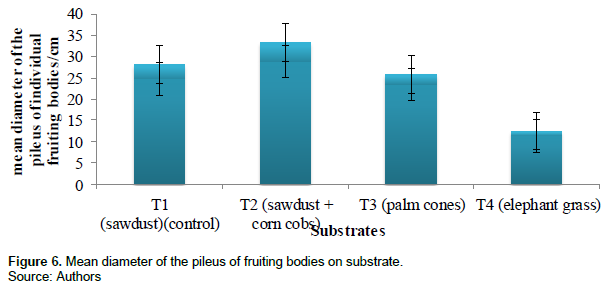
The mean diameter of individual fruiting bodies measured (which is the distance round the pileus) using a tailor’s tape in cm was recorded. The highest mean diameter was recorded in treatment 2 (29.0 ± 4.3 cm), treatment 1 (24.7 ± 3.5 cm), treatment 3 (23.5 ± 2.3 cm) andtreatment 4 (11.3 ± 1.2 cm). Figure 6 and Table 2 show the mean diameter of the pileus of individual fruiting bodies measured in cm of the different substrates.
Number of individual fruiting bodies in a cluster
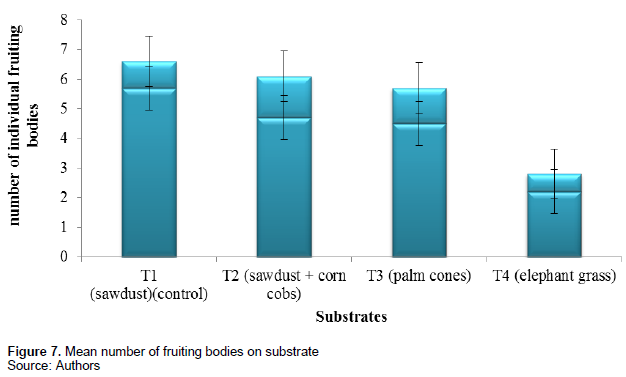
The mean number of individual fruiting bodies per treatment was recorded by counting. This number was almost equal in treatment 1, 2 and 3 (4.5 ± 1.2) and least in treatment 4 (2.2 ± 0.6). Figure 7 and Table 2 show the mean number of individual fruiting bodies of the different substrates.
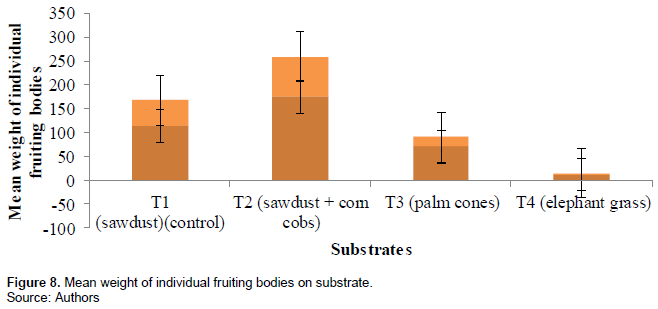
Weight of individual fruiting bodies
The mean fresh weight of individual fruiting bodies was measured in grams and recorded. The highest mean weight of individual fruiting bodies was found in treatment 2 (175.8 ± 84.3 g), second in treatment 1 (114.1 ± 55.0 g), third in treatment 3 (70.9 ± 20.6 g) and least in treatment 4 (11.5 ± 3.8 g). Figure 8 and Table 2 show the mean weight of individual fruiting bodies of the different substrates.
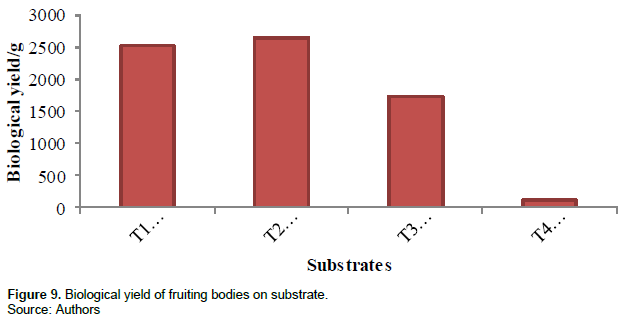
Biological yield
This is the total fresh weight of fruiting bodies on the various treatments measured in grams using an electronic scale. Biological yield was highest in treatment 2 (2645 g), second in treatment 1 (2525 g), third in treatment 3 (1721 g) and least in treatment 4 (110 g). Figure 9 and Table 2 show the biological yield of the different substrates.
