ABSTRACT
Pesticides are identified as hazardous contaminants due to high toxicity and persistence in the environment. In this context, it is necessary to monitor these pollutants in the environment in situ, with the use of low cost analytical techniques and of quick response. Biosensors are presented as a complementary analytical tool to more complex techniques, such as chromatography. The present work aims to develop, in a simple and low cost way, an amperometric biosensor for the detection of simazine in aqueous samples from the inhibition of the enzyme peroxidase extracted from the pinto bean (Phaseolus vulgaris L.). For the development of the biosensor, the enzyme peroxidase was extracted in sodium phosphate buffer solution, of pH 6.0, and pre-purified by ammonium sulfate precipitation 40% (w / v) salt saturation to 50.0 ml of the protein extract. Peroxidase was characterized by electrophoresis and immobilized on silica-titanium oxide, synthesized by the sol-gel method in the proportions of 70% / 30% respectively. The biosensor showed linearity in the concentration range between 1.0 and 6.0 μgL-1 of simazine (R2 = 0.989) in the potential range 0.20 V to 0.30 V vs Ag / AgCl by the square wave voltammetry technique. The curve was obtained in the presence of 2.0 mgL-1 phenol and 3% H2O2 (v / v)), where the current density signal decreased in the presence of successive additions of the simazine inhibitor. The biosensor was applied to a standard simazine-fortified sample and detected the presence of simazine at the concentration of 35.05 μgL-1. Even showing a relative percentage error of 12.63%, when compared with the result of the analysis by high performance liquid chromatography, the biosensor is useful to monitor the presence or absence of the simazine pesticide in the solution.
Key words: Simazine, Biosensor, Carioca beans (Phaseolus vulgaris L.).
Simazine (2,4-Bis(ethylamino)-6-chloro-1,3,5-triazine) is a selective herbicide for weeds grouped into the triazine class (Guo et al., 2014). It constitutes several formulations of pesticides applied mainly in corn, beans, sugarcane and soybean (Morgante et al., 2012; Keren et al., 2015). The continuous and excessive use of this pollutant severely damages soil and water bodies (Gunasekara et al., 2007) due to its toxicity and persistence time in the environment (Parte et al., 2017; Odukkathil and Vasudevan, 2013), in addition to offering a risk to people exposed to this pesticide (Alavanja et al., 2013). Studies report that Simazine can trigger allergies and diseases such as cancer (Sato et al., 1998; Scognamiglio et al., 2013). Monitoring of agricultural pesticides has been studied in the field of development of amperometric biosensors, since these are devices that have high sensitivity and specificity, with the advantages of miniaturization (Mihos et al., 2014), easy transportation and reduced analysis time, being able to determine concentrations in scale of mgL-1a μgL-1 (Zamora-Sequeira et al., 2019; Gil and De Melo, 2010). Enzymatic biosensors are assembled by immobilizing functionalized proteins that act specifically for a particular substrate (Yulaev et al., 2001; Campanella et al., 2011). Generally purified commercial enzymes are used in the assembly, which makes the device more expensive (Zamora-Sequeira et al., 2019). One of the proposition of this work is to assemble an enzymatic biosensor from the peroxidase enzyme extracted from the Carioca Bean (Phaseolus vulgaris L.) grains. This legume is a rich, low-cost protein peroxidase rich source that can be extracted and applied to detect herbicides such as simazine. The success of the herbicide detection is based on its interaction by inhibition over the peroxidase. In order to the bio-reaction to occur, the active peroxidase site must be available and free of deformations, so that a molecule of hydrogen peroxide (natural substrate) oxidizes the ferroprotoporphyrin (Fe+3) group and produces the Fe+4/Fe+5 highly unstable intermediates (Pérez Galende et al., 2015; Aisha et al., 2016; Jang and Moon, 2011). In this step the phenol in solution can act as an electron mediator, recovering the initial oxidation state (Fe+3 from the native enzyme) and repeating the cycle. The selectivity of peroxidase can be optimized by immobilization of the enzyme on a SiO2-TiO2 composite, which blocks the direct transfer of electrons from the electrode and makes it depend only on phenol as electron donor. From then on, the reduction current of the quinones, product obtained from phenol oxidation, can be evaluated at the solution-electrode interface (Morales et al., 1996).
