ABSTRACT
Trichoderma species is a heterotrophic saprobion organism, which competes with other microorganisms, preventing them from developing. This fungus has mechanisms of action, among which are: production of antibiotics and metabolites, and hyperparasitism, which also promotes the induction of systemic resistance in plants. In this study, five native Trichoderma strains which were collected from agricultural land in Tamaulipas, Mexico were evaluated. The objective of this research was to identify the secondary metabolites produced by native strains of Trichoderma spp. under in vitro conditions and evaluation of the effect of these compounds on the growth of the pathogen Fusarium oxysporum and the germination of tomato seeds (Solanum esculentum). The production of Trichoderma's metabolites was performed by culturing in 150 ml of potato broth supplemented with sucrose (5%) and yeast extract (5%). Inoculation was performed by the addition of a 4 mm disk of active mycelium and allowed to grow at 25C ± 2°C and 12 h light/dark on a rotary shaker at 150 rpm for three weeks. Subsequently, the extraction of metabolites was performed using liquid: liquid phase which consisted of the filtrate and methylene chloride in a ratio of 1:3, and then the organic phase was recovered. The organic fraction was evaporated at 40°C with the aid of a rotary evaporator (Buchi®); the sample was recovered in 1 ml of methylene chloride. Analysis by GC-MS indicates that Trichoderma isolates produced 41 secondary metabolites of volatile and semi-volatile molecules and was observed, such that the number of compounds varies from the species analyzed and the collection site. The evaluation of the antagonistic activity of the extracts of Trichoderma spp. on F. oxysporum showed no significant differences between treatments (P=0.05); however, it was observed that pigmentation decreased in the mycelium of the pathogen. Regarding the evaluation of the effect of the extracts on the seeds of tomato, it was observed that the compounds of Trichoderma asperellum (TV1) induced the germination and the development of the seedling.
Key words: Volatile compounds, Trichoderma, gas chromatography-mass spectrometry secondary metabolites, Solanum esculentum.
Trichoderma species is a fungus found in the rhizosphere and organic matter of soils, with carbon as its main source of energy (Galarza et al., 2015). Trichoderma harzianum, Trichoderma viride, Trichoderma virens and Trichoderma koningii are the species more utilized in agriculture, pharmaceutical industry and biotechnology, because Trichoderma spp. produces secondary metabolites being the main peptides, peptaiboles, polyketides, pironas and volatile and non-volatile molecules (Vinale et al., 2009; Muller et al., 2011) that have functional processes such as biofungicides, plant growth promoters, enzyme generation, biofuels production, proteins, pigments, and antibiotics (Gajera et al., 2013). The effects of these molecules on plants are diverse, including: induction of resistance, elimination of toxins and deactivation of enzymes produced by phytopathogens during the infection process; in addition to the solubilization of nutrients, which in their elemental form are not bioavailable to plants, the processes are conferred to the antagonist of additional characteristics when used in the field (Dudareva et al., 2013). It has been reported to stimulate plant growth, due to the production of enzymes linked to tolerance to water stress (Mukherjee et al., 2013).
The success of Trichoderma spp. in the rhizosphere is due to its reproductive capacity, as well as its efficiency in the use of soil nutrients; the effectiveness of the genus has been reported against a variety of plant pathogenic fungi (Szabó et al., 2012). Some species of Trichoderma have the potential to synthesize and release enzymes (polysaccharases, cellulases, xylanases and chitinases) that have been implemented in industrial bioprocesses (Nikolajeva et al., 2012). It has also been reported that Trichoderma spp. are capable of producing auxin and gibberellin type growth regulators reported as promoters of the growth of some agricultural crops (Garnica-Vergara et al., 2016).
The antagonic effects of the genus Trichoderma have been reported in several phytopathogens of economic importance (Woo et al., 2014) including Phytophthora infestans (Kerroum et al., 2015), Fusarium fujikuroi (Ng et al., 2015), Pestalotia theae, Fusarium solani, Colletotrichum gloeosporioides MTCC 3439, Colletotrichum lindemuthianum MTCC 8474, Colletotrichum capsici MTCC 3414, Curvularia senegalensis MTCC 8463 and Alternaria alternata MTCC 8459 (Naglot et al., 2015).
Trichoderma has been reported to colonize the root epidermis and cortical outer layers, releasing volatile compounds such as ethylene, alcohols, aldehydes, ketones, and non-volatile compounds as peptides and enzymes capable of inhibiting the growth of the fungi (Yang et al., 2011).