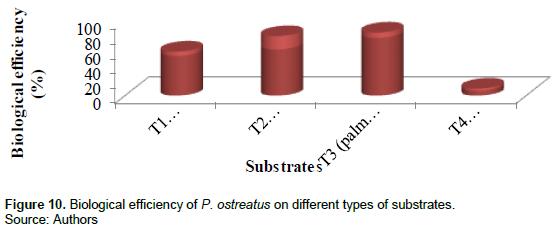
Biological efficiency
The biological efficiency as calculated from the total fresh weights of mushrooms divided by the dry weight of substrates x 100. The highest biological efficiency (77.5%) was obtained in treatment 3, treatment 2 (61.2%), treatment 1 (53.1%) and the lowest in treatment 4(6.3%). Figure 10 and Table 2 show the biological efficiencies of the different substrates.
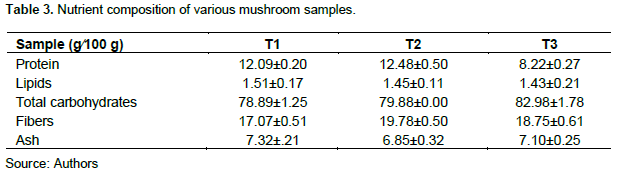
Nutritional analysis
Table 3 shows the composition of various nutrients found in P. ostreatus on different substrates. Protein content was highest in sawdust + corn cobs (12.4 g), lipid concentration highest in sawdust only (1.51 g), total carbohydrate highest in palm cones (82.98 g), fibers highest in sawdust + corn cobs (19.78 g) and total ash highest in sawdust only (7.32 g) per 100 g.
Phytochemical analysis
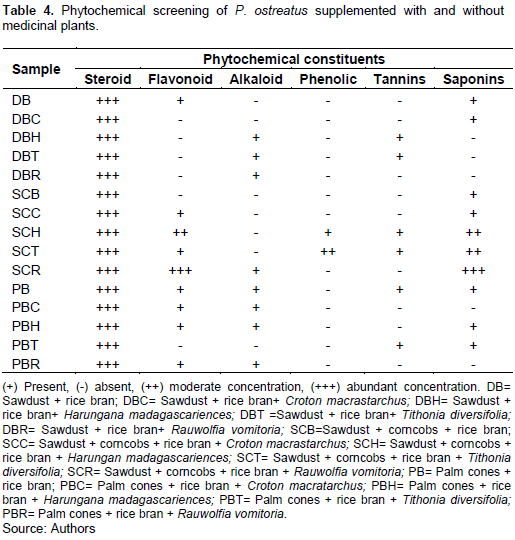
Table 4 shows the various phytochemical compounds and their concentrations found in P. ostreatus on different treatments. According to our finding, steroids were highly concentrated in most of the treatments (those with and without medicinal plants). Supplementation of SCB (Sawdust + corncobs) with R. vomitoria presented the highest concentrations of steroids, flavonoids and saponins (+++) and presence of alkaloids (+). SCB (sawdust + corncobs without supplementation) presented the lowest concentration of bioactive components with steroids highly concentrated (+++), saponins just present (+) and other components absent. Alkaloids were present in some of the treatments supplemented with medicinal plants (DBH, DBT, DBR, SCR, PBC, PBH and PBR).
