Cabbage (Brassica oleracea var. capitata) is a highly nutritive vegetable consumed globally as a major component of salad, shawarma, coleslaw etc. The phytonutrients and antioxidants of cabbage aids in the
the prevention of a number of human diseases such as breast cancer, prostate cancer, vascular inflammatory diseases and high blood
pressure (National Cancer Institute, 2012; Higdon et al., 2017, Joo et al., 2018). Although cabbage has tremendous nutritional and medicinal benefits to the growth, development and health of humans, it has been reported to be susceptible to quite a number of diseases caused by pathogens such as fungi and bacteria (Weinberger and Lumpkin, 2005; Omokore et al., 2008, 2009; Mochiah et al., 2011). Such diseased cabbages when consumed by humans are capable of affecting human immune system negatively, leaving the individuals with a deteriorated health condition. In addition, the presence of pathogens on cabbages precisely reduces their nutritional and market value.
Fungal disease constitutes a menace in vegetable production, and many pathogens have been reported to be associated with vegetable crops in the field as well as at storage and processing stages (Salau and Shehu, 2015). Cabbage can be attacked by pathogens before, during or after cultivation (Kurtzman et al., 1987). Postharvest diseases in cabbage are a result of packing, storage, transport and marketing conditions (Hung et al., 2004). However, it is quite possible for latent infections during cultivation to manifest after harvest, thereby causing bio-deterioration (Barnes and Shaw, 2002).
Till date, the most common method of disease control in cabbage is the chemical control measure. This is expensive and continues to be hazardous to man and the environment. Despite the wide usage and application of chemicals in plant disease control, postharvest diseases are still prevalent, causing huge losses via deterioration. Therefore, attention has drifted towards development of suitable alternative plant disease control measures; one of which is the use of botanicals. Hence, this research was conducted to determine the efficacy of ethanol and aqueous leaf and root extracts of Hyptis suaveolens on fungal pathogens associated with postharvest cabbage spoilage.
H. suaveolens (L.) Poit. (Family: Lamiaceae) is a common weed that is native to tropical America. However, the plant is presently considered a worldwide weed (Chukwujekwu et al., 2005). It is an annual herb found in dense clumps occupying roadsides, rail tracks, wastelands, watercourses, pastures and open forests where the soil is well drained (Carlos et al., 2012; Sharma et al., 2013). It can form dense thickets in all areas of growth. H. suaveolens (pignut) is an obnoxious weed that is distributed throughout the tropics and subtropics (Rajarajan et al., 2014). As reported by Sharma et al. (2013), H. suaveolens is widespread in West and Central Africa, Australia (northern territory and Queensland), China, Indonesia, Papua New Guinea, Solomon Islands, French Polynesia, Federated States of Micronesia (Chuuk and Yap Islands), Niue Islands, Guam and the Hawaiian Islands in the USA. According to Abdullahi et al. (2003), H. suaveolens may be found in abandoned farmlands in West Africa especially in Northern Nigeria.
Collection of plant samples
Diseased and healthy cabbage samples were obtained from major vendors in Ojoo, Sango, Bodija and Agbowo in Ibadan, Oyo State, Nigeria. H. suaveolens samples were collected from Morondiya Distance Learning Centre along Ibadan - Ilorin highway, Nigeria. The plants were identified and authenticated at the herbarium, Department of Botany, University of Ibadan, Nigeria.
Preparation of culture media
The employed nutrient media was the Potato Dextrose Agar (PDA). It was prepared following standard procedure (DifcoTM & BBLTM Manual, 2009; Remel, 2010); by thoroughly mixing 39 g of PDA with 1000 ml of distilled water in a conical flask. The resultant mixture was autoclaved at 103 KNM-2 pressure and 121°C for 15 min, after which it was allowed to cool; thereafter it was acidified using lactic acid (100 drops per 1000 ml) to prevent bacterial growth. The resultant Acidified Potato Dextrose Agar (APDA) was poured into sterilized Petri-dishes and allowed to gel and solidify. This was used for initial isolation and sub-culturing of the fungi.
