ABSTRACT
Coffea arabica, F1 hybrid variety, Ruiru 11 is a highly sought after crop in Kenya due to its alleviated resistance to Coffee Berry Disease and Coffee Leaf Rust coupled with high yield capacity and good cup quality. Access to the variety’s planting materials is limited due to challenges with difficulty in propagation using conventional methods of seed and vegetative propagation; and somatic embryogenesis is regarded as a suitable alternative propagation method. Therefore, the current study aimed to establish an induction protocol in F1 composite hybrid Ruiru 11. The current study investigated the effects of genotype and plant growth regulators, auxins and cytokinins, on induction of embryogenic callus in two composite genotypes of C. arabica L. F1 hybrid variety Ruiru 11. Leaf explants from the F1 hybrid were cultured on half-strength Murashige and Skoog (MS) media supplemented with varied concentrations and combinations of plant growth regulators. Callus formation was evaluated weekly until the 60th day. Genotypic effects were assessed based on the difference on callus induction rates, biomass fresh weights and callus formation. The genotypes tested showed highest callus induction 88% (Code 71) and 100% (Code 93) with respect to the formation of embryogenic calli. Highest fresh weight was obtained at 0.973 ± 0.011 g in Code 71 and 0.649 ± 0.03 g in Code 93 in MS media supplemented with 2,4-D + BAP ( 0.53 + 0.11 µM). The observed results are useful in formulating the best growth regulator concentration suitable for mass in vitro propagation genotypes of Arabica coffee hybrid Ruiru 11 through callus induction in vitro of leaf explants.
Key words: Somatic embryogenesis, callus, auxins, cytokinin, in vitro.
Coffee is one of the most important commodities in the international trade and is cultivated in almost 80 countries where 70% of the total global production is by smallholder farmers (Bunn et al., 2015). In Kenya, the coffee contributes about 1% to the national GDP and 8% of the total agricultural export earnings after tea and horticulture (FAO, 2013). The industry supports around 700, 000 households representing 4.2 million Kenyans (FAO, 2013). Despite this, coffee production has been on the decline falling from an average of 1.5 million bags in the 1970s to 790, 000 bags by 2018 (ICO, 2019). In light of the significance of the commodity to the Kenyan economy, substantial efforts have been directed towards the revitalisation of the coffee sector. The focus entails increase coffee productivity through the adoption of large-scale adoption of high yielding disease resistant F1 hybrid coffee variety Ruiru 11.
Ruiru 11 is a composite hybrid that comprises of about 60 F1 hybrids and each is derived from a cross between several mother plants (catimor) and genetically similar males (Kathurima et al., 2010). The variety combines resistance to coffee berry disease and coffee leaf rust alongside high yield capacity, fine cup quality and compact growth. Adoption of the variety therefore not only promotes increase in production but also reduces cost of production by up to 30% due to resistance to coffee berry disease (CBD) and coffee leaf rust up (KALRO, 2019; Gichimu et al., 2013). Besides, the compact nature of the variety allows for higher density planting of 2,500 to 3000 trees per hectare thereby facilitating increased production with a yield range of 2.5 to 3 tones of clean coffee per hectare under normal management. (Gichimu et al., 2013) compared to other commercial varieties that yield at about 300 kg- 2.0 tons per ha per annum (KALRO, 2019; Gichimu et al., 2013).
Currently, production of the planting materials for the variety relies on cost-intensive conventional methods including hand pollination for hybrid seed production and vegetative propagation through cuttings. The conventional propagation methods of artificial hand pollination and cloning through rooted cuttings have proven insufficient in meeting demands for coffee planting materials in Kenya due to several limitations including the cumbersome nature of the methods, high dependence of the methods on weather conditions, high cost of labour, increased risk of spreading pests and diseases during transportation and low success rate with respect to rate of fruit set and rooting of cuttings (Berthouly and Etienne, 1999). In addition, the methods are inefficient in maintaining the genetic fidelity of the composite hybrid variety (Gichimu et al., 2012).
Several reports exist on the successful regeneration of plants using somatic embryogenesis techniques (Ducos et al., 2007; Mohebodini et al., 2011). In coffee, two methods of somatic embryogenesis (SE) have been studied, direct somatic embryogenesis (DSE), (Hudson, 2015) and indirect somatic embryogenesis (ISE), The ISE approach is preferred for mass propagation in tissue culture (Ducos et al., 2007; Jayaraman et al., 2014; Ahmed and Disasa, 2013) due to the production of a high number of somatic embryos per gram of callus compared to DSE. Despite the success associated with ISE, reports give caution on the specificity of genotype, and its interaction with exogenous plant growth regulators supplemented in the nutrient medium in coffee (Nic-Can et al., 2015). Therefore, this requires empirical tests to optimize in vitro callus induction protocols specific to the F1 composite hybrid, Ruiru 11. The current research was undertaken with these facts in mind and had, as an objective, to investigate the effects of genotype and different combinations of exogenous plant growth regulators on callus induction on two genotypes of C. arabica F1 hybrid, Ruiru 11.
Plant materials
The study was conducted at the Plant Tissue Culture Laboratory at the Coffee Research Institute (Ruiru, Kenya). Twenty seedlings of F1 hybrids Code 71 and Code 93 (Table 1) were used for the study. The seedlings were raised in the greenhouse for a period of 3-6 months.
