ABSTRACT
Azospirillum brasilense strains Ab-V5 and Ab-V6 have been broadly and successfully used in commercial inoculants in Brazil, for both non-legumes and legumes, contributing to increases in grain yields with reduced applications of chemical fertilizers. Azospirillum survival, however, may be very low in liquid inoculant formulations and strategies such as the enrichment with polyhydroxybutyrate (PHB) and biofilm may help both bacterial survival and agronomic performance. The production was quantified for both PHB and biofilm by strains Ab-V5 and Ab-V6 in liquid inoculant formulations. Differences were observed between formulations, strains, and strain x formulation. Cellular PHB concentrations ranged from 7.9 to 40.2% of the cell dry weight after 96 h, and considerable amounts of biofilm were synthesized by both Ab-V5 and Ab-V6. Maximum accumulation of PHB and biofilm occurred with A. brasilense strain Ab-V6 in the formulation FORM2+P3, indicating that it is possible to enrich the inoculants on PHB and biofilm by improving the culture media. Field experiments will now be performed to confirm the agronomic efficiency of the improved inoculant.
Key words: Polyhydroxybutyrate, inoculation, N2 fixation, plant-growth-promoting bacteria, plant-growth-promoting bacteria (PGPB).
Nitrogen (N) is the nutrient most required for plant growth, and agricultural production and productivity are directly related to its availability to crops. The majority of the Brazilian soils are poor in N, requiring an intense management of chemical N-fertilizers, but the country imports over 70% of the N needed for annual production, resulting in high cost for the farmers (Hungria et al., 2013a). Although N-fertilizers can be easily assimilated by plants, they are also subject to severe losses by leaching and emission into gaseous forms, leading to water pollution, ozone-layer depletion and global warming (Hungria et al., 2013a; Sá et al., 2017). In this context, the search for alternative biotechnological products, with an emphasis on microbial inoculants, aiming at the total or partial substitution of N-fertilizers, through innovative practices that are environmentally friendly but capable of maintaining high agronomic yields, has been a major goal of investigation (Hungria et al., 2005; Souza et al., 2015; O´Callaghan, 2016; Pereg et al., 2016; Mahanty et al., 2017).
Biological nitrogen fixation (BNF) represents a cheap and sustainable alternative to N-fertilizers and can be promoted by seed inoculation with elite diazotrophic bacteria, contributing to plant’s N nutrition (Hungria et al., 2005; Malusá et al., 2012; Ormeño-Orrillo et al., 2013). Although the main contribution of BNF is derived from the symbiosis of bacteria collectively known as rhizobia with legumes, other diazotrophic bacteria less specifically associated with plants, associative or endophytically and contributing to lower amounts of nitrogen can also be important to global saving of N-fertilizers (Bashan and de-Bashan, 2010; Ormeño-Orrillo et al., 2013; Pereg et al., 2016). Furthermore, many of these bacteria have addi-tional mechanisms such as the synthesis of phytohormones, induction of plant-stress tolerance and defense genes, among others (Bashan and de-Bashan, 2010; Fukami et al., 2017a, b), which may help to promote plant growth. Due to their multiple beneficial mechanisms, these bacteria have been classified as plant-growth-promoting bacteria (PGPB), and among them, those belonging to the Azospirillum brasilense species are the most studied and employed as inoculants worldwide, with consistent responses to inoculation in all continents, highly contributing to the economy of chemical fertilizers (Okon and Labandera-Gonzalez, 1994; Dobbelaere et al., 2001; Hartmann and Bashan, 2009; Bashan and de-Bashan, 2010; Hungria et al., 2010; Okon et al., 2015; Cassán and Diaz-Zorita, 2016; Pereg et al., 2016; Fukami et al., 2016, 2017a, b).
Liquid inoculants carrying PGPB are easy to handle and can be applied to seeds and by foliar spraying; however, in general, they provide inadequate protection to bacterial cells, leading to early cell death (Stephens and Rask, 2000; Hungria et al., 2005; Tittabutr et al., 2007; Bashan et al., 2014). The improvement of shelf life and maintenance of cell viability can be achieved with the addition of protective molecules such as polymers, which help to maintain water activity and serve as additional supply of carbon and energy to the bacteria (Mugnier and Jung, 1985; Okon and Itzigsohn, 1995; Hungria et al., 2005; Trujillo-Roldán et al., 2013).