Currently, commercial strains of Trichoderma spp. are used in Mexico; however, its effectiveness is diminished by abiotic factors such as the type of climate and soil, which are different from the collection site where they were isolated. Furthermore, there are limited studies in the country which focused on the identification of native compounds of Trichoderma spp. and the evaluation of the compounds in the impact on the inhibition of Fusarium oxysporum and the effect of germination and growth of plants.The objective of this study was to identify the secondary metabolites produced by native strains of Trichoderma spp. under in vitro conditions and the evaluation of Trichoderma extracts on F. oxysporum growth and in the germination of seeds of tomato.
Cultivation of fungal strains
The native strains of Trichoderma were obtained from the Microbiology Laboratory of the Faculty of Engineering and Sciences, belonging to the Autonomous University of Tamaulipas. The organisms were isolated from lots located in the municipalities of the state of Tamaulipas: Padilla, Victoria and Jaumave. The municipality of Padilla is at latitude of 23° 59'26.1" N, 98°56'39.0" W, in which the semi-arid climate (BS1hw) predominates, tempered with summer rains and scarce along the year temperatures range from 1 to 43°C. The average rainfall is of the order of 700 mm3, the predominant soils are vertisols with aptitude for agriculture.
The municipality of Victoria is located at 23°66'37" N, 99°10'81" W. The climate of the area is dry of steppe, very warm (BS1hw) with temperatures ranging from 2 to 40°C. Rainfall occurs in summer with an average precipitation of 926 mm, the soil type is loamy. The municipality of Jaumave is located at 23°24'46.1 "N, 99°23'03.4" W, which has a temperate semi-arid climate (BS1hw), with an annual average temperature of 18 and 22°C; with rainfall in summer from 5 to 10.2% per year. The type of soil is lithosol (LPq) and presents a shallow soil, all the native strains of Trichoderma were isolated from the soil where the Aloe barbadensis Miller is cultivated (CONABIO, 2016).
The cultures were identified by molecular level and maintained on potato dextrose agar (PDA) (Difco®) at 27 ± 2°C. Trichoderma Victoria (TV1) corresponds to Trichoderma asperellum, Trichoderma Padilla (TP1) identified as T. harzianum and the isolated Trichoderma Jaumave corresponds to T. asperellum.
Production and extraction of secondary metabolites by Trichoderma strains
The isolates were grown on solid PDA medium for 7 days prior to the assay. To evaluate the production of metabolites, each isolate was inoculated into 150 ml of potato broth supplemented with sucrose (5%) and yeast extract (5%). The inoculation was performed by the addition of a 4 mm disk of active mycelium. The culture was grown at 25 ± 2°C, 12 h light/dark on a rotary shaker (Labco®) at 150 rpm for three weeks. Each isolate was inoculated in triplicate.
The extraction of metabolites was performed using liquid: liquid phase which consisted of 10 ml of the filtrate and methylene chloride (J.T. Baker®) in a ratio of 1: 3. The sample was stirred until a homogeneous mixture was obtained, then the organic phase was recovered. The organic fraction was evaporated at 40°C with the aid of a rotary evaporator (Buchi®), and then the sample was recovered in 1 ml of methylene chloride. Each concentrated sample was stored at -80°C for further analysis. The procedure was performed in triplicate in each of the samples.
Analysis of secondary metabolites of native strains of Trichoderma
To determine the presence/absence of metabolites, each sample was subjected to thin layer chromatography (TLC) with a mobile phase of hexane: acetone (50:50) (Analytyka®) and as a stationary phase with a silica gel matrix in aluminium plate (Whatman®). 200 μl of non-inoculated culture medium (
by several successive applications of 20 μl at the same point, allowing drying between each application[T1] ) was added to each plate, which was used as a reference, and at another three points, 200 μl of the culture of each isolate was added by successive application. The plate was then air dried for 20 min. The development was performed by exposure of the plate to iodine vapors for 15 min. The procedure was repeated in triplicate for each sample.
The qualitative analysis of the Trichoderma extracts was performed from 3 weeks old samples by sas chromatography-mass spectrometry (GC-MS) on an Agilent® 6890 chromatograph, with an Agilent® 5973N mass detector using a 5% phenyl-methylpolysiloxane column with helium mobile phase. Analysis of each sample was performed in triplicate.