Establishment of callus cultures
The third leaf-pairs were excised from the donor plants and placed in a beaker containing tap water and transferred to the tissue culture laboratory. The leaves were surface
sterilized on both sides using cotton wool dipped in dilute Teepol detergent and rinsed under running tap water. In a sterile laminar flow hood, further surface sterilization was done using 20% commercial bleach (JIK) which contain 3.85% (w/v) sodium hypochlorite for 15 min and rinsed thrice using sterile distilled water. The leaf explants were subsequently immersed in 70% ethanol and rinsed three times with sterile distilled water (Hudson, 2015). These were then dissected into leaf squares
measuring approximately 1 cm × 1 cm, excluding the main vein and edges (lateral apical and basal portions). The explants were inoculated in ½ Murashige and Skoog (MS) media culture media with the adaxial-side down in culture vessels containing three leaf discs per vessel.
Effects of plant growth regulators
Half-strength MS media was supplemented with different plant growth regulators (2, 4-D, IBA, BAP, and KIN) (Table 2), concentrations, sucrose (30 g/L), 100 ml/L inositol, 30 mg/L cysteine-HCL and 40 g/L gelrite. Media pH was adjusted to 5.7 (using 1n NaOH and 1n HCL). The media was sterilized at 121°C under 1.1 psi for 15 min prior to culture. Control experiments where MS media was not supplemented with plant growth regulators and inoculated with explants were used. The culture vessels containing explants were sealed and incubated in dark growth room at 25 ± 2°C. The frequency of callus induction was recorded every two weeks and calculated after 60 days, as
Callus characteristics and growth parameters
The callus characteristics were graded based on morphology, callus score, and color. Callus morphology was characterized after 60 days of culture inoculation based on two characteristics: (i) friable (embryogenic) and (ii) compact (non-embryogenic) as shown in Figure 1. The study evaluated callus score based on growth viability under five categories: NC= no callus, + = very poor, ++ = poor, +++ = good and ++++ = very good for each of the plant growth regulator treatment. Visual analysis of callus induction period was determined as time taken to initiate visible callus from the leaf explants. Callus weights were measured on a precision scale (g) in a sterile laminar flow hood as described by Balbaa et al. (1974). For growth curve, analysis of explant cultures was initiated on the first day of inoculation (day 0). Subsequently, analyses were done at intervals of 7 days until the 70th day. The growth rate was assessed as described by Dung et al. (1981).
Effect of genotype on callus induction
The two genotypes, Code 71 and Code 93, were inoculated in MS Media to test for the callogenesis response. Effects were assessed based on the formation of embryogenic and non-embryogenic calli and callus induction rates (%).
Regeneration
After an incubation period of 45 days for proliferation of embryogenic calli obtained, calli was transferred to half-strength MS regeneration media maintained in a growth room under 16 h photoperiod and 8 h dark cycle. Plantlets with well-developed shoot and root systems were carefully washed off-culture media and transplanted into pots and covered with transparent plastic lids to maintain humidity.
Statistical analysis
The experiments were laid out in completely randomized design and repeated three times with 20 replicates. The results were assessed by a standard analysis of two-way ANOVA variance (Fischer’sTest) using Minitab® 17.1 Software. Each genotype was assessed individually. To test the genotypic differences on Code 71 and Code 93 two-sample t-test was performed.
Callus induction
Callus induction was assessed in leaf cultures of Code 71 and Code 93. Calli was induced within 10 days of inoculation. Cultures in half-strength MS media supplemented with no plant growth hormones did not induce callus in both codes.
Plant growth regulators (PGR) used singly, auxin 2,4-D induced callus whereas, auxin IBA and cytokinin (BAP and KIN) did not induce callus even after 40 days of culture. Highest induction frequency was observed for 2,4-D (0.53 µM) in Code 93 at 97% (Table 3) and 2,4-D (0.33 µM) in Code 71 at 88% (Table 2). The induction frequency, however, varied within concentrations. Low concentration levels of 2,4-D in Code 71 was observed to have increased induction rates, whereas higher concentration levels recorded reduced induction rates (Table 2). The opposite was true for Code 93, which recorded increased induction rates with increased concentration levels of 2,4-D (Table 3).
The results suggest that genotype plays an important role in callus induction. Both the type of PGR and the concentration of the same are therefore important in callus induction in Arabica coffee hybrids.
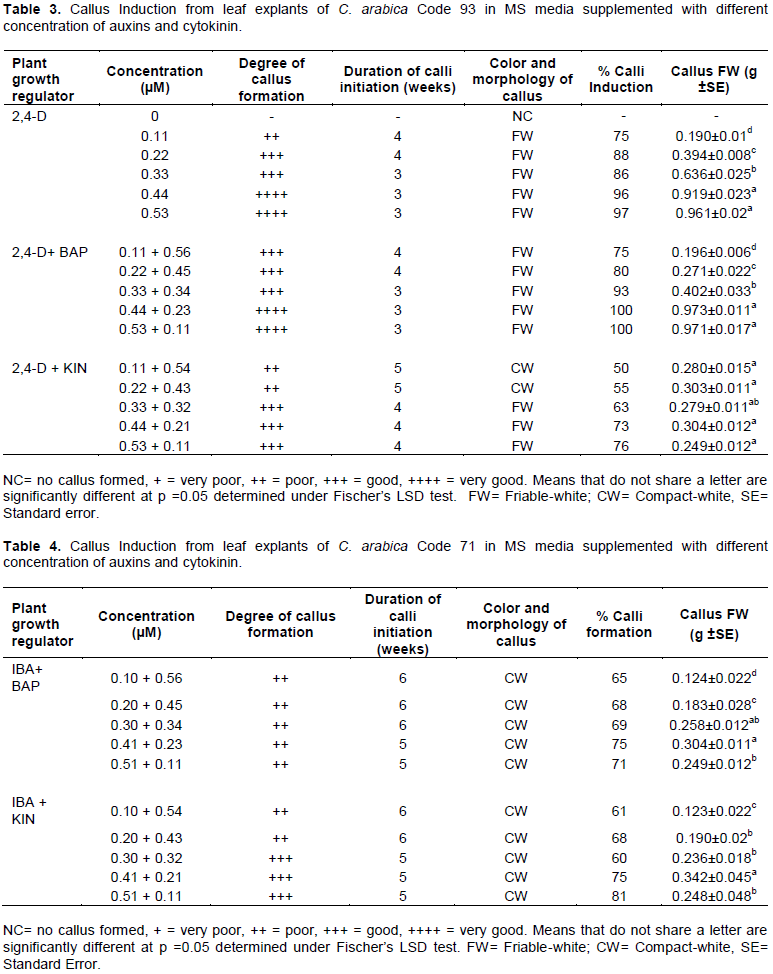
Auxin and cytokinin combinations significantly affected callus induction in both Code 93 and Code 71. 2,4-D + BAP overall recorded highest induction rates in both Code 71 (Table 2) and Code 93 (Table 3). 2,4-D + BAP (0.53 µM + 0.11 µM) resulted in highest induction rates at 100% in Code 93 (Table 3) and 88% in Code 71 (Table 2). The combination also reduced the duration required to observe peak callus in Code 71 by one week. The study also observed that higher concentration levels of auxins (2,4-D and IBA) combined with lower concentrations of cytokinins (BAP and KIN) improved induction rates in both Code 93 (Tables 3 and 5) and Code 71 (Tables 2 and 4).