Cell viability can also be improved by the synthesis of polyhydroxybutyrate (PHB), a reserve polymer that allows bacteria to withstand environmental stresses (Tal and Okon, 1985; Kadouri et al., 2003; Bhat and Subin, 2015). Kadouri et al. (2003) observed that the synthesis and use of PHB as carbon and energy sources by A. brasilense strain Sp7 favored the establishment of this bacterium and its survival in competitive environments under stress conditions. Therefore, it is expected that the accumulation of PHB may favor cell longevity in inoculants. In addition to PHB, biofilm formation provides several benefits to the bacterial community, such as the improvement in bacterial cell-to-cell communication, tolerance of stressful environmental conditions, and host plant colonization (Morris and Monier, 2003; Kreft, 2004; Fukami et al., 2017a). Although studies about the application of biofilms for the benefit of agriculture are scarce, they could contribute to the success of inoculation, protecting bacterial cells from the competition with other soil microbial communities and improving BNF (Jayasinghearachchi and Seneviratne, 2004; Karivaradharajan et al., 2013; Fukami et al., 2017a).
In Brazil, the utilization of A. brasilense strains Ab-V5 and Ab-V6 for maize (Zea mays L.), wheat (Triticum aestivum L.), and brachiarias (Urochloa species), as well as for co-inoculation of soybean (Glycine max (L.) Merr.) and common bean (Phaseolus vulgaris L.) has exponentially grown since 2009 (Hungria et al., 2010, 2013b, 2015, 2016; Hungria, 2011; Marks et al., 2015; Fukami et al., 2016). The objective of this study was to evaluate the production of PHB and biofilm by these two strains of A. brasilense in new formulations of liquid inoculants.
Bacterial strains and growth conditions
The study was performed with A. brasilense strains Ab-V5 (=CNPSo 2083) and Ab-V6 (=CNPSo 2084) from the Diazotrophic and Plant Growth-Promoting Bacteria Culture Collection of Embrapa Soja (WFCC #1213, WDCM #1054). Both strains derived from a selection program for cereals (Hungria et al., 2010), and are currently employed for commercial production of Azospirillum inoculants in Brazil. The strains were grown in liquid media (FORM2+P3 and FORM4+P6) previously developed in our laboratory (Santos, 2017); the formulations are under registration.
Evaluation of cell dry weight and polyhydroxybutyrate
The strains were single cultured in 500 mL of the liquid formulations (FORM2+P3 and FORM4+P6) for 96 h, at 28±2°C, with agitation of 140 rpm. At 48, 72, and 96 h, 10 mL samples of each treatment were withdrawn and centrifuged at 7,690 g for 15 min at 4°C. The supernatant was discarded and the cell mass in the precipitated fraction was washed twice with 5 mL of saline solution (NaCl, 0.85%), followed by further centrifugation, and the supernatant was discarded. The samples were dried at 70°C until constant weight and weighed to determine the cell dry weight (CDW, g L-1) (Belal, 2013). The cells were re-suspended in 12 mL of sodium hypochlorite (NaClO, 5.25%) and incubated for 2 h at 40°C (Karr et al., 1983). The mixture was centrifuged at 2,370 g for 15 min at 4°C, and the supernatant discarded. The precipitated fraction was washed successively with 10 mL of distilled water and 96% ethanol (C2H6O) (Karr et al., 1983; Hawas et al., 2016). The material was oven dried at 70°C, 1 mL of concentrated sulfuric acid (H2SO4) was added, and maintained at 90°C for 30 min, followed by cooling. PHB concentration was obtained by determining the optical density OD235, considering sulfuric acid PA as the blank (Law and Slepecky, 1960). The amount of PHB present in the sample is provided by the estimate of the crotonic acid (C4H6O2) derived from the reaction of the sample with sulfuric acid, using the extinction coefficient of the crotonic acid (1.55 × 104 M-1 cm-1). The experiment was performed with three biological replicates, each with three replicates, for each strain and formulation.