The detection of the Simazine herbicide occurs by evaluation of the cathodic reduction current of the quinones before and after the contact with the inhibitor (Songa et al., 2009; Arduini and Amine, 2014).
Extraction of peroxidase from beans of pinto bean (Phaseolus vulgaris L.)
Pinto bean (P. vulgaris L.) was purchased from EPAMIG (Minas Gerais-Brazil), lot FELPI 001. The grains were grounded in manual grinder. In the process the fractions of shell and pulp were separated using a 0.3 mm nylon sieve. The extracts were obtained by blender homogenization from 50.0 g of bean powder, 5.0 g of polyvinylpyrrolidone (PVP) in 150.0 ml of pH 6 sodium phosphate buffer 0.1 mol.L-1 for 5 min. The obtained homogenate was gauze filtered to remove larger fibers and the filtrate was subjected to centrifugation on a rotation of 13584 × g at 2°C for 30 min. The extracts were stored at -20°C for further analysis.
Pre-purification of pinto bean extract
In a 0°C ice bath system, a 50.0 ml aliquot of crude enzymatic extract was subjected to precipitation in ammonium sulfate 40% (w/v). After precipitation, the sample was centrifuged at 13584 × g under 4°C refrigeration for 20 min. The protein precipitate was resuspended in 5.0 ml pH 6 sodium phosphate buffer. After concentration, the extract was dialyzed using 12 kDa pore aperture cellulose semipermeable membrane versus pH 6 buffer solution. The procedure was performed for 12 h in a refrigerated chamber at 4°C.
Characterization of peroxidase extracted by SDS-PAGE
The peroxidase present in pinto beans was characterized by electrophoresis under denaturing conditions (SDS-PAGE), as described in the literature (Laemmli 1970), with modifications. For the resolution gel were added 0.8 ml of Milli-Q water, 4.125 ml of acrylamide/bis-acrylamide solution (30: 0.8), 5.0 ml of Tris-HCl-SDS 0.75 M/pH 8.8 buffer, 75 μl of 10% (w/v) APS (ammonium persulfate) and 5.0 μl TEMED(N,N,N',N'-tetramethylethylenediamine). The application gel was prepared by mixing 1.925 ml of Milli-Q water, 0.5 ml of acrylamide/bisacrylamide solution (30:0.8), 2.5 ml of 0.25 M/pH 6.8 Tris-HCl-SDS Buffer, 75.0 µl of 10% (w/v) APS and 7.5 μl of TEMED. Protein denaturation was achieved by mixing 20.0 μl of crude bean extract and 20 μl of 0.6 mol.L-1 pH 6.8 Tris-HCl buffer in the presence of β-mercaptoethanol. The sample was heated in a water bath at 100°C for 5 min. The running conditions were 200 V, 60 mV and 70 W, lasting 45 min. The gel was stained with 0.01 M silver nitrate solution in the presence of alkaline solution containing chloroform. After revealing the protein bands, the gel was photographed and the digital image analyzed by GelAnalyzer 2010 software.
Determination of total protein content
The total protein content of the bean extract was determined according to the method described by Bradford (1976). The readings were performed in UV-Visible spectrophotometer at 595 nm wavelength. The obtained values were compared with the standard curve of bovine serum albumin (BSA) in concentrations of 0.10 to 1.40 mg.ml-1.
Determination of peroxidase activity
The enzymatic activity of peroxidase was measured following the procedure of Queiroz et al., (2007). The extracts were diluted to 1:10 in 0.1 mol.L-1 pH 6 sodium phosphate buffer. In three test tubes, 500 μl of enzyme extract, 500 μl of 3% (v/v) hydrogen peroxide and 2.0 ml guaiacol 2.5 mg/100 ml were added and incubated in a water bath for 3 min. The reagent mixture was homogenized and immediately poured into quartz cuvette. The readings were performed on UV-visible (Shimadzu UV-1800) spectrometer, at 470 nm wavelength, at intervals of 1 s, for 300 s. The activity was investigated, in triplicate, by monitoring the tetraguaiacol formed in the enzymatic reaction. An enzyme unit was defined as the amount of enzyme that caused the increase of 0.001 unit of absorbance per minute of reaction.