Evaluation of Trichoderma extracts on F. oxysporum growth
The pathogen, F. oxysporum was isolated from the wilt infected roots of Aloe barbadensis Miller plants, which were collected from the Microbiology Laboratory of the Faculty of Engineering and Sciences, Autonomous University of Tamaulipas. The culture was identified by molecular level and maintained on potato dextrose agar (PDA) (Difco®) at 27 ± 2°C. In this experiment, the Trichoderma extracts (TV1, TP1, TJ1) were evaluated.
The antagonist effect was evaluated in Petri dishes with sterile PDA medium, 100 μL of the extract of Trichoderma spp. was later dispersed throughout the surface of the Petri dish, then a 4 mm disc of F. oxysporum was placed in the center of the Petri dish and incubated at 28 ± 2°C for 7 days. This procedure was performed in triplicate for each Trichoderma extract. A control was added to the evaluation, which consisted of applying 100 μL of sterile distilled water to a Petri dish with PDA medium and then placing a 4 mm disc of F. oxysporum in the middle of the surface. The variables to be evaluated were growth and coloration of the mycelium.
The mycelium growth inhibition (%) of F. oxysporum was measured at 7 days of incubation. The antagonistic effect of the test fungi was estimated by measuring their radial growth in comparison with the control plates by the following formula: I = [(C-T) / C] × 100, where I is the % inhibition in mycelia growth; C is the growth of pathogen in control plates; T is the growth of pathogen in dual culture plates.
Evaluation of Trichoderma extracts in the germination of seeds of tomato (Solanum esculentum)
Seeds of tomato were purchased from commercial market and were surface disinfected in 80% ethanol for 60 s followed by a 20% bleach solution for 2 min with constant agitation. In Petri dishes with sterile PDA medium (Difco®), 200 μL of the Trichoderma extract was added, then dispersed over the entire surface of the Petri dish, then 5 tomato seeds were previously disinfected, incubated at 28 ± 2°C for 7 days.
Five replicates were used per volatile exposure condition and the experiments were repeated three times. A control was added to the evaluation, which consisted in applying 200 μL of sterile distilled water to a Petri dish with PDA medium. The variables to be evaluated were percentage of germination, length of root and stem. The data were analyzed using an analysis of variance (ANOVA), when it was necessary, the comparison of means was performed with the Tukey method (P=0.05). The analysis was analyzed with the statistical program Statistical Analysis System (SAS, version 9).
Production and extraction of secondary metabolites by Trichoderma strains
Differences in the content and diversity of metabolites were observed between strains selected according to thin layer chromatography (TLC) analysis. Comparisons of the samples at the 3rd week showed the presence of more metabolites as compared to the first and second week extracts evaluated. The analysis of metabolites by GC-MS showed that all the native strains of Trichoderma produced secondary metabolites; it was also found that the similarity of the metabolic profiles presented was greater among the isolates of the same species than when the isolate of T. harzianum was compared with any of T. asperellum. The analyzed metabolites are volatile and semi-volatile, such as aromatics, fatty acids, alcohols and hydrocarbons in general, including the alkanes (C4-C9) shown in the three isolates (Table 1).
Analysis of the spectra showed that the number of compounds depended on the species analyzed. The species of T. asperellum (TJ1 and TV1) produced the highest amount of compounds; however, T. harzianum (TP1) produced molecules with higher percentage of abundance. The compounds detected, 10 corresponded to hydrocarbon compounds, 5 were phenolic compounds, 6 corresponded to organic acids, and 5 were unidentified compounds. In this study, six compounds shared between two of the isolates of T. asperellum and eight compounds of exclusive distribution for a single isolated (TP1) was found.