Isolation of fungal pathogens
Fungi responsible for spoilage in cabbage were isolated from the infected cabbage samples. Diseased tissues were excised from the periphery of infected cabbage using sterilized scalpel. The diseased tissues were surface-sterilized by placing them in 80% ethanol for 2 min after which they were immediately rinsed in two changes of sterile distilled water (Amadi et al., 2013). The sterilized diseased tissues were then plated unto APDA with the aid of inoculation needles. The inoculated APDA plates were incubated at room temperature (28 ± 2°C) and observations were made daily for emergence of culture (Babu et al., 2008). The mycelia of the resulting fungi were sub-cultured unto APDA plates and incubated for 7 days. Several sub-culturing unto APDA plates was done until pure cultures were obtained. Thereafter, agar slants were prepared and used to preserve fungal isolates until they were needed.
Identification of fungal isolates
The isolated fungi were identified based on mycelia growth patterns and microscopic examinations (Jonathan et al., 2013). Slides of pure cultures of the fungal isolates were prepared for microscopic observation and identification. Culture and morphological characteristics of the isolates were observed and noted and formed part of the criteria used for identification (Barnett and Hunter, 1987; Domsch et al., 1993). Detailed morphological characteristics of the fungi such as hyphae (septation), reproductive structure (sporangia/conidia) in chain or single; the type of spore, etc. were observed and recorded (Amadi et al., 2013).
Pathogenicity test
Pathogenicity test was carried out according to Koch’s postulate. Six healthy cabbages were surface-sterilized using 80% ethanol and inoculated with test fungi (Amadi et al., 2013). Sterile cork borers were used to remove cylindrical discs (3 mm diameter) from the healthy cabbages. Mycelia plugs (3 mm diameter) were excised from 7 days old pure cultures of the fungal isolates using cork borers and plugged into the pores made in the cabbages. However, some of the cabbage were inoculated with sterile APDA discs instead and these served as the control. After inoculation, the cabbage discs were replaced and the points of inoculation sealed with Vaseline to prevent contamination. The inoculated cabbage were incubated at room temperature (28 ± 2°C) in the laboratory. These cabbage were examined for appearance of disease symptoms after 48 h and subsequently on daily basis for 7 days. Re-isolation of fungal pathogens unto PDA plates was done from inoculated cabbage that showed disease symptoms. The characteristics of the resultant fungal isolates were compared with that of the original cultures of the fungal pathogens in order to confirm they were the same. Likewise, the fungal isolates were re-inoculated into healthy fruits for confirmation as the implicated pathogens.
Preparation of plant extracts of H. suaveolens
Two types of extracts were employed in this research (aqueous and ethanol extracts). The extracts were prepared using leaves and roots of the plant according to the method described by Babu et al. (2008), Alo et al. (2012) and Rajarajan et al. (2014).
Fresh samples of
H. suaveolens were harvested and thoroughly washed using tap water and rinsed with distilled water. These washed samples were then taken to the laboratory where mature healthy non-infected leaves and roots were harvested and dried at room temperature for one week. The dried leaves and roots were then pulverized into fine powder.
Prior to use, the powdered samples were preserved in air tight bottles. In conical flasks, 150 g of each powder were soaked in 750 ml of each extraction solvents (water and ethanol), while stirring vigorously was performed with a glass rod for proper extraction after which the flasks were covered with rubber corks. The mixtures were allowed to stand for 48 h at room temperature with occasional shaking and then filtered through a double layered muslin cloth and Whatman filter paper (No. 1) into separate clean conical flasks (Rahman et al., 2009). The filtrates were concentrated by evaporation to dryness in an evaporating dish (Arikpo et al., 2013). Crude extracts obtained were stored in glass bottles at 4°C prior to use (Akueshi et al., 2002).