Synthesis of SiO2-TiO2 oxide
The silica-titanium oxide was synthesized by the sol-gel method, according to the procedure described in Oliveira (2012). Proportions were prepared for synthesis of the material with 70% SiO2 and 30% TiO2. To a round bottom flask it was added 133.0 ml of tetraethylorthosilicate (TEOS 98%) along with 133.0 ml of anhydrous ethanol. After homogenization, 11.00 ml of 3.5 mol.L-1 HCl was added in order to promote the prehydrolysis of tetraethylorthosilicate. The solution was left under oil bath at 60°C for 3 h with stirring. After this time, 66.00 ml of titanium butoxide (ButOTi) and 11.0 ml of 3.5 mol.L-1 HCl were added and left for a period of 20 h at 60°C. At the end of this step the material was transferred to a becker and kept in oven at 60°C for a period of three days for complete evaporation of solvent residues still present in the medium. After drying, the material was deagglomerated with the aid of a glass stick and subjected to vacuum (10-3 mmHg) for removal of residual solvent. The samples were prepared in KBr pellets and analyzed by NICOLET Magna-IR 760 spectrophotometer
Imobilization of peroxidase in SiO2-TiO2 oxide
The peroxidase enzyme was immobilized on silica-titanium oxide by homogenizing 0.125 g of SiO2-TiO2, 1.0 ml of pre-purified extract containing 2500 U/ml and 100 μl of 0.5% (v/v) pH 6.0 glutaraldehyde on a petri dish. The mix was allowed to dry at room temperature (25°C) for 30 min. After drying, the oxide was removed with the aid of a spatula and the obtained powder containing peroxidase enzyme was added to the carbon paste.
Scanning Electron Microscopy (SEM)
The morphological aspect of the carbon paste modified with SiO2-TiO2 was obtained using the scanning electron microscopy technique, using JEOL model JSM 6360-LV with 20 kV and 1000 x magnification. The mapping of the Si, Ti and O elements was carried out in a semi-qualitative way, by electron dispersive spectroscopy (EDS) coupled to the SEM. For analysis the samples immobilized on conductive double-sided tape and covered with gold.
Preparationofthe graphite-SiO2-TiO2-Peroxidase paste
The carbon paste was previously prepared by the addition of0.2934 g of graphite powder and 0.0754 g of mineral oil. With aid of porcelain mortar and pistil the mixture was homogenized for 5 min. Finally, 0.125 g of the silica-titanium oxide containing the immobilized enzyme was added.
Eletroanalytical system for biosensor use (working electrode)
The modified carbon paste (graphite-SiO2-TiO2-peroxidase) was added to the 0.4239 cm2 area of the working electrode containing a copper wire. The system was composed by Ag/AgCl reference electrode (KClsat), platinum auxiliary electrode and working electrode. The amperometric determinations were obtained using a potentiostat/galvanostat (AutoLab 128n) and square wave voltammetry technique. The potential range studied was -0.20 to +0.50 V vs Ag/AgCl, frequency of 100 Hz, and amplitude of 0.005 V. The current measurements were obtained after 2 min of biosensor/solution contact. The electrolytic solutions without addition of simazine were prepared in phenol concentration 2.0 mg.L-1 and 3.0% (v/v) hydrogen peroxide in pH 7 phosphate buffer. For each current acquisition the modified carbon paste was removed and new paste was added to the electrode.
Inhibition curve
The enzymatic inhibition curve was constructed from 0.5-10.0 μg.L-1 of simazine in 2.0 mg.L-1 phenol solution at pH 7 and 1.0 ml of 3.0% (v/v). Each point of the curve was obtained by recharging the working electrode with the modified carbon paste containing the enzyme. The cathodic current measurements for the presence of quinine were determined by square wave voltammetry. The data were collected after 2 min of contact between the working electrode and the substrate solution. After application of the working potential, the biosensor presented a current response after 12 s.