From the obtained values of CDW and the absolute PHB concentration of each sample, it was possible to estimate the relative PHB concentration (%), as follows:
Biofilm formation
Biofilm formation was evaluated after Christensen et al. (1985), with some modifications. The strains were single cultured in 100 mL of the liquid formulations (FORM2+P3 and FORM4+P6) and also in NFb medium (Döbereiner, 1991) for 24 h at 28±2°C, with agitation of 140 rpm. The cultures were then diluted to an OD600 of 0.2 and 100 μL of cell suspensions, and transferred to each well of polystyrene microplates with U-bottom (Deltalab S.L.), for each of the three culture media. Plates were incubated in a humid chamber at 28±2°C for 14 days. The culture medium was carefully removed from each well and the plates were dried at 60°C for 1 h, followed by three washes by immersion in 0.9% NaCl and dried at 60°C for 1 h. Next, 100 μL of 0.1% crystal violet (C25N3H30Cl. aqueous solution) were added per well and kept for 20 min. Plates were washed three times by immersion with distilled water. After drying for 1 h at 60°C, 100 μL of 96% ethanol were added to each well and the plates were allowed to stir gently until all the crystal violet was dissolved. Finally, the OD570 was determined. The experiment was performed with three biological replicates, each with three replicates, for each strain and formulation.
Statistical analysis
The experimental design was entirely randomized in 2 × 2 factorial, always with three biological replicates, each with three replicates.
The data were submitted to the analysis of homogeneity of variance and the means were compared by the Tukey’s test (p ≤ 0.05) using the STATISTICA 7.0 program.
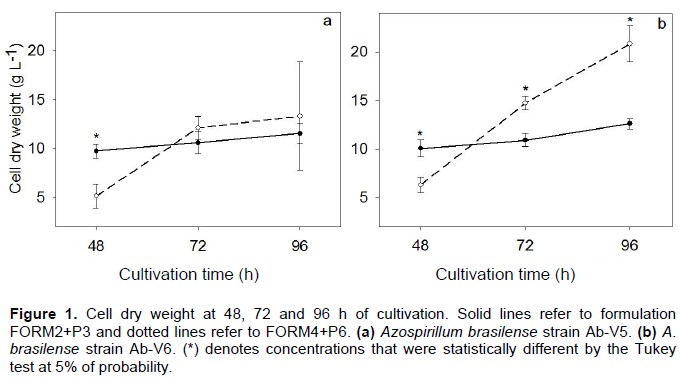
Increases in CDW were observed during the 96 h of growth in both liquid formulations, FORM2+P3 and FORM4+P6, for both strains of A. brasilense, Ab-V5 and Ab-V6 (Figure 1). Differences were observed between the strains, being always higher with Ab-V6. Differences were also observed due to the interaction strain × formulation; growth of Ab-V5 was favored by the composition of FORM2+P3, especially in the first 72 h, while for Ab-V6 the CDW at 72 and 96 h was considerably higher in FORM4+6 (Figure 1).
The production of PHB ranged from 2.13 to 5.07 g L-1 in FORM2+P3 and from 1.16 to 1.76 g L-1 in FORM4+P6, being always higher in the first formulation (Figure 2). Differences between strains were also observed, being always higher in Ab-V6 than in Ab-V5 when grown in FORM2+P3, but with similar and considerably lower accumulation when both strains were grown in FORM4+P6 (Figure 2).
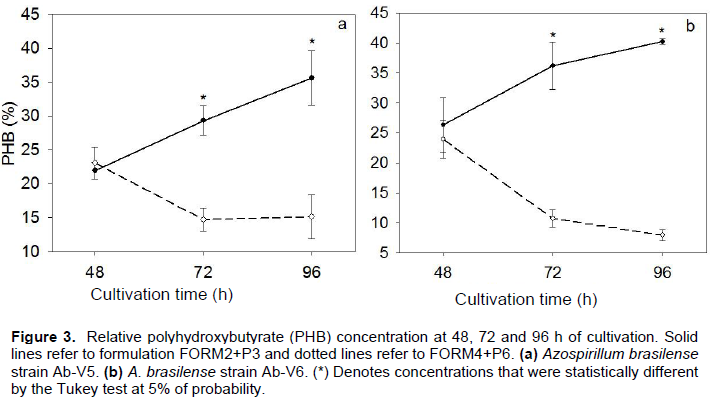
With the CDW values and the PHB concentration, it was possible to estimate the relative PHB concentration (%), which ranged from 7.9 to 40.2% of the CDW (Figure 3). PHB concentration was higher after 96 h of growth for both strains in FORM2+P3. However, in FORM4+P6 the concentration decreased after 48 h, reaching the minimum value after 96 h (Figure 3). Interestingly, although our strains were different in growth capacity, the concentration of PHB was similar for both, but the differences obtained in different media were noticeable, being 4-fold higher for Ab-V6 in FORM2+P3 than in FORM4+P6 (Figure 3).