DISCUSSION
Effects of different substrates on the growth and yield of P. ostreatus
Mycelia growth was faster in palm cones (T3) (14.4 ± 0.8 days) and slowest in sawdust (24.2 ± 1.3 days). Formation of pinhead appeared earlier in palm cones (21.8 ± 2.1 days) after the period of incubation and last in sawdust (46.3 ± 1.7 days). Pin head formation is affected by temperature and humidity; below 17°C pinhead formation is delayed. Composition of growth medium also affects pinhead formation time (Pathmashini et al., 2008); shortest time of primordial initiation in corncobs and palm cones has been reported by Ajonina and Tata (2012). Pinhead formation was prevented in some replications as a result of fungal infection; two replications of the treatment 1 and 1 replication of treatment 2 (sawdust and corn cobs + sawdust). This prevented the expected mycelium growth. Earnshaw et al. (2012) and Oseni et al. (2012) have reported similar infections and their detrimental effects on mycelium.
Days from pinhead formation to harvesting was significantly similar in the treatments 1, 2, 3 (corn cobs and palm cones) when compared with the control (sawdust) (6.8 ± 0.4 days) and higher in treatment 4 (elephant grass) (11.7 ± 0.7 days). The time from the pinhead formation to the first harvest for P. ostreatus was around 6±1 days, agreeing with those of Iqbal et al. (2005) who conducted similar research and who reported 46±3 days for this stage of development. Height of fruiting bodies differed on the different substrates. T2 (corn cobs + sawdust) presented the highest height (19.5 ± 3.3 cm) while the least height was recorded in T4 (elephant grass) when compared with sawdust (control). This result is in line with the findings of Rambey et al. (2018) who reported that sawdust can be mixed with corncobs to enhance productivity. Diameter of pileus of the fruiting bodies was higher in corncobs + sawdust (29.0 ± 4.3 cm) and lowest in elephant grass (11.3 ± 1.2 cm) when compared with sawdust (control) in P. ostreatus. Pileus diameter on corn cobs + saw dust was statistically similar. The outcome is in line with the findings of Ajonina and Tatah (2012) who reported highest diameter of pileus in corncobs and palm cones during their experiment. Mean number of effective fruiting bodies in the various treatments were significantly similar in treatment 1, 2 and 3 (4.7 ± 1.4) (corncobs + sawdust and palm cones) and lower in treatment 4 (elephant grass) when compared with sawdust. This outcome is similar to the findings of Ajonina and Tatah (2012) who reported highest number of fruiting bodies in sawdust and corn cobs. These results might be due to the presence of glucose, fructose and trehalose in the substrate as reported by Kitamoto et al. (1995). The highest weight of individual fruiting bodies of P. ostreatus was recorded in corncobs + sawdust (175.8 ± 84.3 g) and lowest in elephant grass (11.5 ± 3.8 g) when compared with sawdust. This result is similar to the findings of Rambey et al. (2018) who confirmed best yield of P. ostreatus when cultivated on corncobs mixed with sawdust.
Biological yield varied significantly because of different substrate compositions. The highest biological yield (2645 g) was obtained in corncobs + sawdust and the lowest biological yield (110 g) was obtained in elephant grass. Biological yield in corncobs+ sawdust was significantly similar with biological yield on sawdust (control). Biological yield value of 2645 g obtained on the sawdust + corn cobs substrate was higher than the biological yield value of 213.5 g reported by Hawrez (2018) on wheat straw. Biological efficiency is the most important parameter in mushroom growing. Highest biological efficiency (77.1 ± 7.1%) was recorded in palm cones, (61.1 ± 17.7%) in corncobs + sawdust, (53.0 ± 5.8%) in sawdust (control), and lowest in elephant grass (6.3 ± 3.1%). The highest biological efficiency value of (77.1%) obtained on the palm corn substrate was higher than the biological efficiency (66.41 %) reported by Hawrez (2018) on wheat straw substrate.
Nutritional analysis
Nutritional composition of mushroom is affected by many factors among which the composition of the substrate is of major importance. This can be supported by the results in the findings where Pleurotus florida and P. ostreatus grown on sawdust gave a significant nutritional value than that cultivated on corn cobs (Shah et al., 2004). During our trial we discovered that protein content was highest in sawdust + corn cobs substrate (12.48 g/100 g) and least in palm cones (8.22 g/100 g). It is well known that the protein content of mushrooms varies with the type of substrate as a result of the differences in nutrient supply (Gupta et al., 2013). This result is similar to that of Jin et al. (2018) who reported a protein value closer to our finding but contradicts the findings of Kinge et al. (2016) and Hawrez (2018) who reported of protein value higher than that of our findings.