Characterization of pinto bean extract
The peroxidase extracted from carioca beans (P. vulgaris L.) showed total activity of 2500 U/ml, with a protein content of 87.37 mg/ml, which characterizes a specific activity of 66.02 U.mg-1. The bean was characterized by electrophoresis as shown in Figure 1. The molecular mass of the commercial peroxidase is presented in literature as around 40 kDa (Sigma Aldrich 2017), while the peroxidase extracted from the bean (P. vulgaris L.) was identified at approximately 41.68 kDa.
Characterization of carbon paste and silica-titanium oxide
Medium Infrared (FTIR-MID)
After oxide synthesis, the pulverized sample (<90 mechs) was analyzed by medium infrared (IFRED-MID) in KBr paste. The main bands for characterization of silica-titanium oxide are presented in the spectrum of Figure 2. The vibrational frequencies of SiO2 can be identified by wide band at 3448 cm-1 corresponding to the symmetrical stretching vibrations of the silanols (SiO-H) groups. The absorption at 1079 cm-1 is due to axial vibrations of the Si-O group (Navarrete et al., 1996). The presence of titanium was observed by the vibrations at 960 cm-1 for the asymmetric stretching of the Ti-O-Si bond and the bands between 450 and 791 cm-1 corresponding to Ti-O and Ti-O-Ti vibrations (Davis and Liu, 1997). By the absorption profile in the spectrum it can be inferred that the results obtained show that grafted TiO2 are dispersed in the silica matrix.
Scanning electron microscopy (SEM)
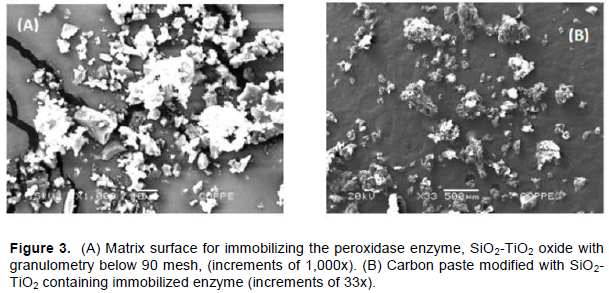
The SiO2-TiO2 oxide prepared by sol-gel was reduced to particle size below 90 mesh and subjected to scanning electron microscopy (SEM) together with the modified carbon paste containing the enzyme, as shown in Figure 3A and B. As Figure 3A shows that the material exhibits diversity of sizes and shapes, which does not interfere on the purpose used. Also by the electron micrograph it is observed that the area selected for Energy-dispersive X-ray spectroscopy (EDS) analysis showed uniform distribution for SiO2-TiO2 by all material, and the formation of individual TiO2 and/or SiO2 agglomerates (islands) does not occur. According to microscopic scanning analysis (Figure 3B), it was possible to visualize the formation of enzyme-SiO2TiO2-Graphite agglomerates, which can provide a high concentration of active sites of the dispersed enzyme on the SiO2-TiO2 surface available for catalysis. In the stage of immobilization cross-links are formed between enzymes and glutaraldehyde and enzymes and support. Such bonds occur through terminal carboxylic and amine groups present in the molecular structure of the enzyme and the titanium oxide, grafted onto the surface of the silica. This immobilization was advantageous due to the increase of the enzyme-matrix contact surface with consequent increase in the rate of enzymatic catalysis.