Both strains produced similar amounts of biofilm in the formulations FORM2+P3 and FORM4+P6 (Figure 4). A. brasilense Ab-V5 synthesized considerable amounts of biofilm in the three culture media, with no differences between them. On the contrary, Ab-V6 strain produced less biofilm in NFb medium and higher in FORM2+P3 and FORM4+P6 (Figure 4).
Cell dry weight accumulation and accumulation of polyhydroxybutyrate
Differences in CDW accumulation were observed between A. brasilense strains Ab-V5 and Ab-V6, with more growth of Ab-V6, and also as a combined effect of strains × formulation, with more growth of Ab-V5 in FORM2+P3, while the growth of Ab-V6 was favored in FORM4+6. Although the genomes of the two strains show high identity of nucleotides (unpublished genomes, but with average nucleotide identity of 99.99%), differences in the CDW and growth in different formulations point out to probable differences in their metabolism. Other mechanisms controlling cell growth, such as quorum sensing (Boyer and Wisniewski-Dyé, 2009), deserve to be investigated, and indeed, Fukami et al. (2017a) have recently shown differences between Ab-V5 and Ab-V6 in quorum sensing mechanisms.
Differences were also observed between the strains and formulations in the production of PHB, overall being higher in Ab-V6 and in FORM2+P3. Variation in PHB can be attributed both to the composition of the culture medium and to the metabolism of each strain, as suggested by Kamnev et al. (2012). In Azospirillum, synthesis of PHB depends on the medium composition and higher PHB values can result in cell flocculation (or aggregation) or in cell changes known as cyst-forms. For example, in a study with A. brasilense strain MTCC-125, cell flocculation and higher PHB production (0.13 g L-1 of CDW) were observed in medium with cationic compounds (Joe and Sivakumaar, 2009), but the concentration was far lower than in our study with both Ab-V5 and Ab-V6.
PHB concentration (%) represented up to 40.2% of the CDW, being favored by FORM2+P3 with both strains. In Azospirillum lipoferum strain Az-204, Vendan and Thangaraju (2007) reported the production of 2.9 mg of PHB per gram of CDW (0.29%) after 96 h of culture in minimal salts medium, considerably lower than with our strains. In contrast, Kamnev et al. (2012) reported that after two days of growth in N-deficient medium and absence of malate, the PHB values obtained with strains Sp7 and Sp245 of A. brasilense were 24 and 32% of the CDW, respectively. In another study, Fallik and Okon (1996) reported that PHB in A. brasilense ATCC 29729 (Cd) reached 40% of CDW, similar values to those found in our study with strains Ab-V5 and Ab-V6.
Variation in PHB concentration may be related to the consumption of this biopolymer by the cells, as observed by Ratcliff et al. (2008) in the cultivation of Sinorhizobium meliloti under nutrient shortage. According to Tal and Okon (1985), the synthesis of PHB is favored under oxygen limitation and by the C:N ratio of the culture medium. In a study by Belal (2013), the best C:N ratio for the PHB production by Rhizobium etli E1 and Pseudomonas stutzeri E114 was 20:1. A similar ratio resulted in higher production of PHB by Bacillus cereus (Belal and Farid, 2016) and by Pseudomonas boreopolis J1 (Hawas et al., 2016). This should also explain the results of our study, as for both strains, higher PHB was obtained in FORM2+P3, with C:N ratio 15:1, than in FORM4+P6, with C:N ratio 6:1. In addition, culture media with high C:N ratio can positively influence the aggregation and flocculation of bacterial cells, while low C:N ratio results in more dispersed bacterial growth (Sadasivan and Neyra, 1985; Burdman et al., 1998, 2000), that may improve cell survival and root colonization.
Several beneficial properties of PGPB have been attributed to the synthesis of PHB. It may represent an important carbon and energy source for both cell growth and for the biological nitrogen fixation process in A. brasilense, in addition to improve cellular resistance against environmental stresses, such as UV radiation, heat, osmotic pressure, osmotic shock, and desiccation (Tal and Okon, 1985; Kadouri et al., 2003). Flocculation or cyst-forms, also attributed to PHB, is another important property; Joe and Sivakumaar (2009) observed that flocculating cells of A. brasilense strain MTCC-125 are rich in PHB and have high tolerance of dissection, while higher PHB accumulation in A. lipoferum Az-204 increased the percentage of cysts forms, and consequently, the tolerance of desiccation and high temperature (40°C) (Vendan and Thangaraju, 2007). Therefore, as indicated by Kadouri et al. (2003), survival of Azospirillum in inoculants may be favored by PHB, allowing increased cell viability. In addition, according to the same authors, apparently, the PHB as carbon and energy sources for A. brasilense under stress conditions favor the establishment of this bacterium and its survival in competitive environments, although no differences in root colonization were observed (Kadouri et al., 2003). Meanwhile, in field experiments, A. brasilense in peat inoculants enriched with PHB (40%) presented better and more consistent results in panicle length and dry weight of maize and foxtail millet (Setaria italic) (Fallik and Okon, 1996). Altogether, these reports suggest that the FORM2+P3 would be highly beneficial as inoculant formulation to both Ab-V5 and Ab-V6 strains, favoring the maintenance of cell viability.