Lipid content ranged from 1.43 to 1.51 g/100 g. Lipid content of oyster mushroom was affected by different substrates. Lipid content was maximum in sawdust (1.51 g/100 g) which was the control and minimum in palm cones (1.43 g/100 g). This result is similar to the findings of Barros et al. (2015) who reported lipid content range from 1.16-1.67 g/100 g for P. ostreatus but contradicts the report of Kinge et al. (2016) who reported a higher value of lipid content to that of our findings. Total carbohydrate ranged from 78.89 to 82.98 g/100 g. Total carbohydrates content varies depending on the type of substrates used. Total carbohydrate content was highest in palm cones (82.98 g) and minimum in sawdust (78.89g). The results of our finding were higher than the reported 61.9% for Croatian wild variety of P. osreatus studied by Beluhan and Ranogajec (2011), but less than the reported values of 73.2 to 78.1% in P. ostreatus studied by Fernandes et al. (2015). Crude fibre content ranged from 17.07 to 19.78 g/100 g. Crude fibre content varies depending on the type of substrate used. Fibre content was maximum in sawdust + corncobs (19.78 g) and minimum in the control which is saw dust only (17.07 g). This result is similar to the findings of Kinge et al. (2016) who reported a crude fibre content value maximum in corncobs (16.69 g) and minimum in sawdust (5.08 g) but contradicted by the findings of Teke et al. (2021) who reported a crude fibre value of 30.69 g obtained from the analysis of eight most preferred wild mushroom species from the Kilium-Ijim mountain forest in Cameroon. Total ash content varied depending on the type of substrate material used. Ash content ranged from 6.85 to 7.32 g/100 g. Total ash content was recorded as maximum in sawdust (7.32 g) which is the control and minimum in sawdust + corncobs (6.85 g). This result is similar to that of Hawrez (2018) who reported the ash content value in the range of 6.38 to 7.48 g/100 g.
Phytochemical analysis
Lindequist et al. (2005) stated that the nutritional and chemical compositions of mushroom are responsible for their medicinal values. The phytochemical analysis of P. ostreatus when supplemented with 4 medicinal plants revealed the presence of the following bioactive components; steroids, flavonoids, alkaloid, phenolic, tannins and saponins present in different concentrations in the different substrates and replications. Flavonoids, phenolic, tannins and saponins were present in P. ostreatus while alkaloid was absent (Kinge et al., 2016). During our experiment, we observed an increase in the concentration of bioactive components in P. ostreatus. The concentration and type of bioactive component varied depending on the substrate material used and the type of medicinal plant supplement. This is similar to the findings reported by Lindequist et al. (2005) who stated that the nutritional and chemical values of mushrooms are responsible for their medicinal values and equally, nutritional value of mushroom is affected by the type of substrate material used (Kinge et al., 2016).
The increase in the phytochemical component and thus the medicinal component of P. ostreatus is as a result of the supplementation with medicinal plants as medicinal plants contain phytochemical components which give them their medicinal properties. This result is in line with the findings of some researchers who reported an increase in the yield of oyster mushroom as a result of phytochemical components. This has been supported by an increase in the concentration of substances present in Neem cake which contain a mixture of tetranor triterpenoids (Govindachari et al., 1998), flavonoid, nimbosterol, liminoids, quercentinm, tannic acid, and substances found in citrus lemon, that is, volatile oil (Zeringue and Bhatnagar, 1994), limonene, alpha-pinene, beta-pinene, citral, coumarins, and bioflavonoids (Sammbamurty and Subrahmanyan, 2000). According to our findings, the concentrations of flavonoids, phenolic, tannins and saponins was highest in sawdust+ corn cobs + R. vomitoria. Alkaloids which are reported to be absent in oyster mushroom (Kinge et al., 2016) were present in some of the replications with medicinal plants in our trial. Alkaloids were present in replications: PBH, DBT, DBR, SCR, PBC, PBH, and PBR. Therefore, the supplementation of substrate with medicinal plants does not only improve on the growth and yield of the oyster mushroom according to Nazir et al. (2010) but also improves on the medicinal value.
