ABSTRACT
The objective of this study was to optimize a protocol for in vitro micropropagation of selected grape vine varieties. Preliminary studies were conducted to optimize the duration of sterilization of the explants. For shoot initiation experiment nodes of the three varieties of grape vines were cultured on MS medium (Murashige-Skoog’s, 1962) supplemented with five different concentrations of 6-Benzylaminopurine (BAP) and the control. Various experiments were carried out to optimize shoot multiplication using MS medium supplemented with different concentrations of BAP alone or in combination with Indole-3-butyric acid (IBA). To optimize root induction, different concentrations of Indole-3-acetic acid (IAA) were used. Sterilization of explants using 1% of NaOCl for 7 min duration was optimum. Chenin blanc showed high percentage of survival rate (96%) followed by Ugni blanc and Canonannon (88%) at 0.5 mg/L BAP. Among the different concentrations and combination of Plant Growth Regulators (PGRs) used for multiplication, maximum mean number of shoots 7.2, 6.7, and 6.1 was achieved at 1 mg/L BAP combined with 0.1 mg/L IBA for Chenin blanc, Canonnanon and Ugni blanc, respectively. All varieties induced root for all the treatments used including the control but good roots were found on MS medium supplemented with 2 and 4 mg/L of IAA. The plantlets were acclimatized in the glasshouse and survival percentage was 92% for Chenin blanc followed by 78.6 and 73.9% for Ugni blanc and Canonannon, respectively. Thus, the achievements of this study will play a big role in the grape vine culture program.
Key words: Vitis vinifera L., acclimatization, explant, cytokinin, auxin.
Grapevine (Vitis vinifera L.) is perennial woody fruit crop growing in the tropical, subtropical and temperate regions (Anupa et al., 2016). It is one of the most economically important crops in the world with average production of 67.5 million tons each year (Kurmi et al., 2011; Lazo-Javalera et al., 2016). Grapes are used for wine production, fresh fruit, dried fruit and juice production. From the total grape production about 71% is used for wine production, 27% as fresh fruit and the remaining 2% as dried fruit (Munir et al., 2015).
Grape has high nutritional value and energy content. It is a rich source of calcium, iron, vitamin C, A, E, amino acid and phosphorous (Jaladet et al., 2009; Yerbolova et al., 2013; Munir et al., 2015). Chemically, grape contains complex compounds which prevent various types of diseases including cancer, heart disease, degenerative nerve disease, Alzheimer disease, retinal disorder, constipation and viral disease (Khan et al., 2015; Munir et al., 2015).
Ethiopia is one of the countries in Africa which have huge potential for the production of grapes due to its favorable climatic condition. Currently in Middle East and Europe, there exist a growing interest for grapes and grape products from Ethiopia (Kassa Melese, 2015). This has a considerable economic significance as a potential source of national revenue. As a result, the government gives high priority to the development and expansion of vineyards to utilize the grape production potential. However, the health and economic significance of grapes might be treated with several types of disease including viral, bacterial and fungal. All these diseases primarily originate from the infected propagating grapevines achieved from the conventional asexual method of propagation. In addition, the conventional method of grapevine propagation (cutting) is time consuming. A planted grapevine needs four to five years to produce propagation materials by cuttings due to its long juvenility period. Grapevine juvenility is one of the principal natural problems hindering grapevine production (Anupa et al., 2016). Hence, the growth of viticulture industry can be maintained through mass production of disease free grapevine materials. In vitro propagation methods of grapevine has emerged as a powerful tool for mass propagation and producing disease-free planting material under aseptic condition. Moreover, this allows for large scale production of plantlets with high phytosanitary and genetic quality in limited space, short time and irrespective of the seasons (Melyan et al., 2015).
The propagation of grapes via micropropagation or tissue culture approach has been commercialized around the world. It was applied for selected Vitis genotypes using the culture of intact or fragmented shoot apical meristems, axillary-bud microcuttings or through adventitious bud formation (Kurmi et al., 2011; Khan et al., 2015). All types of grapevines do not give similar types of response for specific medium composition. The degree of response is highly dependent on the particular genotype, culture environment, culture medium and hormonal treatment. Hence, it is vital to develop new protocol for rapid multiplication of the available grapes varieties found in Ethiopia. Thus this study aims to develop a protocol for multiple shoot regeneration from node through tissue culture.