Preparation of extract concentrations
The used extract concentrations were 20, 40, 60, 80 and 100%. These extract concentrations were prepared by serial dilutions using the method adopted by Mahesh and Satish (2008), Ivoke et al. (2009) and Ademe et al. (2013) which involves reconstituting the crude extracts obtained in their respective extraction solvents. For instance, 80 ml of 100% extract was diluted with 20 ml of the respective extraction solvent to obtain an 80% extract concentration; 60 ml of 100% extract was diluted in 40 ml of the respective solvent to obtain 60% extract concentration, and so on.
Application of leaf and root extracts
The method used for testing fungitoxic properties of plant extracts was a modification of the poisoned food technique (Nene and Thapliyal, 1993; Suleiman and Ogundana, 2010). Different concentrations of leaf and root extracts (1 ml each) were placed on sterile Petri-dishes, molten APDA medium was added and the Petri-dishes were swirled gently to permit even distribution of the plant extracts. However, 1 ml of the respective extraction solvent was added in place of plant extract in some plates and in some others, only APDA was added. These served as Control 2 and Control 1, respectively. After the APDA solidified, mycelia plugs (5 mm diameter) of fungi, taken from the edge of 5-days old cultures were put in the center of the APDA (Umesh, 2013). The inoculated plates were incubated at room temperature for 10 days. However, the effect of the extracts on radial growth of fungal isolates was examined daily and the radial growth (cm) of each fungus was measured for 10 days consecutively after inoculation at an interval of 24 h (Babu et al., 2008).The experiment was setup in triplicates and laid out in a completely randomized design (CRD).
Evaluation of effects of extracts on growth of fungal isolates
The antifungal activity of the extracts was evaluated by measuring the inhibition zones against the tested fungi. Since the experiment was carried out in triplicates, the mean and % inhibition of mycelia growth were determined. Growth inhibition (%) was calculated using the following equation (Odebode, 2006):
R1= radial growth of the pathogen in control medium. R2 = radial growth of the pathogen in the treated/test medium.
Statistical analysis
Inhibitory effects of extract concentrations on fungal growth were compared using one-way Analysis of Variance (ANOVA) coupled with Least Square Difference (LSD) post hoc multiple pairwise comparisons. Difference in fungal growth inhibition between ethanol and aqueous extracts were compared using Student t-Test. Level of significance was set at p < 0.05. Statistical analysis was conducted using SPSS® version 20.0 (IBM Corp., Armonk, USA). Charts were prepared in Microsoft Office Excel® (Microsoft Inc., Redmond, USA).
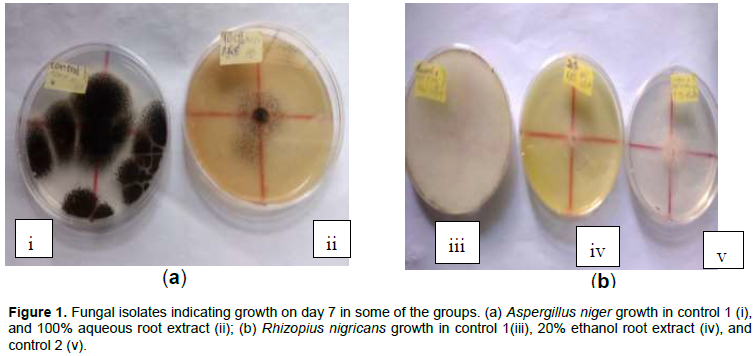
Growth inhibition of A. niger and R. nigricans by extracts
The isolated fungi associated with postharvest cabbage deterioration were
Aspergillus niger and
Rhizopus nigricans (Figure 1(ai and iii). Ethanol and aqueous extracts of
H. suaveolens were potent against
A. niger and
R. nigricans isolates from the cabbage. Ethanol root extracts were highly effective as growth inhibitors of both isolates. Ethanol leaf extract of the plant was only highly effective against
A. niger, but weakly effective against
R. nigricans. Aqueous root extracts of
H. suaveolens were similarly highly potent against
A. niger, but was only weakly inhibitory to
R. nigricans growth. Aqueous leaf extracts of
H. suaveolens irrespective of concentration did not inhibit growth of either of the fungal isolates. The inhibitory activities of the different extract concentrations on
A. niger and
R. nigricans are presented in Tables 1 and 2, respectively. Generally, inhibition of growth of the fungal species decreased based on duration of exposure; effects were in the order: Day 5 effects > day 7 effects > day 10 effects.