Callus characteristics
Preliminary callus structures were visually observed at the cut edges of the leaf discs after 7 days of culture and proliferation was observed for a period of 8 weeks (Figure 1a and b). A white callus morphology was recorded across all treatments in Code 71 and Code 93. The color of resultant calli did not affect proliferation rates across all treatments.
Media supplemented with 2,4-D induced good friable callus (Figure 1b) formation (++) across all treatments in Code 71 (Table 2) and Code 93 (Table 3). Higher concentration levels of 2,4-D combined with lower concentration levels of BAP induced very good friable calli (++++) in Code 71 and Code 93 whereas, lower levels of 2,4-D and higher levels of BAP recorded good friable callus formation (+++) in Code 71 (Table 2) and Code 93 (Table 3). On the other hand, IBA combined with KIN and BAP induced compact calli (Figure 1a) across all treatments in Code 71 (Table IV) and Code 93 (Table 4). The browning of callus was observed after extended periods in culture, which could indicate the onset of cell death (Figure 1 c and d).
Callus induction time
The shortest period for maximum callus proliferation in Code 71 was recorded in MS media supplemented with 2,4-D + BAP (Table 2) and Code 93 (Table 3). 2,4-D recorded increased initiation time with a difference of 5-6 weeks in Code 71 (Table 2) but, reduced initiation time (3-4 weeks) in Code 93 (Table 3).
These observations indicate that PGRs treatments had an effect both on the time to and rate of callus proliferation. Auxin-cytokinin combinations (2,4-D, IBA) and (BAP and KIN) recorded longer initiation periods in Code 71 and Code 93. Time is a critical factor in the determination of efficient induction protocols. However, accompanying factors including biomass, callus morphology, and induction frequency, are important.
Biomass growth measurement
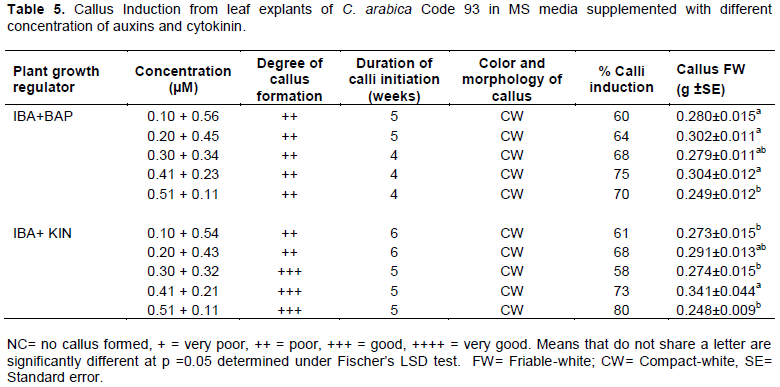
Results from fresh weight measurements indicated a significant difference in weights in embryogenic calli between PGR treatments. ANOVA analysis indicated that 2,4-D (0.33 µM) recorded highest fresh weight (0.490 ±0.013 g FW) in Code 71 (Figure 3) and 2,4-D (0.53 µM) recorded highest weight (0.961±0.02 g FW) in Code 93 (Figure 3). The results show a significant difference in embryogenic response. Code 71 showed increase in biomass with increase in 2,4-D levels but a decrease in embryogenic response in high concentration levels of 2,4-D. On the other hand, Code 93 showed a general increase in embryogenic response with increased concentration levels of 2,4-D. Auxin and cytokinin combinations, 2,4-D + BAP (0.53 + 0.11 µM) recorded highest weights in Code 71 yielding 0.649±0.026 g FW (Figure 2a) and in Code 93, (0.44 + 0.23 µM) recorded highest yield at 0.973±0.011 g FW (Figure 2a). 2,4-D + KIN (0.33 + 0.32 µM) recorded highest weights in Code 71 of 0.238±0.016 g FW (Figure 2b). The combination of 2,4-D + KIN (0.44 + 0.32 µM) yielded 0.304±0.012 g FW in Code 93 (Figure 2b).