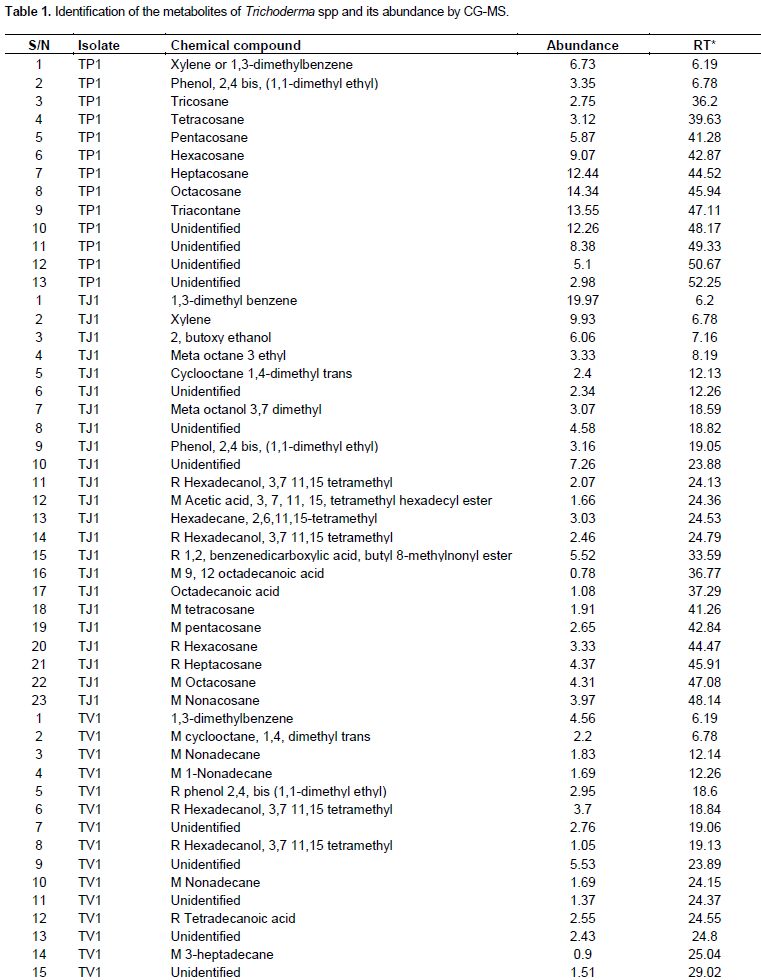
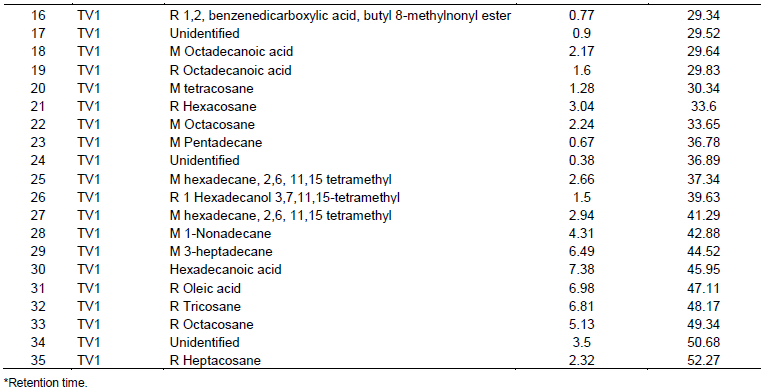
The isolated T. asperellum (TV1) produced 35 compounds with relative abundances between 0.38 and 7.38% and retention times between 6.19 and 52.27 min, of which 17 are hydrocarbon, 4 phenolic, 6 organic acids and 8 molecules were unidentified. In the case of T. asperellum (TJ1), 23 compounds was detected, with relative abundances varying between 0.78 and 19.97% with retention times between 6.19 and 48.14 min of which two are hydrocarbon, one phenolic, five organic acids, four alcohols and one was unidentified. The analysis of the T. harzianum (TP1) isolate showed the presence of 13 compounds, with relative abundances ranging from 2.75 to 12.44% with retention times between 6.19 and 52.25 min of which eight are hydrocarbon, one phenolic and four unidentified.
In this study, a correspondence of compounds between the two species of Trichoderma was found when sharing a compound between both species, such as phenol, 2,4 bis, 1,1-dimethyl ethyl. The correspondence of compounds produced by each isolate of T. asperellum was 11.56%, since six compounds were shared, among 1,3-dimethyl benzene, M octadecainoic acid, M tetracosan, R octadecainoic acid, R heptacosan and R hexacosane. All volatile compounds, which act in an antibiotic way against plant pathogenic fungi and can promote the growth of the same, as well as grant them systemic resistance. In this study, volatile metabolites of n-hydrocarbons type T. harzianum and T. asperellum were identified (Table 1). According to these results, C4 to C9 alkanes were identified, with a predominance of C5-C8 (Table 2).
The native strains of Trichoderma produced secondary metabolites, the compounds analyzed are volatile and semi-volatile in nature, these molecules are related to the interaction between the fungi and the environment, it was observed that the number of compounds varies between species, intraspecific variation as a possible result of the different environmental conditions to which they were submitted at their respective collecting sites, T. asperellum species (TJ1 and TV1) produced the highest amount of compounds, however, T. harzianum (TP1) produced molecules with greater percentage of abundance.