CONCLUSION
Cultivation of oyster mushroom (P. ostreatus) on corn cobs substrate mixture with sawdust has a significant positive effect on the growth and yield of P. ostreatus. The nutritional content of oyster mushroom depends on the type of substrate material used. Protein and fibres content was highest in sawdust + corn cobs substrate making it the best substrate under investigation. Phytochemical components were present in oyster mushroom supplemented with medicinal plants more than in the control. Best result was presented by supplementation of sawdust + corn cobs with R. vomitoria. R. vomitoria is an anti-cancer plant. Oyster mushroom substrate can be supplemented with this plant during cultivation and the mushroom giving to cancer patients as a treatment. Therefore, mushroom growers are encouraged to supplement their oyster mushroom substrates with medicinal plants to improve on its medicinal value.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors are thankful to the Director of Mushroom Programme Training and Research Center (MUPTAREC) Bamenda and workers for their assistance during the experimental work. They thank Department of Chemistry, Faculty of Science, University of Buea, Southwest Region, Cameroon for assisting in the phytochemical analysis.
REFERENCES
|
Ajonina AS, Tatah LE (2012). Growth performance and yield of oyster mushroom (Pleurotus ostreatus) on different substrates composition in Buea Southwest of Cameroon. Science Journal of Biochemistry 6 p. |
|
|
Alakeh MN, Tamungang NEB, Kenneth M, Blaise YN, Biosengazeh, NF (2020). Trace Element Status and Environmental Implications of Soils and Zea mays from Farmed Dumpsites in the Bamenda Metropolis, North-West Cameroon. Applied and Environmental Soil Science. |
|
|
Anagho GB (2008). Mushroom Cultivation, a Manual for Farmers. Pp.19. |
|
|
Association of Official Analytical Chemists (AOAC) (1984). Official Methods of Analysis, 13thed. AOAC, Washington D.C. pp. 987-1012. |
|
|
Barros L, Pereira C, Ferreira ICFR (2008). Optimized analysis of organic acids in edible mushrooms from Portugal by ultra-fast liquid chromatography and photodiode array detection. Food Analytical Methods 6(1):309-316. |
|
|
Barros L, Cruz T, Baptista P, Estevinho LM, Ferreira IC (2015). Wild and commercial mushrooms as source of nutrients and nutraceuticals. Food and Chemical Toxicology 46(8):2742-2747. |
|
|
Beluhan S, Ranogajec A (2011). Chemical composition and non-volatile components of creation wild edible mushrooms. Food chemistry 124(3):1076-1082. |
|
|
Bhushan S, Kalia K, Sharma M, Singh B, Ahuja PS (2008). Processing of apple pomace for bioactive molecule. Critical Reviews in Biotechnology 28(4):285-296. |
|
|
Bonatti M (2004). Evaluation of Pleurotus ostreatus and Pleurotus sajor-caju nutritional characteristics when cultivated in different lignocellulosic wastes. Food Chemistry 88(3):425-428. |
|
|
Cardoso JCP, Demenjour PLMM, Paz MF (2013). Cultivo do cogumelo comestíve Pleurotus ostreatus embagaço de bocaiuva e de cana-de-açúcarpelatécnicajun-cao. Evidência 13(1):31-40. |
|
|
Carel JC, Hallmann C, Pecquet C, Bismut E, Raverdt C, Sola-Cazagns A (2013). Infusion allows tolerance induction and diabetes treatment in a type 1 diabetic child with insulin allergy. Diabetes and Metabolism 39(2):174-1773. |
|
|
Chang ST, Lau OW, Cho KY (1981). The Cultivation and Nutritional Value of Pleurotus sajor-caju. European Journal of Applied Microbiology and Biotechnology 12(1):58-62. |