Calibration curve for simazine
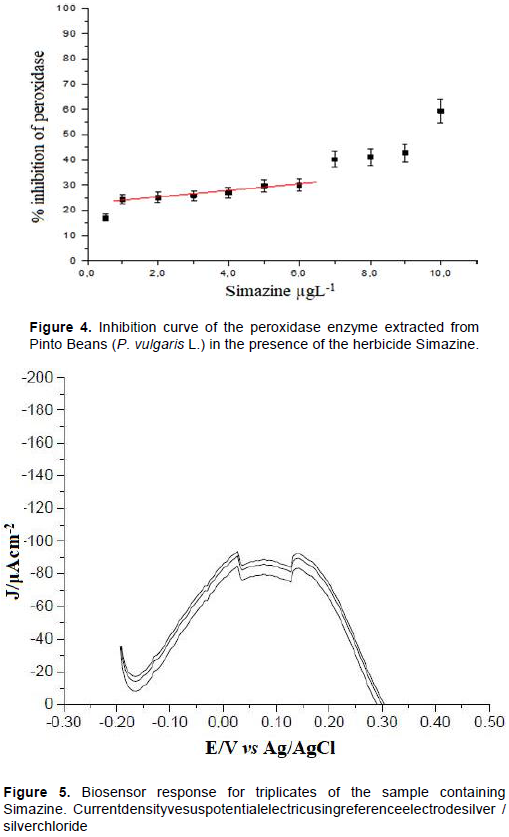
The percent inhibition of the immobilized peroxidase enzyme was determined from the cathodic current density values for quinone reduction. The inhibition curve was constructed by increasing concentrations of simazine from 0.5 to 10 μg/L at pH 7.0. Current data were obtained at +0.14 V vs Ag/AgCl, in the absence and presence of the inhibitor (simazine). In the absence of the inhibitor, the biosensor had a maximum current density of -120.10 J/μAcm-2, from the enzymatic catalysis of phenol oxidation (2.0 mg/L phenol and 3% H2O2 (v/v)). In the presence of the inhibitor, the cathodic current measurements were obtained after 2 min of contact with the solution containing simazine. The inhibition curve of peroxidase as a function of the concentration of simazine is shown in Figure 4. As shown in Figure 4 for established experimental conditions, the biosensor showed linearity in the concentration range 1.0 to 6.0 μg/L (R2 = 0.989% inhibition = 1.073 [simazine] + 22.8) of Simazine. The modified carbon paste containing the peroxidase enzyme used to construct the calibration curve was also used to determine simazine in sample01.
Application of biosensor on samples
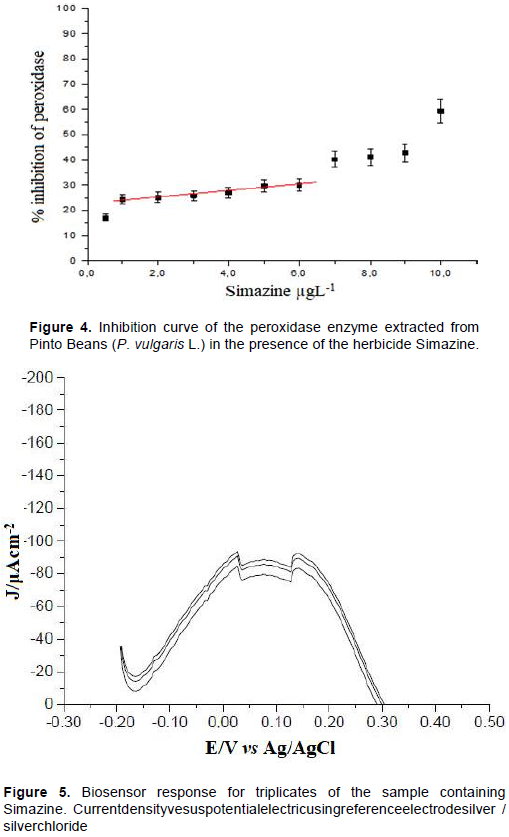
The herbicide Simazina has its commercialization controled, regulated by law in several countries (Heri et al., 2008;Xie et al., 2019; Conama, 2005) in view of its high toxicity to humans and the environment. Thus, to evaluate the response of the biosensor a sample of Simazina was assigned by the Oswaldo Cruz Foundation (Fiocruz-Brazil) and analyzed by square wave voltammetry. For analysis using the biosensor the sample was diluted (10 times) in pH 7 phosphate buffer and applying scanning potential from -0.20 V to +0.50 V vs Ag/AgCl. The measurements were obtained in triplicate, from the same batch of modified carbon paste, as shown in Figure 5.