Explant collection and surface sterilization
Three varieties of grape namely Chenin blanc, Ugni blanc and Canonannon, were used in this study. Nodes having 2 to 3 cm length were collected from stock plant grown in greenhouse and thoroughly washed with tap water containing ‘Tween 20’. To identify the optimum sterilization time for sodium hypochlorite (NaOCl) ten nodes of variety Chenin blanc were sterilized for different time duration (5, 7 and 9 min) and cultured on MS medium supplemented with 0.5 mg/L of BAP.
Shoot initiation
Sterilized explants were cultured on MS media supplemented with BAP (0.5, 1, 2, 3 and 4 mg/L) including the control. Then the culture was incubated in a growth room at 27°C for four weeks. Twenty five explants were cultured for each treatment.
Shoot multiplication
The induced plantlets were transferred in to MS medium supplemented with BAP (0.25, 0.5, 1.0, 2.0, 3.0 and 4.0 mg/L) and four different concentrations of BAP (0.5, 1, 2 and 4 mg/L) in combination with 0.1 mg/L IBA (Table 6). All the cultures were incubated in a growth room at 27°C for 4 weeks.
Rooting
Shoots having one centimeter and more length were cut 2 mm below their basal node and planted into the medium containing Indole-3-acetic acid (IAA) at different concentrations ( 0, 0.5, 1.0, 2.0 and 4.0 mg/L). The cultures were incubated in a growth room at 27°C for 4 to 6 weeks.
Acclimatization
Plantlets with well developed roots were transferred into small pots containing sterile soil, compost and sand in the ratio of 2:1:1, respectively and covered by plastic bags for one week in glasshouse.
Experimental design and data analysis
All experiments were replicated and laid out in completely randomized design (CRD). Analysis of variance was conducted using JMP SAS computer software version 8.0. Means were compared using the least squares means procedure. Number of explants survived, number of shoots, nodes, roots per explants, shoots height and root length were recorded in a month interval.
Sterilization of explants
The success of plant tissue culture relies on the sources of the explants and sterilizing periods (Garg et al., 2014; Malyan et al., 2015). The effect of sterilizing duration on the surface sterilization of the explants using 1% sodium hypochlorite is shown in Table 1. The result revealed that, 100% clean cultures were obtained when the explants were treated for 7 and 9 min. However, all explants treated for 9 min died due to prolonged sterilization time.