Synthesis of biofilm
In this study, an interesting interaction of strain × formulation in the biofilm formation was found; the culture medium did not affect the production by strain Ab-V5, but in Ab-V6, it was significantly lower in the NFb medium. According to Donlan and Costerton (2002), some parameters may influence the biofilm formation, such as nutrient availability, temperature, microbial species and cell number. The composition of the NFb medium, relatively poor in nutrients, and lower in C source than our formulations could explain the lower biofilm production by strain Ab-V6. The results obtained with Ab-V6 corroborate the idea proposed by O'Toole et al. (2000), of continuous production of biofilm by bacteria under non-limited nutrient conditions. Watnick and Kolter (2000) suggested that biofilm-forming microorganisms can return to mobile lifestyles when nutrient availability becomes scarce, a likely response to the search for new sources of nutrients; the change in lifestyle may result in the colonization of new environments (Costerton et al., 1995). However, intriguingly, biofilm formation in Ab-V5 was not affected by the medium composition and as biofilm is also related to quorum sensing (Boyer and Wisniewski-Dyé, 2009; Fukami et al., 2017a), this is an additional evidence pointing out that the differences between the two strains might be related to this mechanism.
In Azospirillum spp., biofilm formation is key for root colonization; more specifically, bacteria migrate towards the root by chemotaxis and are attached to the root system, where they proliferate and form micro-colonies, which are fixed to the roots by means of biofilms (Compant et al., 2010; Santi et al., 2013). Noteworthy, Jayasinghearachchi and Seneviratne (2004) observed improvement on N2 fixation parameters of soybean when a bradyrhizobial-fungal biofilm inoculant (Bradyrhizobium elkanii SEMIA 5019 with Penicillium species) was used. Therefore, an inoculant enriched in biofilm could benefit plant growth, by improving root colonization by elite PGPB strains.
Inoculants carrying A. brasilense strains Ab-V5 and Ab-V6 have been exponentially commercialized in Brazil and other South American countries for both non-legume and legume crops (Hungria et al., 2010, 2013b, 2015, 2016; Hungria, 2011; Marks et al., 2015; Fukami et al., 2016). However, the successful performance of the bacteria relies on their survival under stressing conditions and on an adequate colonization of roots, and PHB accumulation and biofilm formation are keys for the achievement of both processes. Therefore, inoculants enriched with PHB and biofilm are of agronomic interest. In this study, the production of PHB and biofilm by two strains of A. brasilense in new formulations of liquid inoculants was evaluated, and the maximum accumulation of PHB and biofilm with A. brasilense strain Ab-V6 in the formulation FORM2+P3 was identified. Field experiments will now be performed to confirm the agronomic efficiency of this inoculant.
The authors have not declared any conflict of interests.
REFERENCES
|
Bashan Y, de-Bashan LE (2010). How the plant growth-promoting bacterium Azospirillum promotes plant growth- a critical assessment. Adv. Agron. 108:77-136.
Crossref
|
|
|
|
Bashan Y, de-Bashan LE, Prabhu SR, Hernandez J (2014). Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 378:1-33.
Crossref
|
|
|
|
|
Belal EB (2013). Production of Poly-hydroxybutyric acid (PHB) by Rhizobium etli and Pseudomonas stutzeri. Curr. Res. J. Biol. Sci. 5:273-284.
|
|
|
|
|
Belal EB, Farid MA (2016). Production of Poly-β-hydroxybutyric acid (PHB) by Bacillus cereus. Int. J. Curr. Microbiol. Appl. Sci. 5:442-460.