Growth inhibition of A. niger by 20, 40, 60, 80 and 100% ethanol leaf and root extracts of H. suaveolens were significantly different from control 1 (p < 0.0001). A. niger growth inhibition by 20, 40, 60, 80 and 100% aqueous extract of H. suaveolens was significantly different from control 1 (p < 0.0001). Compared to aqueous leaf extracts, all concentrations of ethanol leaf extracts used were significantly more inhibiting on
A. niger growth at days 5, 7 and 10 (
p < 0.05) (Table 1). Growth inhibition of
A. niger by ethanol and aqueous roots were similar except for 40 and 100% concentrations on day 5, and 100% concentration on day 10. Duration and % growth inhibition of
R. nigricans by ethanol leaf extracts were low compared to inhibition of
A. niger (Table 2). Ethanol leaf extracts and control 2 (ethanol solvent only) significantly reduced
R. nigricans growth on day 5 (p < 0.05); by days 7 and 10, the activity had ceased except for 60% concentration.
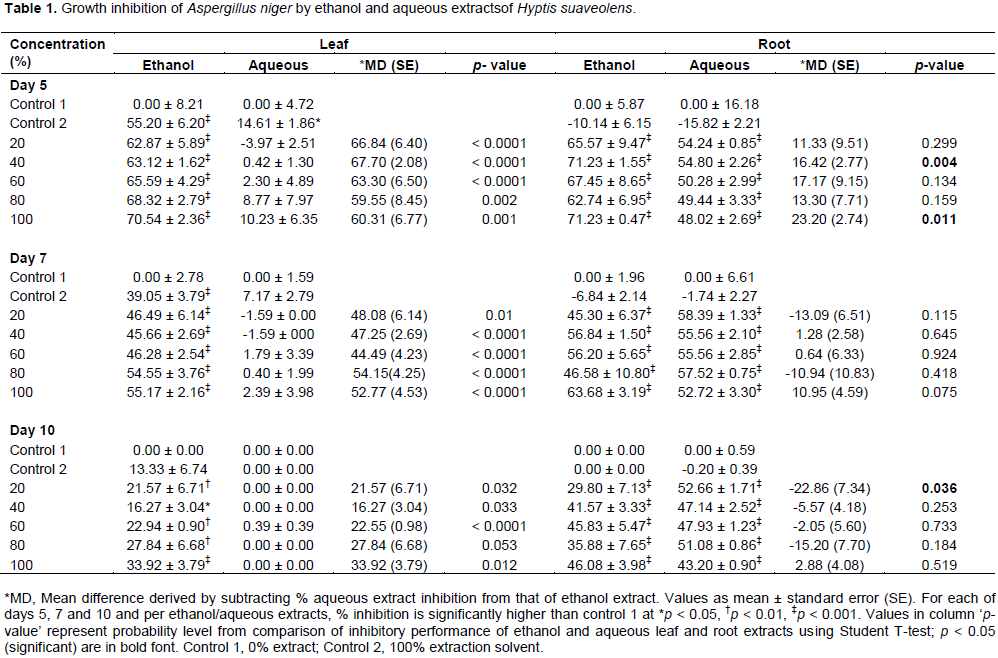
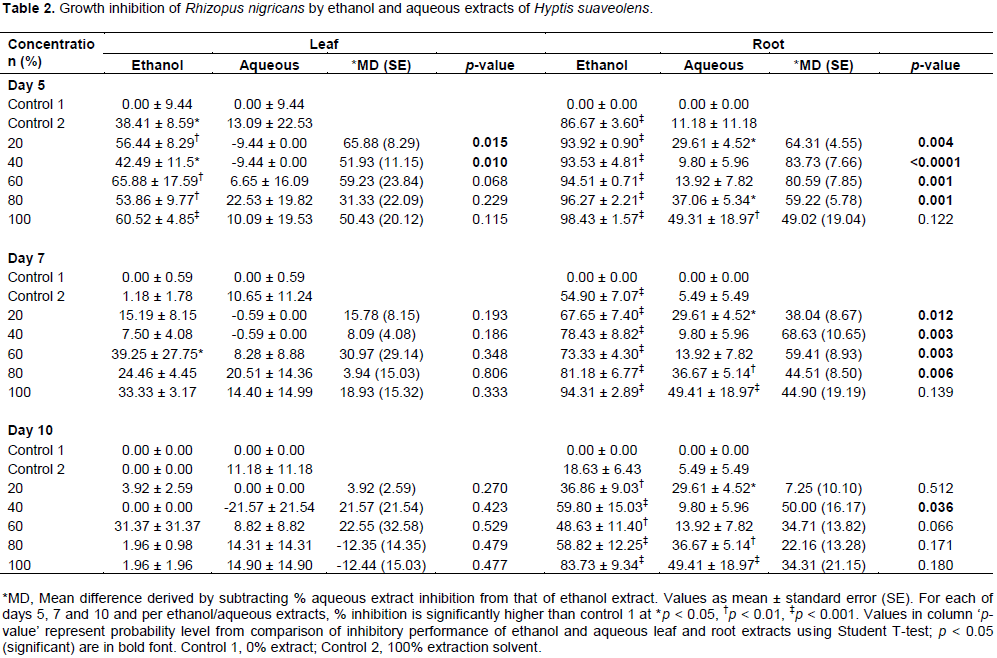
The solvent ethanol appeared to assist inhibition of R. nigricans by H. suaveolens on day 5. Aqueous leaf extract was also unable to inhibit R.nigricans growth at all used concentrations; all detected inhibitory activities were not different from control 1 (p > 0.05). Ethanol root extract of H. suaveolens had a very high inhibitory effect on R. nigricans; the inhibitory activity was concentration and duration of exposure dependent. The two highest concentrations (80 and 100%) of the extracts inhibited R. nigricans growth most effectively, though activities decreased on day 10 