Evaluation of Trichoderma spp. extracts on F. oxysporum growth The evaluated extracts of T. asperellum (TV1, TJ1) and T. harzianum (TP1) did not inhibit the growth of the phytopathogen F. oxysporum, because growth percentages were observed for 81 to 94.37% (Table 3); however, in all treatments with extracts of Trichoderma spp. the color of the phytopathogen mycelium turned white, this is in comparison with the control, which is red-pink color (Figure 1).
Evaluation of Trichoderma extracts in the germination of seeds of tomato S. esculentum
The evaluated extracts of T. asperellum (TV1, TJ1) and T. harzianum (TP1) promoted the germination of the tomato seeds; however, the control treatment did not germinate. Seedlings that were germinated (root and stem) were measured; it was observed that the treatment T. asperellum (TV1) showed the highest growth in the variable stem with 5.5 cm and root 3.10 cm, in contrast with the treatment T. asperellum (TJ1) that belongs to the same species that registered a stem growth of 0.51 and 0.46 cm of root. The treatment TP1 belonging to T. harzianum promoted a growth of 0.9 cm of stem and root of 0.45 cm (Table 3). In this study, it was observed that tomato seedlings exposed to the metabolites of Trichoderma showed a development in the main root as shown in Figure 1.
In this study, 3 Trichoderma spp. were screened and demonstrated that all of these strains produced secondary metabolites. However, no effect was observed on the decrease in growth of the phytopathogenic mycelium. On the other hand, the evaluation of the effect of metabolites in tomato seeds showed that it increases the percentage of germination, the growth of root and stem. The study showed that a 3-week of fermentation Trichoderma spp. has a greater amount of metabolites, compared to the production of metabolites of the extracts of weeks 1 and 2.
The isolated T. asperellum (TV1) produced 35 compounds with relative abundances between 0.38 and 7.38%, of which 17 are hydrocarbons, 4 phenolics, 6 organic acids and 8 molecules unidentified. Twenty three T. asperellum (TJ1) compounds were detected, with relative abundances varying between 0.78 and 19.97%, of which two are hydrocarbons, one phenolic, five organic acids, four alcohols and one was unidentified. The analysis of the T. harzianum (TP1) isolate showed the presence of 13 compounds, with relative abundances ranging from 2.75 to 12.44%, of which eight are hydrocarbons, one phenolic and four unidentified. These data are consistent with the studies reported (Lee et al., 2016) where there are differences in metabolite production even among organisms of the same species.
The spectrum analysis showed that the number of compounds depended on the species analyzed. The species of T. asperellum (TJ1 and TV1) produced the highest amount of compounds; however, T. harzianum (TP1) produced molecules with higher percentage of abundance. Of all the compounds detected, 10 corresponded to hydrocarbon compounds, five were phenolic compounds, six corresponded to organic acids, five were unidentified compounds, and some of them to date have reported plants, nevertheless, none has been attributed to Trichoderma spp. This study has found six compounds shared between two of the isolates of T. asperellum and eight compounds of exclusive distribution for a single isolate (TP1). These results are consistent with the studies (Mukherjee et al., 2012) which reported that Trichoderma possesses fungistatic mechanisms that prevent the development of phytopathogenic fungi, as well as the capacity to synthesize volatile substances involved in the complex responsible for this phenomenon, these components are: carbon dioxide, ethanol, acetaldehyde, acetone, propanol, isobutanol and isopentanol, which in different concentrations intervene in the regulation of the fungistatic mechanism.
In this study, a correspondence of compounds between the two species of Trichoderma was found when sharing a compound between both species, such as phenol, 2,4 bis, 1,1-dimethyl ethyl. The correspondence of compounds produced by each isolate of T. asperellum was 11.56%, since six compounds are shared, among which are 1,3-dimethyl benzene, M octadecainoic acid, M tetracosan, R octadecainoic acid, R heptacosan and R hexacosane. All volatile compounds act in an antibiotic way against plant pathogenic fungi and can promote the growth of the same, as well as grant them systemic resistance. In the present work, T. asperellum isolates (TJ1 and TV1) were produced, even when the same species were involved. This is due to the fact that these organisms were collected at different sites, where interaction with environmental factors, the type of soil, precipitation, among others would be affecting the mechanisms of action of Trichoderma. It has been elucidated that the diversity of metabolites produced by Trichoderma have evolved for communication or defense, against phytopathogens, in addition to these, stress conditions influence the expression of the volatile metabolites (Polizzi et al., 2011).