Crossref
|
|
|
|
|
Bhat SG, Subin RS (2015). Bacterial polyhydroxyalkanoates production and its applications. In. Bhat SG, Nambisan P (eds.), Microbial Bioproducts. Directorate of Public Relation and Publication, Kerala, India. pp. 70-96.
|
|
|
|
|
Boyer M, Wisniewski-Dyé F (2009). Cell-cell signalling in bacteria: Not simply a matter of quorum. FEMS Microbiol. Ecol. 70:1-19.
Crossref
|
|
|
|
|
Burdman S, Jurkevitch E, Shwartsburd B, Hampel M, Okon Y (1998). Aggregation in Azospirillum brasilense: effects of chemical and physical factors and involvement of extracellular components. Microbiology 144:1989-1999.
Crossref
|
|
|
|
|
Burdman S, Jurkevitch E, Soria-Diaz ME, Serrano AMG, Okon Y (2000). Extracellular polysaccharide composition of Azospirillum brasilense and its relation with cell aggregation. FEMS Microbiol. Lett. 189:259-264.
Crossref
|
|
|
|
|
Cassán F, Diaz-Zorita M (2016). Azospirillum sp. in current agriculture: From the laboratory to the field. Soil Biol. Biochem. 103:117-130.
Crossref
|
|
|
|
|
Compant S, Cle’Ment C, Sessitsch A (2010). Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 42:669-678.
Crossref
|
|
|
|
|
Costerton JW (1995). Overview of microbial biofilms. J. Ind. Microbiol. 15:137-40.
Crossref
|
|
|
|
|
Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH (1985). Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006.
|
|
|
|
|
Dobbelaere S, Croonenborghs A, Thys A, Ptacek D, Vanderleyden J, Dutto P, Labandera-Gonzalez C, Caballero-Mellado J, Aguirre JF, Kapulnik Y, Brener S, Burdman S, Kadouri D, Sarig S, Okon Y (2001). Responses of agronomically important crops to inoculation with Azospirillum. Aust. J. Plant Physiol. 28:871-879.
|
|
|
|
|
Döbereiner J (1991). The genera Azospirillum and Herbaspirillum. In. Ballows A, Trüper HG, Dworkin M, Harder W, Shleifer K, (eds.), The Prokaryotes. Springer-Verlag, New York. pp. 2236-2253.
|
|
|
|
|
Donlan RM, Costerton JW (2002). Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193.
Crossref
|
|
|
|
|
Fallik E, Okon Y (1996). Inoculants of Azospirillum brasilense: Biomass production, survival and growth promotion of Setaria italica and Zea mays. Soil Biol. Biochem. 28:123-126.
Crossref
|
|
|
|
|
Fukami J, Abrantes JLF, del Cerro P, Nogueira MA, Megías M, Ollero FJ, Hungria M (2017a). Revealing different strategies of quorum sensing in Azospirillum brasilense strains Ab-V5 and Ab-V6. Arch. Microbiol.
|
|
|
|
|
Fukami J, Nogueira MA, Araujo RS, Hungria M (2016) Accessing inoculation methods of maize and wheat with Azospirillum brasilense. AMB Express 6(3):1-13.
Crossref
|
|
|
|
|
Fukami J, Ollero FJ, Megías M, Hungria M (2017b). Phytohormones and induction of plant-stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth. AMB Express 7:153.
Crossref
|
|
|
|
|
Hartmann A, Bashan Y (2009). Ecology and application of Azospirillum and other plant growth-promoting bacteria (PGPB). Eur. J. Soil Biol. 45:1-2.
Crossref
|
|
|
|
|
Hawas LME, El-Banna TE, Belal EBA, El-Aziz AA (2016). Production of bioplastic from some selected bacterial strains. Int. J. Curr. Microbiol. Appl. Sci. 5:10-22.
Crossref
|
|
|
|
|
Hungria M (2011). Inoculação com Azospirillum brasilense: Inovação em Rendimento a Baixo Custo. Documentos, 325, Embrapa Soja, Londrina, Brazil, 37p.
|
|
|
|
|
Hungria M, Campo RJ, Souza EM, Pedrosa FO (2010). Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 331:413-425.
Crossref
|
|
|
|
|
Hungria M, Loureiro MF, Mendes IC, Campo RJ, Graham PH (2005). Inoculant preparation, production and application. In. Werner W. Newton WE (eds.), Nitrogen Fixation in Agriculture, Forestry, Ecology and the Environment. Springer, Dordrecht, Amsterdam. Pp. 223-254.