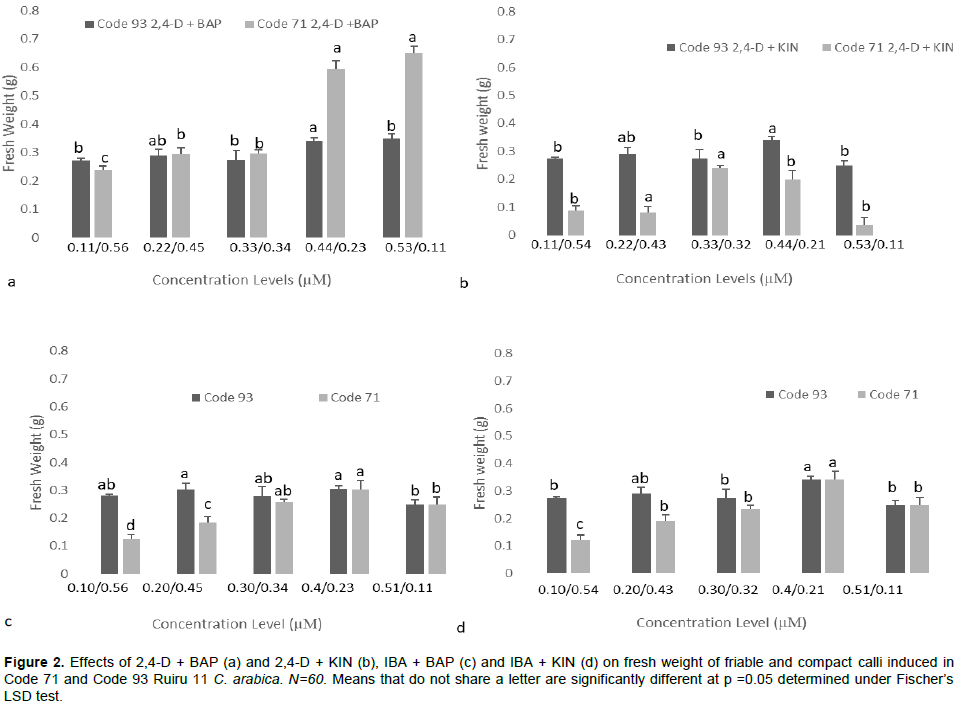
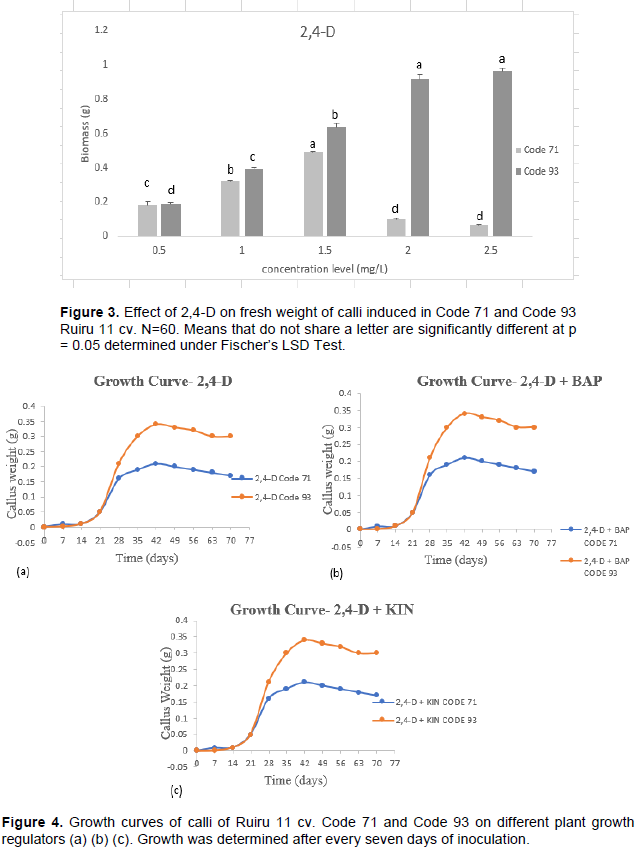
Callus growth curve
The callus growth curve is necessary to identify the suitable point of transfer in coffee tissue culture. The growth curve of calli indicated three distinct phases: lag phase, exponential phase, and linear phase (dos Santos and de Souza., 2016; Daffalla et al., 2019). Lag phase and exponential phase represent cell growth ideal for cell division and cell multiplication of callus. Linear phase is characterized by reduced cell division (dos Santos and de Souza, 2016). Prolonged linear phase promotes phenolic compound production, which is not amenable for callus proliferation. This may explain the browning observed in cultures. Figure 4 indicates growth curves recorded on embryogenic calli obtained from 2,4-D + BAP (Figure 4b) 2,4-D (Figure 4a) and 2,4-D + KIN (Figure 4c) in Code 71 and Code 93. Dedifferentiation of leaf calli was evident through a lag phase from the day of inoculation to the 14
th day of culture inoculation; exponential phase from the 21
st day to the 42
nd day of exposure and linear phases from the 42
nd day of culture. The growth curves showed a peak in growth from the 28
th day to the 42
nd day with a subsequent decrease in biomass. The prolonged linear phase suggests that a decrease in growth rate results from the scarcity of nutrients or drying of solidifying agent or accumulation of toxic substances (Daffalla et al., 2019).
The results, therefore, suggest that transfer to new growth media is necessary to increase survival rates (cell viability) and sustain callus growth after the 42nd day.
Genotype
Genotype played a significant role in callus induction in cultures. The study observed varying responses in embryogenic calli induction in Code 71 and Code 93. Code 93 recorded callus induction at a 50-100% rate in the different plant growth regulators supplemented in MS media (Tables 3 and 4) whereas, Code 71 induced at a range of 45-88% (Tables 2 and 4). 2,4-D (0.53 µM) in Code 93 showed higher mean difference in embryogenic response at 0.961 g FW (SD 0.0913). By comparison Code 71 under similar treatment was observed to have numerically lower means at 0.0672 g FW (SD 0.00182). According to the t-test analysis, the results showed significant difference in means derived for Code 71 (Mean = 0.06721; SD = 0.00814) and Code 93 (Mean = 0. 9612; SD = 0.0913) under conditions p-value (0.000) and t-value (-43.63).