compared to day 5, but both concentrations retained a very significantly higher activity compared to control 1 (p < 0.0001). Aqueous root extract also showed a duration dependent effect against R. nigricans growth, but the activities were generally lower than ethanol root extracts. Aqueous root extracts concentrations of 20, 80 and 100% retained same level of significantly higher inhibitory activity when compared to control 1 against R. nigricans on days 5, 7 and 10 (p < 0.05).
Figures 2 and 3 show the difference between growth inhibitions caused by ethanol leaf and aqueous leaf extracts, and between ethanol root and aqueous root extracts on A. niger and R. nigricans. In Figure 2, differential growth inhibition of A. niger was observed. Ethanol leaf extract showed over 60% greater performance than aqueous leaf extract on day 5; though the difference decreased by days 7 and 10 as an indicative of generally observed decline in inhibitory potency of the extract as days progressed. Despite the decline in inhibitory activities of all concentrations of ethanol leaf extract against A. niger, it retained a positive differential inhibition compared to aqueous leaf extract which was completely ineffective against the fungus. Ethanol and aqueous root extracts had low differential inhibitory activities; both were similarly very potent against A. niger. On day 5, ethanol root extract had higher inhibitory effect against A. niger; this was only retained by 100% concentration by days 7 and 10. This indicates that aqueous root extract retained potency against A. niger than ethanol root extract for the duration of the study.