Crossref
|
|
|
|
|
Hungria M, Mendes IC, Mercante FM (2013a). A Fixação Biológica do Nitrogênio como Tecnologia de Baixa Emissão de Carbono: Avaliação nas Culturas do Feijoeiro e da Soja. Documentos, 337, Embrapa Soja, Londrina, Brazil, 22p.
|
|
|
|
|
Hungria M, Nogueira MA, Araujo RS (2013b). Co-inoculation of soybeans and common beans with rhizobia and azospirilla: Strategies to improve sustainability. Biol. Fertil. Soils 49(7):791-801.
Crossref
|
|
|
|
|
Hungria M, Nogueira MA, Araujo RS (2015). Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A new biotechnological tool to improve yield and sustainability. Am. J. Plant Sci. 6:811-817.
Crossref
|
|
|
|
|
Hungria M, Nogueira MA, Araujo RS (2016). Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: an environment-friendly component in the reclamation of degraded pastures in the tropics. Agric. Ecosyst. Environ. 221:125-131.
Crossref
|
|
|
|
|
Jayasinghearachchi HS, Seneviratne GA (2004). A bradyrhizobial-Penicillium spp. biofilm with nitrogenase activity improves N2 fixing symbiosis of soybean. Biol. Fertil. Soils 40:432-434.
Crossref
|
|
|
|
|
Joe MM, Sivakumaar PK (2009). Role of certain cationic compounds on the enhancement of flocculation in Azospirillum brasilense MTCC-125: Bioinoculation effect on growth of sunflower. Die Bodenkultur: J. Land Manag. Food Environ. 60:5-13.
|
|
|
|
|
Kadouri D, Jurkevitch E, Okon Y (2003). Involvement of the reserve material poly-β-hydroxybutyrate in Azospirillum brasilense stress endurance and root colonization. Appl. Environ. Microbiol. 69:3244-3250.
Crossref
|
|
|
|
|
Kamnev AA, Tugarova AV, Tarantilis PA, Gardiner PHE (2012). Comparing poly-3-hydroxybutytateaccumulation in Azospirillum brasilense strains Sp7 and Sp245: The effect of copper (II). Appl. Soil Ecol. 61:213-216.
Crossref
|
|
|
|
|
Karivaradharajan S, Prasanna R, Kumar A, Pattnaik S, Chakravarty K, Shivay YS, Singh R, Saxena AK (2013). Evaluating the influence of novel cyanobacterial biofilmed biofertilizers on soil fertility and plant nutrition in wheat. Eur. J. Soil Biol. 55:107-116.
Crossref
|
|
|
|
|
Karr DB, Waters JK, Emerich DW (1983). Analysis of Poly-,3-Hydroxybutyrate in Rhizobium japonicum Bacteroids by Ion-Exclusion High-Pressure Liquid Chromatography and UV Detection. Appl. Environ. Microbiol. 46:1339-1344.
|
|
|
|
|
Kreft JU (2004). Biofilms promote altruism. Microbiology 150:2751-2760.
Crossref
|
|
|
|
|
Law JH, Slepecky RA (1960). Assay of poly-β-hydroxybutyric acid. J. Bacteriol. 82:33-36.
|
|
|
|
|
Mahanty T, Bhattacharjee S, Goswami M, Bhattacharyya P das B, Ghosh A, Tribedi P (2017) Biofertilizers: a potential approach for sustainable agricultrue development. Environ. Sci. Pollut. Res. 24:3315-3335.
Crossref
|
|
|
|
|
Malusá E, Sas-Paszt L, Ciesielska J (2012). Technologies for beneï¬cial microorganisms inocula used as biofertilizers. Sci. World J 2012:1-12.
Crossref
|
|
|
|
|
Marks BB, Megías M, Ollero FJ, Nogueira MA, Araujo RS, Hungria M (2015). Maize growth promotion by inoculation with Azospirillum brasilense and metabolites of Rhizobium tropici enriched on lipo-chitooligosaccharides (LCOs). AMB Express 5:71.
Crossref
|
|
|
|
|
Morris CE, Monier JM (2003). The ecology significance of biofilm formation by plant-associeted bacteria. Annu. Rev. Phytophatol. 41:429-453.
Crossref
|
|
|
|
|
Mugnier J, Jung G (1985). Survival of bacteria and fungi in relation to water activity and the solvent properties of water in biopolymer. Appl. Environ. Microbiol. 50:108-114.
|
|
|
|
|
O’Callaghan M (2016). Microbial inoculation of seed for improved crop performance: issues and opportunities. Appl. Microbiol. Biotechnol. 100:5729-5746.