In the present work, T. asperellum isolates (TJ1 and TV1) produced different type of volatile compounds; this is due to the fact that these organisms were collected at different sites, where interaction with environmental factors, the type of soil, precipitation, among others would be affecting the mechanisms of action of Trichoderma, so it has been elucidated that the diversity of metabolites produced by Trichoderma have evolved for communication or defense, against phytopathogens, in addition to these, stress conditions influence the expression of the volatile metabolites.
In this work, volatile metabolites of n-hydrocarbons type T. harzianum and T. asperellum were identified (Table 1). According to these results, C4 to C9 alkanes were identified, with a predominance of C5-C8 (Table 2). It has been reported that C8 (octanone) compounds induce conidiation and also have an antimicrobial and antiviral action (Polizzi et al., 2011). In addition, Trichoderma species such as T. harzianum, Trichoderma atroviride and T. asperellum are used as plant protection agents and as growth promoters (Stoppacher et al., 2010). Studies focusing on bioremediation with Trichoderma strains in soils contaminated by hydrocarbons showed that T. harzianum, Trichoderma pseudokoningii and T. viride possess the ability to degrade pyrene and to use it as a carbon source (Ravelet et al., 2000). Others studies report that T. harzianum contributes in pollutant degradation in 65 and 33.7% of monoaromatic compounds in concentrations of 50 and 100 mg L-1, respectively (Saraswathy and Hallberg, 2002). The implementation of chromatographic techniques, studies have been carried out on the chemical profile of the secondary metabolites produced by Trichoderma and its effect on the biochemical, molecular and physiological processes of plants with potential in the field (Stoppacher et al., 2010).
The native strains of Trichoderma produced secondary metabolites, the compounds analyzed are volatile and semi-volatile in nature, these molecules are related to the interaction between the fungi and the environment; it was observed that the number of compounds varies between species, intraspecific variation as a possible result of the different environmental conditions to which they were submitted at their respective collecting sites. T. asperellum species (TJ1 and TV1) produced the highest amount of compounds; however, T. harzianum (TP1) produced molecules with greater percentage of abundance. Studies carried out by Martínez-Medina et al. (2014) reported that the effectiveness of the native strains of the antagonist differs due to the associated abiotic factors, highlighting mainly the site of collection, type of soil, average temperature and humidity.
Specific criteria, such as mass spectral fusion factors and retention indices, the application of the method resulted in the identification of 41 volatile and semi-volatile compounds. However, studies report the identification of more than 141 compounds when analyzing 11 species of Trichoderma by chromatographic methods (Lee et al., 2016). Other works reported more than 278 compounds when using different columns and mobile phases in the chromatograph; in this sense, the mobile phase is related to the detection of the compounds analyzed, due to the polarity level that is present (Siddiquee et al., 2012). The number of compounds identified may be influenced by the number of species analyzed, the number and variety of culture conditions and conditions of analysis (preparation method, separation and analysis conditions).
Among the metabolites observed, the presence of the 1,3-dimethyl benzene compound in the TJ1 and TV1 isolates, with 19.97 and 4.56% of abundance, respectively was found. For TP1 isolates, the triacontane alkane stands at 13.55%, which has been reported as a plant growth regulator (Cai et al., 2013) (Table 1). Studies reported have demonstrated that volatile secondary metabolites play a key role in Trichoderma mycoparasitism and its interaction with plants (Piechulla and Degenhardt, 2014).
Regarding the evaluation of the effect of the secondary metabolites produced by the isolates of Trichoderma spp., it was observed that the phytopathogen mycelium coloration turned white in comparison with the control, which is red-pink colored. This effect is possibly due to the production of secondary metabolites of Trichoderma spp. which have an antibiosis effect; so the mycelia of the phytopathogen decreased, because the color of the phytopathogen is correlated with the virulence of it (Hermosa et al., 2012).
Studies that focused on the interaction of Trichoderma with the roots of plants have reported the appearance of auxin-like metabolites released by Trichoderma and perceived by the roots, altering multiple hormonal mechanisms that control the growth and development of plants in "normal" or stress conditions (Bae et al., 2009; Garnica-Vergara et al., 2016). Therefore, when the root system is colonized, the association is enhanced, providing protection in this area against pathogenic microorganisms, and also develops a root system that improves the absorption of nutrients and water in the plant which have demonstrated that volatile metabolites play an important role in mycoparasitism (Contreras-Cornejo et al., 2015).