The difference in response rates explanation to this could be attributed to genealogy influences.
Regeneration
Regeneration of Code 71 and Code 93 was carried out on half-strength MS media supplemented with proline 4 mg/L, sucrose 20 g/L and GA3 at 3 mg/L. Efficiency rate in formation of well-developed shoot and root system was determined as a percentage. Code 71 regenerated 68.2% and Code 93 at 75.3%. Two-way ANOVA analysis indicated regeneration potential for Code 71 at 523.8 ± 0.25 and Code 93 at 706.5 ± 0.11. The results show variation in Code interaction with half-strength MS media in regeneration potential at probability 0.05.
Callus induction
From the results observed on the control experiments with no plant growth regulators in culture media, there was no callus induction in all the trials. This is expected given that both plant growth regulators are paramount in stimulating growth and differentiation in cells and tissues under physiological process. Callus induction is a physiological process that requires the influence of plant growth regulators for cell division and differentiation. Auxins are effective in stimulation of cell elongation and vascular differentiation while cytokinins are critical in stimulation of cell division (Gaspar et al., 1996; Kumar et al., 2016). Gaspar et al., (1996) elucidates that effective callus induction is as a result of positive interaction between endogenous PGR and exogenous PGR. Exogenous PGR biological activity can be equivalent to or be in excess of endogenous PGR to influence physiological activity including callus induction. The present observations, therefore, support the results obtained hitherto by other researchers in coffee and other crop species. Similar results were obtained by Ahmed and Disasa, (2013) in C. arabica leaf explants and dos Santos and de Souza (2016) in Capsicum annum.
The PGR used singly, auxin 2,4-D induced callus in all treatments in Code 71 and Code 93. This agrees with and reemphasize the observations by Maciel et al., (2016) in Coffea arabica L., Rashmi and Trivedi (2014), in Nerium odorum (Apocynaceae), Dos Santos and De Souza (2016) in Capsicum annum, Gopitha et al. (2010) in Saccharum officinarum (Sugarcane), and Lee et al. (2011) in Morus alba (Mulberry) all whom observed embryogenic response in callus formation using 2,4-D.
Unlike auxins, the single treatment with cytokinins (KIN and BAP) did not yield any embryogenic response. This is expected from the observation because Gaspar et al., (1996) points to the fact that cytokinins are suitable for cell division, with BAP and KIN being the most commonly used. The authors further observed that cytokinins are effective in callus formation when combined with auxins; this was observed to be true for the coffee genotypes investigated through this study. The reason is that cell division is a joint action that requires a synergistic relation between auxins and cytokinins (Varshney et al., 2013). In plant cells, for auxins to be effective, the PGR has to be protected from oxidative denaturization through molecular conjugation enabling storage and consequential, gradual release for enzymatic action (Gaspar et al., 1996).
The level of concentration of the respective PGRs was observed to have an important impact on the rate of callus induction, with different genotypes displaying different reaction patterns with low concentration levels of 2,4-D (0.11, 0.22 and 0.33 µM) resulting in higher induction rates in code 71 whereas a linear increase in induction rates was observed in Code 93 with increased concentration levels. This is in line with the observations by Molina et al. (2002) and Rezende et al. (2012) in C. arabica. Overall, Code 93 recorded highest induction rates compared to Code 71 which indicate that induction rates are strongly influenced by genotype which, supporting the observations by Jiménez (2001) that embryogenic response to PGR is directly related to genotype.
Auxin and cytokinin combinations significantly affected callus induction in both Code 93 and Code 71. Similar observations have indeed been made in other studies including Gatica-Arias et al. (2008), Maciel et al. (2016) and Aga and Khillare (2017) in C. arabica callus induction using auxin and cytokinin combinations.
Auxins (2,4-D and IBA) combined with cytokinin (BAP and KIN) obtained callus formation across all treatments in Code 71 and Code 93. However, difference in embryogenic response was observed within the treatments. The differences were specific to auxin and cytokinins concentration and type along with genotype. The present study obtained highest induction rates in 2,4-D + BAP in both Code 71 and 93. Contrary, studies by Etienne et al., (2018), Gatica-Arias et al., (2008) report of best callus induction was obtained in treatments supplemented with 2,4-D + KIN. For PGR to be effective, Gaspar et al., (1996) elucidate that endogenous PGR interacts with exogenous PGR by which exogenous PGR biological activity can either be equivalent to or in excess to endogenous PGR. The interaction with endogenous PGR is specific, and cell and tissue responses greatly rely on plant species, the genotype of species and explant source. In most cases, auxin and cytokinin interaction can either be synergistic or antagonistic, whereby, auxins inhibit cytokinin action and vice versa. This is reflected by the induction competence between Code 71 and Code 93.
Callus morphology
Assessment of callus induction in tissue culture is mandatory to determine formation of embryonic and non-embryonic calli. The reason is because somatic embryogenesis can only be determined as a result of differentiation of callus cells into embryos (Ikeuchi et al., 2013). Embryogenic calli are preferred due to characteristic loosely aggregated cells of low densities which have high cell viability for embryogenesis in coffee (Leva et al., 2012; dos Santos and De Souza, 2016). The present study obtained embryogenic calli in auxin 2,4-D and auxin-cytokinin, 2,4-D + BAP across all treatments in Code 71 and Code 93. Similar observations are reported by Molina et al. (2002), Maciel et al. (2016) and Aga and Khillare (2017) in C. arabica; Durrani et al. (2017) Solanum spp, and Gopitha et al. (2010) Saccharum officinarum.