Crossref
|
|
|
|
|
O'Toole G, Kaplan HB, Kolter R (2000). Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79.
Crossref
|
|
|
|
|
Okon Y, Itzigsohn R (1995). The development of Azospirillum as a commercial inoculant for improving crop yields. Biotechnol. Adv. 13:415-424.
Crossref
|
|
|
|
|
Okon Y, Labandera-Gonzales C, Lage M, Lage P (2015). Agronomic applications of Azospirillum and other PGPR. In. de Brujin FJ (eds.), Biological Nitrogen Fixation. John Wiley and Sons Inc., Hoboken. Pp. 921-933.
|
|
|
|
|
Okon Y, Labandera-Gonzalez C (1994). Agronomic applications of Azospirillum: an evaluation of 20 years worldwide field inoculation Soil Biol. Biochem. 26:1591-1601.
Crossref
|
|
|
|
|
Orme-o-Orrillo E, Hungria M, Martínez-Romero E (2013). Dinitrogen-fixing prokaryotes. In. Rosemberg E, De Long EF, Lory S, Stackebrandt E, Thompson F (eds.), The Prokaryotes - Prokaryotic Physiology and Biochemistry. Springer-Verlag, Berlin Heidelberg. pp. 427-451.
|
|
|
|
|
Pereg L, de-Bashan LE, Bashan Y (2016). Assessment of affinity and specificity of Azospirillum for plants. Plant Soil 399:389-414.
Crossref
|
|
|
|
|
Ratcliff WC, Kadam SV, Denison RF (2008). Poly-3-hydroxybutyrate (PHB) supports survival and reproduction in starving rhizobia. FEMS Microbiol. Ecol. 65:391-399.
Crossref
|
|
|
|
|
Sá JCM, Lal R, Cerri CC, Lorenz K, Hungria M, Carvalho PCC (2017). Low-carbon agriculture in South America to mitigate global climate change and advance food security. Environ. Int. 98:102-112.
Crossref
|
|
|
|
|
Sadasivan L, Neyra CA (1985). Flocculation in Azospirillum brasilense and Azospirillum lipoferum: Exopolysaccharides and cyst formation. J. Bacteriol. 163:716-723.
|
|
|
|
|
Santi C, Bogusz D, Franche C (2013). Biological nitrogen fixation in non-legume plants. Ann. Bot. 111:743-767.
Crossref
|
|
|
|
|
Santos MS (2017). Desenvolvimento de novas formulações de inoculantes líquidos para Azospirillum brasilense estirpes Ab-V5 e Ab-V6. Thesis. Londrina, Brasil: Universidade Estadual de Londrina.
|
|
|
|
|
Souza RD, Ambrosini A, Passaglia LM (2015). Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 38:401-19.
Crossref
|
|
|
|
|
Stephens JHG, Rask H (2000). Inoculant production and formulation. Field Crops Res. 65:249-258.
Crossref
|
|
|
|
|
Tal S, Okon Y (1985). Production of the reserve material polybetahydroxybutyrate and its function in Azospirllum brasilense Cd. Can. J. Microbiol. 31:608-613.
Crossref
|
|
|
|
|
Tittabutr P, Payakapong W, Teaumroonga N, Singletonb PW, Boonkerda N (2007). Growth, survival and field performance of bradyrhizobial liquid inoculants formulations with polymeric additives. Sci. Asia 33:69-77.
Crossref
|
|
|
|
|
Trujillo-Roldán MA, Valdez-Cruz NA, Gonzalez-Monterrubio CF, Acevedo-Sánchez EV, Martínez-Salinas C, García-Cabrera RI, Gamboa-Suasnavart RA, Marín-Palacio LD, Villegas J, Blancas-Cabrera A (2013). Scale-up from shake flasks to pilot scale production of the plant growth-promoting bacterium Azospirillum brasilense for preparing a liquid inoculant formulation. Appl. Microbiol. Biotechnol. 97:9665-9674.
Crossref
|
|
|
|
|
Vendan RT, Thangaraju M (2007). Development and standardization of cyst based liquid formulation of Azospirillum bioinoculant. Acta Microbiol. Immunol. Hung. 54:167-177.
Crossref
|
|
|
|
|
Watnick P, Kolter R (2000). Biofilm, city of microbes. J. Bacteriol. 182:2675-2679.
Crossref
|
|