The analysis by GC-MS indicates that Trichoderma isolates produced 41 secondary metabolites of volatile and semi-volatile molecules and it was observed that the number of compounds varied from the species analyzed and the collection site. The metabolites analyzed are volatile and semi-volatile, such as aromatic compounds, fatty acids, alcohols and general hydrocarbons, including the alkanes (C4-C9) shown in all the three isolates.
The evaluation of the antagonistic activity of the extracts of Trichoderma spp. on F. oxysporum showed no significant differences between treatments (P=0.05); however, it was observed that pigmentation decreased in the mycelium of the pathogen. Moreover, in the evaluation of the effect of the extracts on the seeds of tomato, it was observed that the compounds of T. asperellum (TV1) induced the germination and the development of the seedling. The present study shows the wide range of compounds produced by native Trichoderma spp. However, it is necessary to search for strains to evaluate them as potential agents of biological control due to their possible specific adaptations, since they constitute a reservoir of genetic material and potential synthesis of particular antagonistic compounds. This underscores the importance of exploring the chemical diversity of compounds secreted by Trichoderma strains for the purpose of implementing them in potential agricultural applications.
The authors have not declared any conflict of interests.
The authors are grateful for the doctoral scholarship of CONACYT (291025) to the first author and the support of PROMEP. They also thank the Laboratory of Biotechnology and Genetics of the Institute of Applied Ecology of the UAT for the use of facilities and equipment.
REFERENCES
|
Bae H, Sicher RC, Kim MS, Kim SH, Strem MD, Melnice RL (2009). The beneficial endophyteTrichoderma hamatum isolate DIS 219b promotes growth and delays the onset of drought response in Theobroma cacao. Journal of Experimental Botany 60(11):3279-3295.
Crossref
|
|
|
|
Cai F, Guanghui Y, Ping W, Zhong W, Lin F, Qirong S, Wei C (2013). Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiology and Biochemistry 73:106-113.
Crossref
|
|
|
|
|
CONABIO (2016). Portal de Geoinformación. Available from
View. Accessed may. 5, 2017.
|
|
|
|
|
Contreras-Cornejo HA, López-Bucio JS, Méndez-Bravo A, Macías-Rodríguez L, Ramos-Vega M, Guevara-García A, López-Bucio J (2015). Mitogen-activated protein kinase 6 and ethylene and auxin signaling pathways are involved in Arabidopsis root-system architecture alterations by Trichoderma atroviride. Molecular Plant-Microbe Interactions 28(6):701-710.
Crossref
|
|
|
|
|
Dudareva N, Klempien A, Muhlemann JK, Kaplan I (2013). Biosynthesis,function and metabolic engineering of plant volatile organic compounds. New Phytology 198(1):16-32.
Crossref
|
|
|
|
|
Gajera H, Domadiya R, Patel S, Kapopara M, Golakiya B (2013). Molecular mechanism of TrichodermaTrichoderma as bio-control agents against phytopathogen system. Current Research in Microbiology and Biotechnology 1(4):133-142
|
|
|
|
|
Garnica-Vergara A, Barrera-Ortiz S, Mu-oz-Parra E, Raya-González J, Méndez-Bravo A, Macías-Rodríguez L, Ruiz-Herrera LF, López-Bucio J (2016). The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ethylene insensitive 2 functioning. New Phytology 209(4):1496-1512.
Crossref
|
|
|
|
|
Hermosa R, Viterbo A, Chet I, Monte E (2012). Plant-beneficial effects of Trichoderma and of its genes. Microbiology 158:17-25.
Crossref
|
|
|
|
|
Kerroum F, Karkachi N, Jamal E, Mabrouk K (2015). Antagonistic effect of Trichoderma harzianum against Phytophthora infestans in the North-west of Algeria. International Journal of Agronomy and Agricultural Research 6(4):44-53.
|
|
|
|
|
Lee S, Yap M, Behringer G, Hung R, Bennett JW (2016). Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biology and Biotechnology 3:7.
Crossref
|
|
|
|
|
Martínez-Medina A, Alguacil MDM, Pascual JA, Van Wees SCM (2014). Phytohormone profiles induced by Trichoderma isolates correspond with their biocontrol and plant growth-promoting activity on melon plants. Journal of Chemical Ecology 40(7):804-815.