The currents were converted to current density, presenting values of -89.58, -88.24 and -86.74 J/μAcm-2, with average current density of -88.19 J/μAcm-2. The percentage of inhibition was obtained by applying the inhibition curve (%inhibition= 1.073 [simazine] + 22.8). The inhibition percentage for the analyzed sample was 26.56%, corresponding to a simazine concentration of 35.04 μg.-1 in the sample (dilution factor: FD = 10). In contrast, the sample was analyzed by high performance liquid chromatography (HPLC) from a calibration curve in concentrations of 0.02 to 0.032 μg.ml-1.
The sample analyzed by HPLC showed a concentration of 31.11 μgL-1 Simazine (R2 = 0.997). This result shows that the proposed biosensor presented a relative percentage error of 12.63%, showing that the developed device does not present sufficient confidence in the detection of the herbicide. However, even with an error greater than 10%, the biosensor can be considered efficient for the verification of the presence or absence of simazine in aqueous samples. It is also a promising device considering the cost reduction analysis and manufacturing, compared to classical techniques such as HPLC, which demands time and high investment costs. The proposed biosensor also requires investigative studies regarding several variables that can be optimized to minimize detection errors, such as the storage time of the simazine solution after preparation and the time of contact between biosensor (peroxidase) and agrochemical simazine.
The results show that peroxidase present in the carioca bean extract, even if not totally purified, presented a good selectivity and specificity in relation to the catalytic route for phenol oxidation. This is also due to the fact that carbon paste based biosensors, which use electron mediators such as phenol, immobilizing peroxidase on the titanium silica matrix favours the biosensor selectivity for this molecule. This is due to the electron transfer blockage between the electrode (carbon paste) and the enzyme, which depends exclusively on phenol (electron donor), a fact that has already been observed by Rosatto et al., (1999). The decrease in the peroxidase phenol oxidation product reduction current signal suggests a mechanism of inhibition of the peroxidase active site, and further investigation is needed, which makes this research a basis for this investigation. There are studies based on immunosensors that use the peroxidase conjugation device with antibodies, in which the authors report that the inhibition is competitive (Yulaev et al., 2001).
Many enzymatic biosensors that use partially purified extracts showed good sensitivity measures and low percentual errors, while others had considerable detection errors above 10%. Moccelini et al., (2008) reports the construction of a biosensor from scarlet eggplant (Solanum gilo) extracts containing the peroxidase enzyme for phenolic compound detection, showing relative error of 4.5% and 1.5%, with detection limit for hydroquinone of 2 .0 ×10-5 molL-1 and 3.0 × 10-8 molL-1 rutin.
Santos (2016) developed a biosensor from mushroom extract (Agaricus bisporus) partially purified with ammonium sulfate, containing the tyrosinase enzyme. The author determined the presence of benzoic acid in a guarana sample and obtained a percentual error above 10% in the samples when compared to HPLC measurements. This study reported the presence of interferers in the media which caused errors in the determinations. The bean extract based biosensor containing peroxidase enzyme is a viable alternative of simple elaboration, providing linear response by the basic principles of amperometric detection. The good approximation of the results compared with HPL shows that the device is efficient and selective and can be applied by the enzyme inhibition method in the presence of simazine pesticide, being able to validate the presence or absence of this contaminant in water. Improvement of the device is still necessary so that its performance can reach quantification levels and not just detection, with lower relative error for analysis. The mechanisms that control the inhibition reaction still needs to be investigated more clearly.
Pinto bean (P. vulgaris L.) is a rich source of peroxidase, low cost and easily accessible in several countries. The biosensor based on the extract of the bean grains containing the peroxidase enzyme is a viable alternative of simple elaboration, but with basic principles of amperometric detection, consolidated by several biosensors. Its use is potentialized in view of the need for environmental monitoring of contaminating pesticides on surface and groundwater sources, and it is possible to prepare the analysis in aqueous samples in situ. It was demonstrated that there is an inhibition response, by the decrease of cathodic current, when the biosensor comes in contact with the herbicide simazine, fact that shows the sensitivity of the device in the detection of this pesticide. The improvement of the device is still necessary so that its performance can reach levels of quantification and not only of qualification, with lower relative error for analysis.