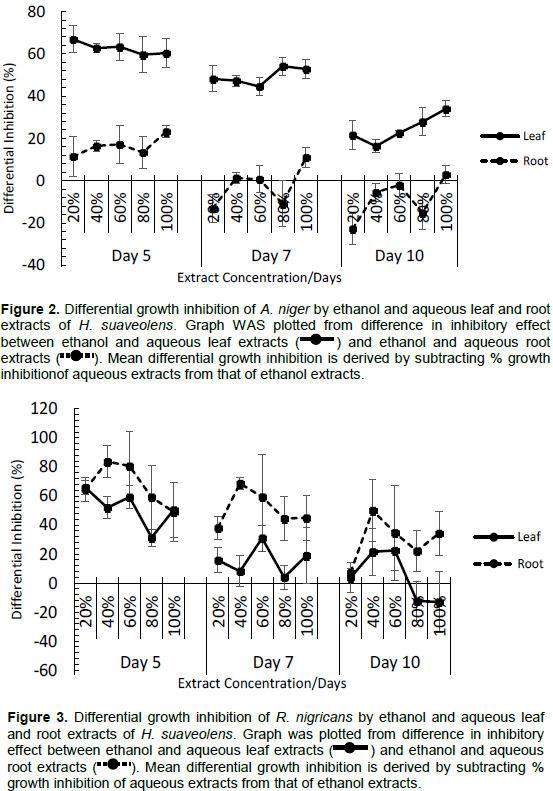
The observed pattern for leaf and root extracts of H. suaveolens against A. niger was the reversed for R. nigricans: Slightly higher differential performance occurred in roots extracts against R. nigricans than differential performance of leaf extracts (Figure 3). This is unlike what occurs for A. niger, where leaf extracts had much higher differential performance than root extracts. This reverse action is an indicative of poor performance of ethanol and aqueous leaf extracts as growth inhibitors of R. nigricans. Only on day 5, ethanol leaf extract was inhibitive to R. nigricans growth; by days 7 and 10, the inhibitory activity was completely lost attaining same level as aqueous leaf extract. The higher root differential inhibition compared to leaf is also indicative of very high growth inhibitory effect of ethanol root extract on R. nigricans and relatively low inhibitory effect of some concentrations of aqueous root extract.
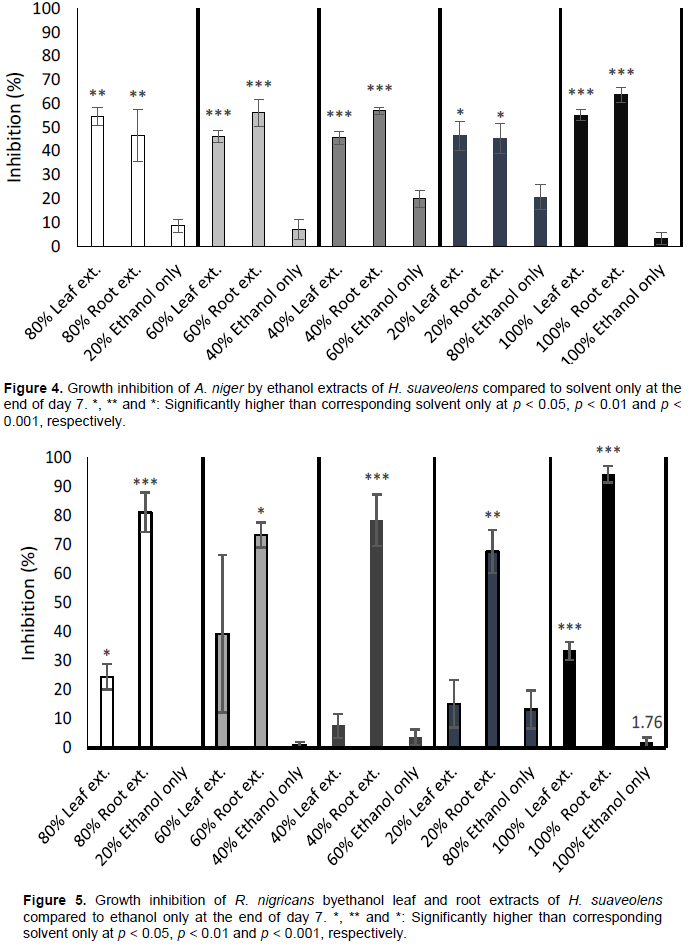
Performance of only solvent against extracts
The outcomes of experimental setups to evaluate the fungi growth inhibitory performance of only the extraction solvent against different concentrations of the extracts on day 7 post-treatment are shown as Figures 4 and 5. This distinguishes the additional inhibitory activity of the extracts where inhibition is observed. If inhibition is due to solvent only or extracts, this helps clarification. Generally, all concentrations of leaf and root ethanol extracts inhibited growth of
A. niger and
R. nigricans compared to ethanol only. The 20, 40, 60 and 80% concentrations of ethanol only (that is, the respective quantities serially diluted with water) had some inhibitive activities against
A. niger; this activity was highest at 80% concentration (Figure 4). But their activities were much lower when compared to ethanol leaf and root extracts of
H. suaveolens. At 100% ethanol concentration, inhibition of
A. niger growth was abrogated. The pattern of growth inhibition
R. nigricans by concentrations of ethanol only compared to the leaf and root extracts of same solvent was similar to that of
A. niger. Though growth inhibition of
R. nigricans by 60 and 80% ethanol only was similar to corresponding 40 and 20% leaf extracts, respectively (Figure 5).