|
|
Cohen R, Persky L, Hadar Y (2002). Biotechnological applications and potential of wood degrading mushrooms of the genus Pleurotus. Applied microbiology and biotechnology 58(5):582-594. |
|
|
Dawoud MEA, Eweis M, Rizk MA (2005). Chemical control of some edible mushroom pathogenic bacteria. N. Egypt Journal of Microbiology 12:24-37. |
|
|
Dias ES, Koshikumo ÉMS, Schwan RF, Silva R (2003). Cultivation of the mushroom Pleurotus sajor-cajuin different agricultural residues. Ciência e Agrotecnologia 27:1363-1369. |
|
|
Earnshaw VA, Smith LR, Chaudoir SR (2012). Stereotypes about people living with HIV: implications for perceptions of HIV risk and testing frequency among at-risk populations. AIDS Education and Prevention. 24(6):574-581. |
|
|
Fan L, Soccol CR, Pandey A (2000). Produção de cogumelo comestível Pleurotus emcasca de café e avaliação do grau de detoxificação do substrato. Simpósio de Pesquisa dos Cafés do Brasil 1:687-670. |
|
|
Fernandes A, Barros L, Martins A, Herbert P, Ferreira ICFR (2015). Nutritional characterization of Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm. produced using paper scraps as substrate. Food Chemistry 169:396-400. |
|
|
Govindachari TR, Suresh G, Gopalakrisnan G, Banumathy B, Masilamani S (1998). Identification of anti-fungal compounds from the seed oil of Azadirachta indica. Phytoparasitica 26(2):109-116. |
|
|
Gupta A, Sharma S, Saha S (2013). Yield and nutritional content of P. sajor caju on wheat straw supplemented with raw and detoxified mahua cake. Food Chemistry 141(4):4231-4239. |
|
|
Hawrez AN (2018). Effect of different substrates on the yield and quality of oyster mushroom (Pleurotus ostreatus). Master thesis 60 p. |
|
|
Hyde KD, Xu J, Rapior S, Jeewon R, Lumyong S, Niego AGT, Abeywickrama PD, Aluthmuhandiram JVS, Brahamanage RS, Brooks S, Chaiyasen A (2019). The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Diversity 97(1):1-136. |
|
|
Iqbal S, Bhanger MI, Anwar F (2005). Antioxidant properties and components of some commercially available varieties of rice bran in Pakistan. Food Chemistry 93(2):265-272. |
|
|
Jin Z, Li Y, Ren J, Qin N (2018). Yield, nutritional content and antioxidant activity of P. ostreatus on corncobs supplement with herb recidue. Mycobiology 46(1):24-32. |
|
|
Kinge TR, Adi EM, Mih AM, Nji TM (2016). Effects of substrate on the growth, nutritional and bioactive components of P. ostreatus and P. florida. African Journal of Biotechnology 15(27):1476-1486. |
|
|
Kitamoto Y, Horkoshi T, Hosio N, Ichikawa Y (1995). Nutritional study of fruiting-body formation in Psilocybe panaeoliformis. Transaction of Mycological Society of Japan 16(3):268-281. |
|
|
Lindequist U, Niedemer THJ, Julich WD (2005). The pharmacological potential of mushrooms. Evidence-Based Complementary and Alternative Medicine 2(3):285-299. |
|
|
Loss EMS (2009). Aproveitamento de resíduos da cadeia produtiva do milho para cultivo de cogumelos comestíveis. Dissertation. State University of Ponta Grossa, Paraná, Brasil pp. 1-15. |
|
|
MacNee W (2005). Pathogenesis of chronic obstructive pulmonary disease. Proceedings of American Thoracic Society 2(4):258-266. |
|
|
Mata G, Hernandez DM, Andreu LG (2005). Changes in lignocelluloytic enzyme activitiein six Pleurotus spp. strains cultivated on coffee pulp in confrontation with Trichoderma spp. World Journal Microbiology and Biotechnology 21(2):143-150. |