The authors have not declared any conflict of interests.
REFERENCES
|
Aisha A, Amine A, Achi F, Saliha BB, Cubillana-Aguilera L, José M Palacios-Santander, Abdoullatif B, Abdelhamid E (2016). A novel amperometric inhibition biosensor based on HRP and gold sononanoparticles immobilised onto Sonogel-Carbon electrode for the determination of sulphides. International Journal of Environmental Analytical Chemistry 96(6):515-529.
Crossref
|
|
|
|
Alavanja MCR, Ross MK, Bonner MR (2013). Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA: A Cancer Journal for Clinicians 63(2):120-142.
Crossref
|
|
|
|
|
Arduini F, Amine A (2014). Biosensors Based on Enzyme Inhibition. Biosensors Based on Aptamers and Enzymes 140:299-326.
Crossref
|
|
|
|
|
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72:248-254.
Crossref
|
|
|
|
|
Campanella L, Eremin S, Lelo D, Martini E, Tomassetti M (2011). Sensors and Actuators B : Chemical Reliable new immunosensor for atrazine pesticide analysis. Sensors and Actuators: B. Chemical 156(1):50-62.
Crossref
|
|
|
|
|
CONAMA (2005). RESOLUÇÃO No 357, DE 17 DE MARÇO DE 2005. Retrieved from
View
|
|
|
|
|
Davis RJ, Liu ZF (1997). Titania-silica: A model binary oxide catalyst system. Chemistry of Materials 9(11):2311-2324.
Crossref
|
|
|
|
|
Gil ES, Melo GR (2010). Electrochemical biosensors in pharmaceutical analysis. Brazilian Journal of Pharmaceutical Sciences 46(3):375-391.
Crossref
|
|
|
|
|
Gunasekara AS, Troiano J, Goh KS, Tjeerdema RS (2007). Transport of simazine in unsaturated sandy soil and predictions of its leaching under hypothetical field conditions. Journal of Contaminant Hydrology 94(3-4):166-177.
Crossref
|
|
|
|
|
Guo Q, Wan R, Xie S (2014). Simazine degradation in bioaugmented soil: urea impact and response of ammonia-oxidizing bacteria and other soil bacterial communities. Environmental Science and Pollution Research International 21(1):337-343.
Crossref
|
|
|
|
|
Heri W, Pfister F, Basel S, Parshley BC, Nabors JB (2008). The triazine herbicides: 50 years revolutionizing agriculture, pp. 31-43.
Crossref
|
|
|
|
|
Jang J, Moon K (2011). Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chemistry 124(2):444-449.
Crossref
|
|
|
|
|
Keren Y, Borisover M, Bukhanovsky N (2015). Chemosphere Sorption interactions of organic compounds with soils affected by agricultural olive mill wastewater. Chemosphere 138:462-468.
Crossref
|
|
|
|
|
Laemmli UK (1970). Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 227(5259):680-685.
Crossref
|
|
|
|
|
Mihos FDCSS, Silva LMC, Salgado AM, Maggesissi MR (2014). Application and construction of a biosensor using graphite rod and bean, phaseolus vulgaris l., for phenol detection. Chemical Engineering Transactions 38:439-444.
|
|
|
|
|
Moccelini SK, Spinelli A, Vieira IC (2008). Enzyme and Microbial Technology Biosensors based on bean sprout homogenate immobilized in chitosan microspheres and silica for determination of chlorogenic acid. Enzyme and Microbial Technology 43:381-387.
Crossref
|
|
|
|
|
Morales A, Cespedes F, Muzoz J, Fabrebregas E, Alegret S (1996). Hydrogen peroxide amperometric biosensor based on a peroxidase-graphite-epoxy biocomposite. Analytica Chimica Acta 332(2-3):131-138.
Crossref
|
|
|
|
|
Morgante V, Flores C, Fadic X, González M, Hernández M, Cereceda-balic F, Seeger M (2012). In fl uence of microorganisms and leaching on simazine attenuation in an agricultural soil. Journal of Environmental Management 95:S300-S305.