Effect of different concentration of BAP for shoot initiation and survival rate
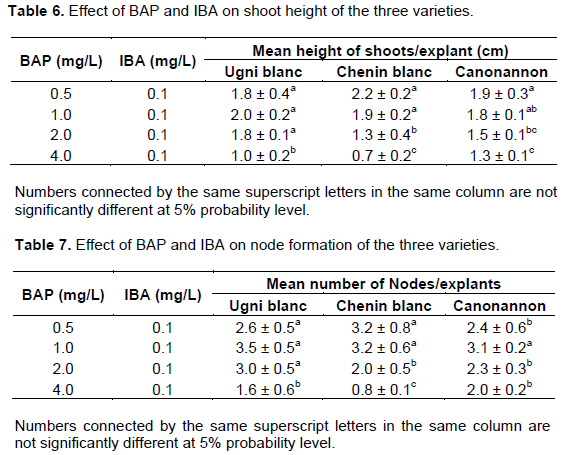
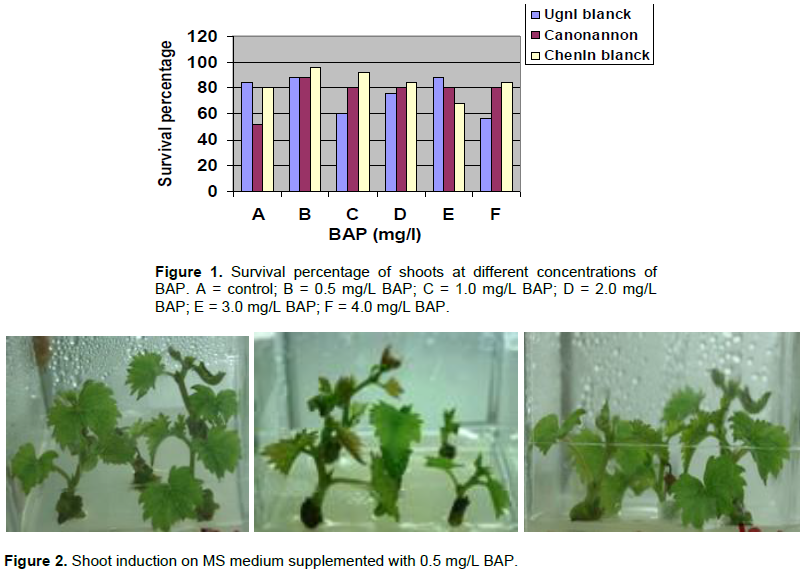
Results of the effect of BAP on shoot initiation and survival percentage for the three varieties of grapes (Figures 1 and 2) showed that shoots were induced in all treatments including the control. Khan et al. (2015) also presented similar results in the culture of a grape nodal segment containing an axillary bud with and without plant growth regulator. Even though shoots could be induced on hormone free medium morphologically best shoots were induced in the medium containing 0.5 mg/L BAP. The survival percentage of shoots showed significant variation at different concentration of BAP (p<0.05). The maximum percentage of survival was 96% for the variety Chenin blanc and 88% for the variety Ugni blanc and Canonnanon. This study were in contrast with the work of Abido et al. (2013) which evaluated the effects of BAP and Naphtaline Acetic Acid (NAA) and their combinations for shoot multiplication of V. vinifera L. cv. Muscat of Alexandria cultured in vitro. Their results indicated that the survival rate and the percent of the explants forming growth were not affected significantly by both plant growth regulator and their interaction.
Effect of different concentrations of BAP and in combination with IBA on number of shoot, node and height
Among the seven BAP concentration used for multiplication, 0.5 mg/L of BAP produced significantly maximum mean number of shoots for all the three varieties (Table 2) (Figure 3). On the other hand, Abido et al. (2013) found highest mean number of shoots at 2 mg/L of BAP and the least number was found in the absences of BAP. Canonannon gave maximum mean number of shoots (5.6) followed by Ugnin blanc (5.3) and Chenin blanc (5.0). The best mean height of shoot was attained at 1 mg/L of BAP for Ugni blanc and Chenin blanc moreover Ugni blanc score maximum height on hormone free medium as Canonannon (Table 3). Chenin blanc scored the maximum mean number of node (3.6) at 0.25 mg/L BAP as compared to Ugni blanc and Canonannon (3.1) (Table 4). The results of this study was in agreement with previous studies which confirmed that BAP is the most effective on inducing shoot proliferating among all other cytokinins in Vitis (Abido et al., 2013).