|
|
Mehta V, Gupta JK, Kaushal SC (1990). Cultivation of Pleurotus florida mushroom on rice straw and biogas production from the spent straw. World Journal of Microbiology and Biotechnology 6(4):366-370. |
|
|
Nazir J, Muhammad AK, Rana B, Gulshan I (2010). Use of medicinal plants in different composts for yield improvement of various strains of oyster mushroom. Pakistan Journal of Botany 42(5):3275-3283. |
|
|
Osemwegie OO, Idu M, Ndukwu BC (2007). Ethnofloristic studies of Ethiopia Council area of Delta State, Nigeria. Journal of Plant Sciences 2(1):1-13. |
|
|
Oseni TO, Dlamini SO, Earnshaw DM, Masarirambi MT (2012). Effect of substrate pre-treatment methods on oyster mushroom (Pleurotus ostreatus) production. International Journal of Agriculture and Biology 14(2):251-255. |
|
|
Park CH, Lim J, Lee Y, Lee B, Kim S, Lee J, Kim S (2014). Optimization and morphology for decolorization of reactive black 5 by Funalia trogii. Enzyme and Microbial Technology 40(7)1758-1764. |
|
|
Pathmashini L, Arulnandhy V, Wijeratnam RSW (2008). Cultivation of oyster mushroom (Pleurotus ostreatus) on sawdust. Ceylon Journal of Science 37(2):177-182. |
|
|
Pokhrel CP, Kalyan N, Budathoki U, Yadav RKP (2013). Cultivation of Pleurotus sajor-caju using different agricultural residues. International Journal of Agricultural Policy and Research 1(2):19-23. |
|
|
Raghuramulu N, Madhavan N, Kalyanasundaram K (2003). A Manual of Laboratory Techniques. National Institute of Nutrition. Indian Council of Medical Research, India pp. 1-38. |
|
|
Raja J, Kurcheve V (1999). Fungicidal activity of plant and animal products. Annals of Agricultural Research 20(1):113-115. |
|
|
Rambey R, Matondang GPN, Siregar EBM (2018). Growth and productivity of oyster mushroom (P. ostreatus) on mixed planting media of coco peat with sawdust. IOP Conference Series: Earth and Environmental Science 454(1):209. |
|
|
Ramos JAA, Barletta M, Dantas DV, Lima ARA, Costa MF (2011). Influence of moon phase on fish assemblages in Estuarine mangrove tidal creeks. Journal of Fish Biology 78(1):344-354. |
|
|
Sammbamurty AVSS, Subrahmanyan NS (2000). Medicinal plants in industry. C.B.S Publishers, New Delhi pp. 22-64. |
|
|
Sanchez A, Ysunza F, Beltran-Gracia M, Esqueda M (2002). Biodegradation of Viticulture Wastes by Pleurotus: A Source of Microbial and Human Food and Its Potential Use in Animal Feeding. Journal of Agricultural and Food Chemistry 50(9):2537-2542. |
|
|
Shah ZA, Ashraf M, Ishhaq, CH (2004). Comparative study on cultivation and yield performance of oyster mushroom (P. ostreatus) on different substrates (wheat straw leaves, saw dust). Pakistan Journal of Nutrition 3(3):158-160. |
|
|
Teke AN, Manju EB, Monah LN, Kinge RK (2021). Nutrient and mineral components of wild edible mushrooms from the Kilum-Ijim forest, Cameroon. African Journal of Food Science 15(4):152-161. |
|
|
Vendrusco F, Albuquerque PCM, Streit F, Esposito E, Ninow J (2008). Apple pomace: a versatile substrate for biotechnological applications. Critical Reviews in Biotechnology 28(1):1-12. |
|
|
Wojewoda W (2003). Checklist of polish large Basidiomycetes. Biodiversity of Poland 6:435. |
|
|
Zeringue HJJ, Bhatnagar D (1994). Effects of neem leaf volatiles on submerged cultures of aflatoxigenic Aspergillus parasiticus. Applied and Environmental Microbiology 60(10):3543-3547. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0