Non-embryogenic calli was also observed in treatments supplemented with 2,4-D + KIN, IBA combined with BAP and KIN across all treatments (Code 71 and Code 93). Contrary, Etienne et al., (2018) reported inducing embryogenic calli using 2,4-D + KIN (0.11 + 0.54 µM) while, Gatica-Arias et al., (2008) reported the same results at 2,4-D + KIN (4.52 +18.56 µM) in C. arabica leaf explants. Ahmed snd Disasa, (2013) on C. arabica leaf explants callus induction using IBA + KIN reported induction of embryogenic call whereas, Mohajer et al. (2012) on Onobrychis sativa, and Wahyuni et al. (2017) on Justicia gendarussa reported non-embryogenic callus induction in media supplemented with IBA + BAP. The results infer that plant growth regulators and genotype of plant can influence varying callus formations- embryogenic and non-embryogenic calli.
Code 71 and Code 93 are obtained from similar coffee variety, Ruiru 11, and similar explant, but, presented varying responses based on formation of embryogenic and non-embryogenic. This is attributed to their genotypic difference (Table 1). Jiménez (2001) suggests that embryogenic and non-embryogenic competence in callus formation in similar explants from genetically identical cells and tissues respond differently to varying stimuli which could also be the case with respect to Codes 71 and 93 in this study.
The present study observed varying callus score in treatments supplemented with auxins and cytokinins. Knowledge on the influence of degree of callus formation in relation to callus induction efficiencies is obscure. However, this study observed varying callus scores in based on plant growth regulator and genotype. The observations indicate that callus score is influenced by plant growth regulator used singly or in combination with auxin-cytokinin recording good calli formation in Code 71 and Code 93. Also, observations suggest that callus score is dependent on genotype of coffee species.
Time is a critical factor when optimizing micropropagation protocols for high-frequency somatic embryogenesis for large-scale propagation in coffee (dos Santos and de Souza, 2016). As such, shorter induction periods are superlative compared to longer induction periods (Samson et al., 2006). With regard to callus induction time, the results suggest that time taken to induce callus is influenced by genotype as well as PGR and concentration level. This parameter is often coupled with embryo formation yield, which should emphasize on high-frequency somatic embryogenesis.
Callus growth curve
Investigation on callus growth curve is paramount to determine the deceleration phase (Dos Santos and De Souza, 2016). A growth curve encompasses phases that include lag phase, exponential phase, linear phase, and deceleration phase. The pattern of the growth curve is dependent on plant species (dos Santos and de Souza, 2016; Daffalla et al., 2019). The present study observed three growth stages; lag, exponential, and linear phase. The growth curve of the study did not obtain the deceleration phase, but, phenolic compound production (synonymous with browning in coffee) was reported. Browning is tantamount with activation of secondary metabolites, phenolic compounds, (Ignacimuthu et al., 1999) considered to be a severe problem in indirect somatic embryogenesis where it inhibits growth resulting in reduced regeneration potential and recalcitrance (Jones and Saxena, 2013). Data in Figure 4 indicate that peak growth competence was achieved in embryogenic calli on the 42nd day with a subsequent slight deceleration in the growth curve. This peak indicates that probably optimal growth was achieved at the 42nd day, which consequently should be the ideal time to transfer calli to new media for callus proliferation hence increase survival rate and improve on callus growth. A similar conclusion was reached by dos Santos and de Souza (2016) working on C. canephora calli. Indicating that genotype has no influence over callus growth curve.
Biomass yield
The embryogenic potential is determined by biomass yield, which is an important factor in coffee somatic embryogenesis. Auxin, 2,4-D used singly in Code 71 (Table 2) showed reduced biomass yield with increased concentration levels. Contrary, Code 93 (Table 3) was observed to increase biomass yield with increased concentration levels. Similar results were obtained with 2,4-D + BAP in Code 93 and Code 71 across all treatments. However, biomass yield presented no effect with induction rates across all treatments in Code 71 and Code 93. The results suggest that genotype and PGR affect biomass yield in Ruiru 11 F1 hybrids Code 71 and Code 93. This observation is important given that optimized micropropagation protocols rely on high-frequency somatic embryogenesis, which is directly related to induction rates; time taken to induce callus and biomass yield.
Therefore, the difference in embryogenic response in Code 93 and Code 71 was varied based on assessed parameters in the present study through student t-test. This further confirms the conclusion by Nic-can et al. (2015) that somatic coffee embryogenesis is highly dependent on the genotype of coffee, explant source, and type and concentration of plant growth regulator. The results obtained have shown a strong inclination on the influence of auxins and cytokinins and concentration levels on callogenesis, meaning that both PGR choice and concentration levels are an important consideration in the optimization of protocols for somatic embryogenesis in the species. The results suggest that the difference in growth response is also attributed to the diversity in the genealogy of F1 hybrid Ruiru 11 Code 71 and Code 93. Molina et al. (2002) observed that coffee genealogy plays a crucial role in embryogenic capacity in genotypes which is based on the genealogy of progenies and embryogenic response is under a strong genetic control (Orians, 2000; Molina et al., 2002). Ruiru 11 is a composite Arabica coffee that comprises of hybridization of diverse coffee species including Robusta coffee and difference in results observed for the two clones may be a reflection of the differences in the residual backgrounds of the respective progenitors for the two clones.