Crossref
|
|
|
|
|
Mukherjee PK, Horwitz BA, Herrera-Estrella A, Schmoll M, Kenerley CM (2013). Trichoderma research in the genome era. Annual Review Phytopathology 51:105-129.
Crossref
|
|
|
|
|
Mukherjee PK, Horwitz BA, Kenerley CM (2012). Secondary metabolism in Trichoderma-a genomic perspective. Microbiology 158(1):35-45.
Crossref
|
|
|
|
|
Muller S, Fleck CB, Wilson D, Hummert C, Hube B, Brock M (2011). Gene acquisition, duplication and metabolic specification: the evolution of fungal methylisocitrate lyases. Environmental Microbiology 13(16):1534-1548.
Crossref
|
|
|
|
|
Naglot A, Goswami S, Rahman I, Shrimali DD, Yadav KK, Gupta VK, Rabha AJ, Gogoi HK, Veer V (2015). Antagonistic potential of native Trichoderma viride strain against potent tea fungal pathogens in North East India. Plant Pathology Journal 31(3)278-289.
Crossref
|
|
|
|
|
Ng LC, Ngadin A, Azhari M, Zahari NA (2015). Potential of TrichodermaTrichoderma spp. as biological control agents against bakanae pathogen (Fusarium fujikuroi) in rice. Asian Journal Plant Pathology 9(2):46-58.
Crossref
|
|
|
|
|
Nikolajeva V, Petrina Z, Vulfa L, Alksne L, Eze D, Grantina L, Gaitnieks T, Lielpetere A (2012). Growth and antagonism of Trichoderma spp. and conifer pathogen Heterobasidion annosum s.l. In vitro at different temperatures. Advances in Microbiology 2(3):295-302.
Crossref
|
|
|
|
|
Piechulla B, Degenhardt J (2014). The emerging importance of microbial volatile organic compounds. Plant, Cell and Environment 37:811-812.
Crossref
|
|
|
|
|
Polizzi V, Adams A, Picco AM, Adriaens E, Lenoir J, Van Peteghem C, De Saeger S, De Kimpe N (2011). Influence of environmental conditions on production of volatiles by TrichodermaTrichoderma atroviride in relation with the sick building syndrome. Building and Environment 46(4):945-954.
Crossref
|
|
|
|
|
Ravelet C, Krivobok S, Sage L, Steiman R (2000). Biodegradation of pyrene by sediment fungi. Chemosphere 40(5):557-563.
Crossref
|
|
|
|
|
Saraswathy A, Hallberg R (2002). Degradation of pyrene by indigenous fungi from a former gasworks site. FEMS Microbiology Letters 210(2):227-232.
Crossref
|
|
|
|
|
Siddiquee S, Cheong BE, Taslima K, Kausar H, Hasan MM (2012). Separation and identification of volatile compounds from liquid cultures of Trichoderma harzianum by GC-MS using three different capillary columns. Journal of Chromatographic Science 50(4):358-367.
Crossref
|
|
|
|
|
Stoppacher N, Kluger B, Zeilinger S, Krska R, Schuhmacher R (2010). Identification and profiling of volatile metabolites of the biocontrol fungus TrichodermaTrichoderma atroviride by HS-SPME-GC-MS. Journal of Microbiological Methods 81(2):187-193.
Crossref
|
|
|
|
|
Szabó M, Csepregi K, Gálber M, Virányi F, Fekete C (2012). Control plant-parasitic nematodes with Trichoderma species and nematode-trapping fungi: The role of chi18-5 and chi18-12 genes in nematode egg-parasitism. Biological Control 63(2):121-128.
Crossref
|
|
|
|
|
Vinale F, Ghisalberti EL, Sivasithamparam K, Marra R, Ritieni A, Ferracane R, Woo S, Lorito M (2009). Factors affecting the production of Trichoderma harzianum secondary metabolites during the interaction with different plant pathogens. Letters in Applied Microbiology 48:705-711.
|
|
|
|
|
Woo SL, Ruocco M, Vinale F, Nigro M, Marra R, Nadia L, Pascale A, Lanzuise S, Manganiello G, Lorito M (2014). Trichoderma based products and their widespread use in agriculture. Open Mycology Journal 8:71-126.
Crossref
|
|
|
|
|
Yang CA, Cheng CH, Liu SY, Lo CT, Lee JW, Peng KC (2011). Identification of antibacterial mechanism of l-amino acid oxidase derived from Trichoderma harzianum ETS 323. FEBS Journal 278(18):3381-3394.
Crossref
|
|