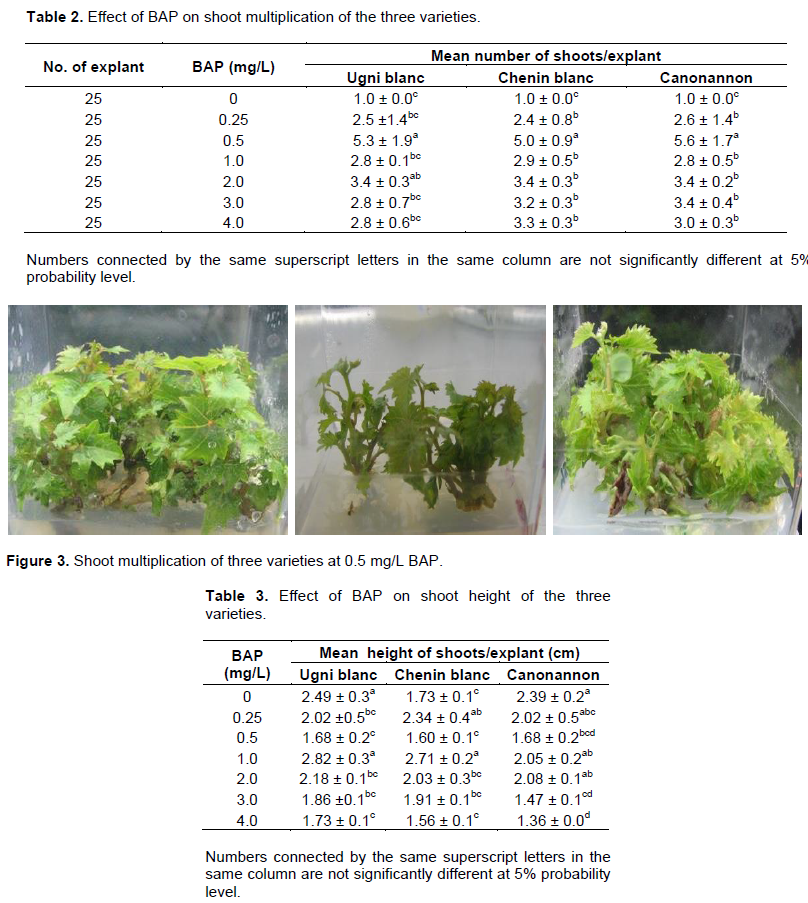
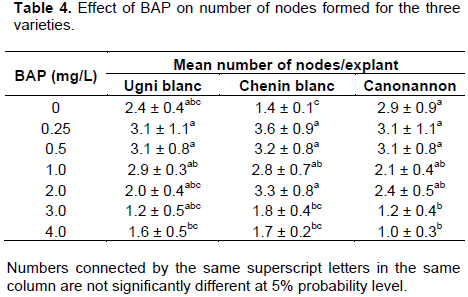
Besides development of multi shoots did not occurred in hormone free medium. The presence of BAP, even at relatively low levels (that is, 0.25 mg/L), enhanced shoot multiplication (Tables 2 and 6). As the concentration increases abnormal growth was observed in all varieties. The shoots become short and bunchy; the leaves were thick and fragile. Tehrim et al. (2013) also confirmed that increasing concentration of BAP results a decrease in the shoot length of grape accessions. High cytokinins concentration in the culture media causes production of ethylene that limits the regeneration of shoots and inhibits the elongation of internodes (Khan et al., 2015).
There was significant difference for the response of BAP concentration in number of shoot among the three varieties. The effect of PGRs on shoot number was not significant between the three varieties. Shoot height and number of nodes showed significant variability at various levels of BAP and among the three varieties. The effect of BAP on shoot height and number of node dependent on the type of the variety since the interaction of PGRs (BAP) with varieties was significant. According to this experiment, increased concentration of BAP has a negative effect to lengthen the induced shoots and the number of nodes. Studies indicated that the combination of different growth regulators and their concentration significantly affected shoot length due to their effect on cell division and cell expansion (Aazami, 2010; Khan et al., 2015). In this study, the combination of BAP with IBA showed significant improvement on the length of shoots and number of nodes.
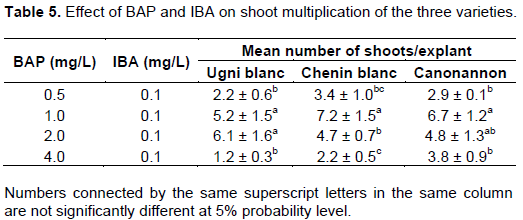
According to the results presented in Table 5, 1.0 mg/L BAP with 0.1 mg/L of IBA gave maximum mean number of shoots (7.2) for the variety Chenin blanc (Figure 4). In this combination, Canonannon had also given 6.7 mean number of shoot which is significantly different from the other combination except 2.0 mg/L BAP with 0.1 mg/L IBA. Kahn et al. (2015) was also reported maximum shoot regeneration frequency (53.33%) at concentrations of BAP and NAA (1.5 and 0.5 mg/L, respectively) and lowest shoot regeneration frequency (6.67%) in control medium (without any growth regulator). Similarly, Abido et al. (2013) achieved the maximum number of proliferated shoots on MS medium containing 3.0 mg/L BAP + 0.2 mg/L NAA.