The study established that combined use of plant growth regulators 2,4-dichlorophenoxy acetic acid [2,4-D] and 2,4-dichlorophenoxy acetic acid [2,4-D] + Benzyl amino purine [BAP] are ideal induction protocol for C. arabica F1 hybrid, Ruiru 11. The study observes that the presence of genotypic difference with respect to response to treatments with plant growth regulators leading to differences in induction rates, response rates and callus morphology. The study recommends that adoption of the induction protocol for Code 71 and Code 93 is necessary to induce callus effectively and regenerate coffee planting materials to meet current demand. It is therefore recommended that further studies be undertaken using representative sample of codes of Ruiru 11 given that the current study had limited number of codes.
The authors have not declared any conflict of interests.
The authors are grateful to the European Union for the financial support through the ACP-EU Project - Boosting coffee productivity in Kenya and Malawi through better access to and use of modern technologies and innovations (Contract number: FED/2013/330-219). The authors would also appreciate the technical staff at the Coffee Research Institute- Breeding Department for their assistance in the completion of the study. The publication of this paper has been made possible through the financial support provided by the Value Chains and Trade theme of CAB International (CABI). This paper has been published with the permission of Institute of Biotechnology Research (IBR)- Jomo Kenyatta University of Agriculture and Technology (JKUAT).
REFERENCES
|
Aga E, Khillare Y (2017). In vitro multiplication of Coffea arabica L. from leaf explants through indirect somatic embryogenesis. Interntational Journal of Botany Studies 2(1):17-22.
|
|
|
|
Ahmed WF, Disasa TT (2013). Somatic embryogenesis of a coffee (Coffea arabica L.) hybrid using leaf explants. The Journal of Horticultural Science and Biotechnology 88(4):469-475.
Crossref
|
|
|
|
|
Balbaa SI, Hilal SH, Haggag MY (1974). Effect of the use of different methods of drying of Digitalis lanata leaves on their quality and glycosidal content. Planta medica 26(05):20-25.
Crossref
|
|
|
|
|
Berthouly M, Etienne H (1999). Somatic embryogenesis of coffee. Somatic Embryogenesis in Woody Plants pp. 259-287.
Crossref
|
|
|
|
|
Bunn C, Läderach P, Rivera OO, Kirschke D (2015). A bitter cup: climate change profile of global production of Arabica and Robusta coffee. Climatic Change 129(1-2):89-101.
Crossref
|
|
|
|
|
Daffalla HM, Elsheikh AM, Ali HA, Khalafalla MM (2019). Callus Maintenance and Cell Line Selection of Grewia tenax. Journal of Herbs, Spices & Medicinal Plants 25(3):1-18.
Crossref
|
|
|
|
|
dos Santos MRA, de Souza CA (2016). Dedifferentiation of Leaf Cells and Growth Pattern of Calluses of Capsicum annuumcv. Etna. Embrapa Rondônia-Artigo em periódico indexado (ALICE). Australian Journal of Basic and Applied Sciences 10(12):362-368.
|
|
|
|
|
Ducos JP, Lambot C, Pétiard V (2007). Bioreactors for coffee mass propagation by somatic embryogenesis. International Journal of Plant Developmental Biology 1(1):1-12.
|
|
|
|
|
Dung NN, Szoki E, Verzar-Petri G (1981). The growth dynamics of callus tissues of root and leaf origin in Datura innoxia mill. Acta Botanica Academiae Scientiarum Hungaricae 27(3-4):325-333.
|
|
|
|
|
Durrani NUS, Ahmad D, Jalal A, Rajab H, Khan MS (2017). The effect of explant sources and growth regulators on callus induction and regeneration in different tomato cultivars. Journal of Animal and Plant Sciences 27(2):481-489
|
|
|
|
|
Etienne H, Breton D, Breitler JC, Bertrand B, Dechamp E, Awada R, Marraccini P, Leran S, Alpizar E, Campa C, Courtel P, Georget F, Ducos JP (2018). Coffee somatic embryogenesis: How did research, experience gained, and innovations promote the commercial propagation of elite clones from the two cultivated species? Frontiers in Plant Science 9:1-21
Crossref
|
|
|
|
|
Food and Agriculture Organization (FAO) (2013). Analysis of Incentives and Disincentives for Coffee in Kenya. Monitoring African Food and Agricultural Policies.
View
|
|
|
|
|
Gaspar T, Kevers C, Penel C, Greppin H, Reid DM, Thorpe TA (1996). Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cellular and Developmental Biology-Plant 32(4):272-289.
Crossref
|
|
|
|
|
Gatica-Arias AM, Arrieta-Espinoza G, Espinoza Esquivel AM (2008). Plant regeneration via indirect somatic embryogenesis and optimisation of genetic transformation in Coffea arabica L. cvs. Caturra and Catuaí. Electronic Journal of Biotechnology 11(1):101-112.
Crossref
|
|
|
|
|
Gichimu BM, Gichuru EK, Mamati GE, Nyende AB (2012). Selection within Coffea arabica cv. Ruiru 11 for high cup quality. African Journal of Food Science 6(18):456-464
|
|
|
|
|
Gichimu BM, Nyende AB, Gichuru EK, Mamati GE (2013). Yield Selection within Coffea arabica cv. Ruiru 11. American Journal of Experimental Agriculture 3(1):76-88
Crossref
|
|
|
|
|
Gichimu BM, Gichuru EK, Mamati GE, Nyende AB (2014). Occurrence of Ck-1 gene conferring resistance to Coffee Berry Disease in Coffea arabica cv. Ruiru 11 and its parental genotypes. Journal of Agricultural and Crop Research 2(3):51-61.