Crossref
|
|
|
|
|
Navarrete J, Lopez T, Gomez R, Figueras F (1996). Surface acidity of sulfated TiO2-SiO2 sol-gels. Langmuir 7463(21):4385-4390.
Crossref
|
|
|
|
|
Odukkathil G, Vasudevan N (2013). Toxicity and bioremediation of pesticides in agricultural soil. Reviews in Environmental Science and Bio/Technology 12(4):421-444.
Crossref
|
|
|
|
|
Oliveira SSD (2012). Protoporfirina-IX imobilizada sobre SiO2-TiO2 preparada pelo processo Sol-Gel: Síntese, Caracterização e Aplicação Fotoquímica. Universidade Federal do Rio de Janeiro (UFRJ).
|
|
|
|
|
Parte SG, Mohekar AD, Kharat AS (2017). Microbial degradation of pesticide : A review. African Journal of Microbiology Research 11(24):992-1012.
Crossref
|
|
|
|
|
Pérez GP, Hidalgo Cuadrado N, Kostetsky EY, Roig MG, Villar E, Shnyrov VL, Kennedy JF (2015). Kinetics of Spanish broom peroxidase obeys a Ping-Pong Bi-Bi mechanism with competitive inhibition by substrates. International Journal of Biological Macromolecules 81:1005-1011.
Crossref
|
|
|
|
|
Queiroz AS, Ester M, Moreira M (2007). Purificação parcial e caracterização cinética da enzima Polifenol oxidase de banana nanica (Musa acuminata ) Partial purification and kinetics caracterization of enzyme Polyphenol oxidase of stunded banana (Musa acuminata). Revista do Instituto de Ciências da Saúde 25(3):239-246.
|
|
|
|
|
Rosatto SS, Kubota LT, Neto GO (1999). Biosensor for phenol based on the direct electron transfer blocking of peroxidase immobilising on silica-titanium. Analytica Chimica Acta 390(1-3):65-72.
Crossref
|
|
|
|
|
Santos VDS (2013). Application of Agaricus bisporus Extract for Benzoate Sodium Detection Based on Tyrosinase Inhibition for Biosensor Development. Chemical Engineering Transactions (32):1831-1836.
|
|
|
|
|
Sato T, Taguchi M, Nagase H, Kito H, Niikawa M (1998). Augmentation of allergic reactions by several pesticides. Toxicology 126(1):41-53.
Crossref
|
|
|
|
|
Scognamiglio V, Pezzotti I, Pezzotti G, Cano J, Manfredonia I, Buonasera K, Giardi MT (2013). A new embedded biosensor platform based on micro-electrodes array (MEA) technology. Sensors and Actuators B: Chemical 176:275-283.
Crossref
|
|
|
|
|
Sigma Aldrich (2017). Peroxidase from horseradish Sigma Type VI. Sigma Aldrich.
View
|
|
|
|
|
Songa EA, Arotiba OA, Owino, JHO, Jahed N, Baker PGL, Iwuoha EI (2009). Electrochemical detection of glyphosate herbicide using horseradish peroxidase immobilized on sulfonated polymer matrix. Bioelectrochemistry 75(2):117-123.
Crossref
|
|
|
|
|
Yulaev MF, Sitdikov RA, Dmitrieva NM, Yazynina EV, Zherdev AV, Dzantiev BB (2001). Development of a potentiometric immunosensor for herbicide simazine and its application for food testing. Sensors and Actuators B: Chemical 75(1-2):129-135.
Crossref
|
|
|
|
|
Xie Y, Luo Y, Singhasemanon N, Goh KS (2019). Modeling Pesticide Aquatic Exposures in California for Regulatory Purposes: Model Review and Scenario Assessment.
Crossref
|
|
|
|
|
Zamora-Sequeira R, Starbird-Pérez R, Rojas-Carillo O, Vargas-Villalobos S (2019). What are the Main Sensor Methods for Quantifying Pesticides in Agricultural Activities? A Review. Molecules 24(14):2659.
Crossref
|
|