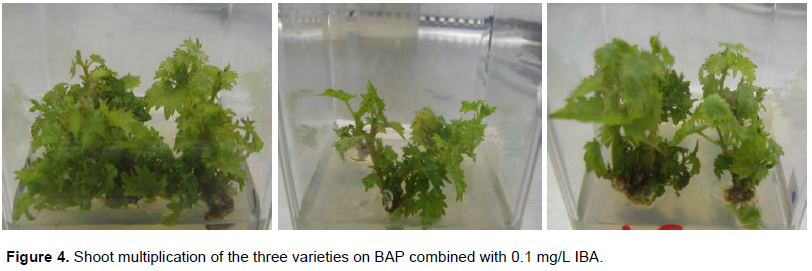
In this study, the combination having 0.5 and 1.0 mg/L BAP with 0.1 mg/L IBA produced the best height for the variety Chenin blanc, Canonannon and Ugni blanc, respectively (Table 6). Kahn et al. (2015) achieved significantly increased shoot length on growth regulating hormones in the combination of 1.0 mg/L BAP + 0.1 mg/L GA3. The variety Ugni blanc gave more number of nodes as compared to Chenin blanc and Canonannon (Table 7).
Different combination of BAP and IBA showed variability in number of shoot, shoot height and number of nodes. The ANOVA revealed that there was significant interaction between PGRs and varieties.
Effect of IAA on root induction
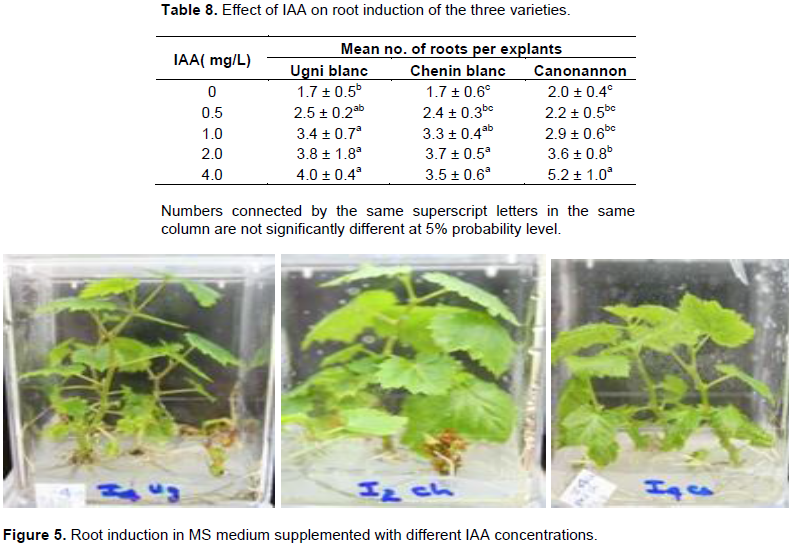
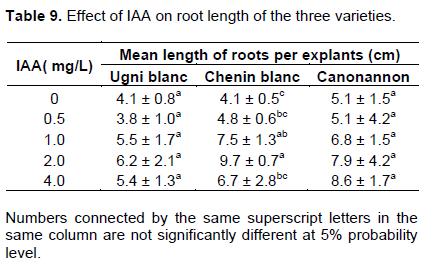
The success of tissue culture depends on the rooting ability of the plant. In the micropropagation of grape, auxins like IAA, IBA and NAA was found effective for inducing in vitro rooting (Kurmi et al., 2011). The results found in the rooting experiment of this study are presented in Table 8 and Figure 5. The results showed the plantlets cultured on rooting media were induced roots in all media supplemented with IAA including hormone free media. The primary roots were visible after two weeks of culturing on MS medium supplemented by different concentration of IAA. However, more developed and mature roots were obtained after a month.
The maximum mean number of roots was counted for Ugni blanc (4) and Canonannon (5.2) in a medium supplemented with 4.0 mg/L IAA and the number of roots were increased with increasing of IAA concentration. On the other hand, the maximum mean no of root for Chenin blanc (3.7) was obtained in a medium that contains 2.0 mg/L IAA.