Crossref
|
|
|
|
|
Gopitha K, Bhavani AL and Senthilmanickam J (2010). Effect of the different auxins and cytokinins in callus induction, shoot, root regeneration in sugarcane. International Journal of Pharma and Bio Sciences 1(3):1-7.
|
|
|
|
|
Hudson AL (2015). Direct Somatic Embryogenesis of Selected Commercial Coffea arabica L. Varieties in Kenya (Doctoral dissertation, JKUAT). View
|
|
|
|
|
ICO (2019). Historical Data on the Global Coffee Trade. International Coffee Organization.
View
|
|
|
|
|
Ignacimuthu S, Arockiasamy S, Antonysamy M, Ravichandran P (1999). Plant regeneration through somatic embryogenesis from mature leaf explants of Eryngium foetidum, a condiment. Plant Cell, Tissue, and Organ Culture 56(2):131-137.
Crossref
|
|
|
|
|
Ikeuchi M, Sugimoto K, Iwase A (2013). Plant callus: mechanisms of induction and repression. The Plant Cell 25(9):3159-3173.
Crossref
|
|
|
|
|
KALRO (2019). Coffee Research Institute: Coffee Varieties. Ruiru 11.
View Accessed August 2019.
|
|
|
|
|
Kathurima CW, Kenji GM, Muhoho SM, Boulanger R, Davrieux F (2010). Discrimination of Coffea arabica hybrids of the composite cultivar ruiru 11 by sensorial evaluation and biochemical characterization. Advance Journal of Food Science and Technology
|
|
|
|
|
2(3):148-154.
|
|
|
|
|
Jayaraman S, Daud NH, Halis R, Mohamed R (2014). Effects of plant growth regulators, carbon sources and pH values on callus induction in Aquilaria malaccensis leaf explants and characteristics of the resultant calli. Journal of Forestry Research 25(3):535-540.
Crossref
|
|
|
|
|
Jiménez VM (2001). Regulation of in vitro somatic embryogenesis with emphasis on to the role of endogenous hormones. Revista Brasileira de Fisiologia Vegetal 13(2):196-223.
Crossref
|
|
|
|
|
Jones AMP, Saxena PK (2013). Inhibition of phenylpropanoid biosynthesis in Artemisia annua L.: a novel approach to reduce oxidative browning in plant tissue culture. PloS One 8(10):e76802.
Crossref
|
|
|
|
|
Kumar S, Singh R, Kalia S, Sharma SK, Kalia R (2016). Recent advances in understanding the role of growth regulators in plant growth and development in vitro-I: conventional growth regulators. Indian Forester 142(5):459-470.
|
|
|
|
|
Lee Y, Lee DE, Lee HS, Kim SK, Lee WS, Kim SH, Kim MW (2011). Influence of auxins, cytokinins, and nitrogen on production of rutin from callus and adventitious roots of the white mulberry tree (Morus alba L.). Plant Cell, Tissue, and Organ Culture 105(1):9-19.
Crossref
|
|
|
|
|
Leva AR, Petruccelli R, Rinaldi LMR (2012). Somaclonal variation in tissue culture: a case study with olive. Recent Advances in Plant in vitro Culture, pp. 123-150.
Crossref
|
|
|
|
|
Maciel ALDR, Rodrigues FA, Pasqual M, Carvalho CHSD (2016). Large-scale, high-efficiency production of coffee somatic embryos. Crop Breeding and Applied Biotechnology 16(2):102-107.
Crossref
|
|
|
|
|
Mohajer S, Taha RM, Khorasani A, Yaacob JS (2012). Induction of different types of callus and somatic embryogenesis in various explants of Sainfoin (Onobrychis sativa). Australian Journal of Crop Science 6(8):1305-1313.
|
|
|
|
|
Mohebodini M, Mokhtar JJ, Mahboudi F, Alizadeh H (2011). Effects of genotype, explant age and growth regulators on callus induction and direct shoot regeneration of Lettuce (Lactuca sativa L.). Australian Journal of Crop Science 5(1):92-95
|
|
|
|
|
Molina DM, Aponte ME, Cortina H, Moreno G (2002). The effect of genotype and explant age on somatic embryogenesis of coffee. Plant Cell, Tissue and Organ Culture 71(2):117-123.
Crossref
|
|
|
|
|
Nic-Can GI, Galaz-Ávalos RM, De-la-Peña C, Alcazar-Magaña A, Wrobel K, Loyola-Vargas VM (2015). Somatic embryogenesis: Identified factors that lead to embryogenic repression. A case of species of the same genus. PLoS One 10(6):e0126414.
Crossref
|
|
|
|
|
Orians CM (2000). The effects of hybridization in plants on secondary chemistry: implications for the ecology and evolution of plant-herbivore interactions. American Journal of Botany 87(12):1749-1756.
Crossref
|
|
|
|
|
Rashmi R, Trivedi MP (2014). Effect of various growth hormone concentration and combination on callus induction, nature of callus and callogenic response of Nerium odorum. Applied Biochemistry and Biotechnology 172(5):2562-2570.
Crossref
|
|
|
|
|
Rezende JCD, Carvalho CHSD, Santos ACR, Pasqual M, Teixeira JB (2012). Multiplication of embryogenic calli in Coffea arabica L. ActaScientiarum. Agronomy 34(1):93-98.
Crossref
|
|
|
|
|
Varshney A, Anis M, Aref IM (2013). Potential role of cytokinin-auxin synergism, antioxidant enzymes activities and appraisal of genetic stability in Dianthus caryophyllus L.-an important cut flower crop. In Vitro Cellular and Developmental Biology-Plant 49(2):166-174.
Crossref
|
|
|
|
|
Wahyuni DK, Andriani P, Ansori ANM, Utami ESW (2017). Callus Induction of Gendarussa (Justicia gendarussa) by Various Concentration of 2, 4-D, IBA, and BAP. Biosaintifika: Journal of Biology and Biology Education 9(3):402-408.
Crossref
|
|