Regarding the length of the roots, Chenin blanc and Canonannon induced a maximum mean length of 9.7 cm and 8.6 cm at 2.0 and 4.0 mg/L IAA, respectively. Ugni blanc produced maximum mean length of root (6.2 cm) at 2.0 mg/L IAA. Significant variation in mean number of root among treatments was observed for the varieties Canonannon and Chenin blanc and variety Chenin blanc showed significant variation on the length of roots (Table 9). Shatnawi et al. (2011) found the maximum number of roots per explants in the micropropation of V. vinifera L. via meristem culture at 0.6 mg/L of IBA. Number of root induced and length increased with increasing concen-tration of IAA and the maximum root length was obtained with 0.6 to 0.8 mg/L of NAA. On in vitro propagation of grapevine (V. vinifera L.) Muscat of Alexandria cv, Abido et al. (2013) found the highest rooting percentage, number of roots/shoot and root length (87%, 3.4 and 4.5 cm, respectively) on MS medium supplemented with1.0 mg/L IBA + 0.5 mg/L IAA.
Acclimatization
In this experiment, plantlets about 3 cm tall were transferred into the soil because plantlets less than 3 cm were not survived. Pictures showing the growth condition of grape plantlets in the glasshouse at different time are presented (Figure 6). Moreover those plantlets uncovered with plastic bags started to weaken immediately after being transferred to the soil. This implies the plastic cover protects the plantlets from the external stress for some times until they adapt to the outside environment. After one week, the plastic cover should be removed to avoid fungal development because among the five plantlets remains with the plastic cover all of them were contaminated by fungi. Survival rate of the acclimatized plantlets was 78.6, 92.0 and 73.9% for Ugni blanc, Chenin blanc and Canonanoon respectively.
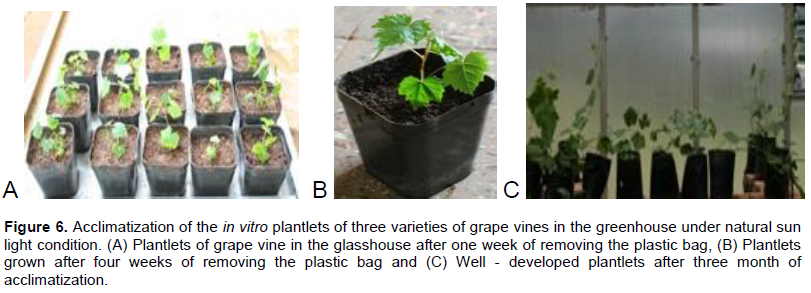
Shatnawi et al. (2011) achieved a survival rate of 95% when the rooted explants were acclimatized ex vitro in the study of in vitro micropropagation of vitis. Only survival percentage of the variety Chenin blanc were maximum but the rest two varieties have relatively low survival percentages. Laslo et al. (2010) was found also to have 90% survival rate in the ex vitro acclimatization of V. vinifera L. In addition, Abido et al. (2013) found 80 to 90% survival rate after they kept plantlets in rooting medium for 34 before acclimatization. In vitro plantlets of grapevine are very sensitive to ex vitro conditions; the success of acclimatization depends on the increases of humidity, reduction of light intensity, and temperature in the first days of acclimatization (Laslo et al., 2010).
The experimental results of this study revealed that seven minutes of sterilization in 1% NaOCl followed by culturing on basal Ms medium supplemented with 0.5 mg/L BAP was found to be effective for sterilization and shoot initiation, respectively. The best multiplication of shoots were obtained at 1.0 mg/L BAP with 0.1 mg/L IBA for the variety Chenin blanc and Canonannon, while 2.0 mg/L BAP with 0.1 mg/L IBA for Ugni blanc. High levels of IAA, 2.0 mg/L for Chenin blanc and 4.0 mg/L for Ugni blanc and Canonannon were found to be effective in enhancing root number and length. In the acclimatization process the survival rate of Chenin blanc was better than the other two varieties. In this in vitro micro propagation of grape vine, it is possible to demonstrate culture establishment, shoot proliferation and rooting by using the different concentrations of BAP, IBA and IAA.
The authors have not declared any conflict of interests.
REFERENCES
|
Aazami MA (2010). Effect of some growth regulators on "in vitro" culture of two Vitis vinifera L. cultivars. Rom. Biotechnol. Lett. 15:5229-5232.
|
|
|
|
Abido AI, Aly MA, Hassanen SA, Rayan GA (2013). In vitro Propagation of Grapevine (Vitis vinifera L.) Muscat of Alexandria cv. For Conservation of Endangerment Middle-East. J. Sci. Res. 13(3):328-337.
|
|
|
|
|
Anupa T, Sahijram L, Samarth R, Rao BM (2016). In Vitro shoot induction of three grape (Vitis vinifera L.) varieties using nodal and axillary explants. Bioscan 11(1):201-204.
|
|
|
|
|
Garg RK, Srivastava V, Kaur K, Gosal SS (2014). Effect of sterilization treatments on culture establishment in J. curcas L. Karnataka. J. Agric. Sci. 27:190-192.
|
|
|
|
|
Jaladet M, Jubrael S, Hali SH (2009). Genetic diversity analysis of a number of grape (Vitis vinifera L.) varieties in Kurdistan region-Iraq using rapid markers. J. Duhok Univ.12(1):17-22.
|
|
|
|
|
Kassa Melese A (2015). Opportunities and Potential in Ethiopia for Production of Fruits and Vegetables: A Graduate Senior Seminar Paper. Afr. J. Basic Appl. Sci. 7(6):328-336.
|
|
|
|
|
Khan N, Ahmed M, Hafiz I, Abbas N, Ejaz S, Anjum M (2015). Optimizing the concentrations of plant growth regulators for in vitro shoot cultures, callus induction and shoot regeneration from calluses of grapes. OENO One 49(1):37-45.
Crossref
|
|
|
|
|
Kurmi US, Sharma DK, Tripathi MK, Tiwari R, Baghel BS, Tiwari S (2011). Plant regeneration of Vitis vinifera (L) via direct and indirect organogenesis from cultured nodal segments. J. Agric. Technol. 7(3):721-737.
|
|
|
|
|
Laslo V, Zapartan M, Vicas S (2010). In vitro response of several cultivars of Vitis vinifera L on media with balanced phytohormone Ratio. Res. J. Agric. Sci. 42(2):269-274
|
|
|
|
|
Lazo-Javalera MF, Troncoso Rojas R, Tiznado Hernandez ME, Martinez-Tellez MA, Vargas Arispuro I, Islas-Osuna MA, Rivera-Dominguez M (2016). Surface disinfection procedure and in vitro regeneration of grapevine (Vitis vinifera L.) axillary buds. Springer Plus 5(1):453.
Crossref
|
|
|
|
|
Melyan G, Sahakyan A, Harutyunyan A (2015). Micropropagation of grapevine (Vitis vinifera L.) seedless cultivar 'Parvana' through lateral bud development. Vitis 54:253-255.
|
|
|
|
|
Munir N, Safdar I, Naz S (2015). Effect of induced mutation for varietal improvement in some local grapevine cultivars. J. Anim. Plant Sci. 25(1):234-242.
|
|
|
|
|
Shatnawi M, Anfoka G, Shibli R, Al-mazra'awi M, Shahrour W, Arebiat A (2011). Clonal propagation and cryogenic storage of virus-free grapevine (Vitis vinifera L.) via meristem culture. Turk. J. Agric. 35:173-184.
|
|
|
|
|
Tehrim S, Mirza MY, Sajid GM (2013). Comparative study of different growth regulators for efficient plant regeneration in grapes. Pak. J. Agric. Res. 26:275-289.
|
|
|
|
|
Yerbolova LS, Ryabushkina NA, Oleichen SN (2013). The Effect of Growth Regulators on in vitro Culture of Some Vitis vinifera L. Cultivars. World Appl. Sci. J. 23(1):76-80.
|
|