The obtained results revealed that A. niger and R. nigricans are the most common pathogens responsible for postharvest cabbage spoilage in Ojoo, Sango, Bodija and Agbowo in Ibadan, Oyo State, Nigeria, and probably other parts of the globe. This is in line with the submission of Junghare et al. (2014) which reported the close association of Aspergillus sp. and Rhizopus sp. with vegetable spoilage. The results also showed that leaf and root extracts from H. suaveolens can be used effectively to inhibit the growth of fungi associated with postharvest bio-deterioration of cabbage. Thus, H. suaveolens possesses antifungal properties as reported by Okonogi et al. (2005) and Sharma et al. (2013).
Antifungal activities observed in the present study corroborates the works of Parichad and Krittaporn (1999) and Ahmad et al. (2013) who found that leaf and root extracts of H. suaveolens possess antimicrobial properties. However, the growth inhibition caused by the root extracts was significantly higher than that caused by leaf extracts in the present study. Similar findings were noted by Olofsdotter et al. (2002) and Zhang and Fu (2010) who suggested that root extracts exudes higher amount of the bioactive compounds than the leaves and fruits.
A. niger was more sensitive to the inhibitory effect of the leaf and root extracts of
H. suaveolens. Sharma et al. (2013) had made a similar observation, where it was suggested that various extracts from
H. suaveolens showed better antifungal activity against
A. niger when compared with other fungi. However, the effectiveness of ethanol and aqueous root extracts in inhibiting the growth of the two pathogens differed significantly from each other. This supports the findings of Enyiukwu et al. (2013) who emphasized the influence of extraction solvents on the solubility of the active ingredient(s) in plant extracts. The inhibitory effect exerted by 80 and 100% extract concentrations on mycelia growth of
A. niger and
R. nigricans were higher than that caused by other concentrations. This agrees with the report of Babu et al. (2008) who observed higher inhibition of fungal growth at higher concentrations of plant extracts.
Duration of exposure to extracts had impact on the mycelia growth of isolated pathogens. This is in accordance with the report of Sobowale et al. (2010) which suggested that there is a relationship between duration of contact and growth inhibition of fungal pathogens.
Ethanol and aqueous leaf and root extracts of H. suaveolens possess fungitoxic properties that might be effective as phytofungicides against fungi responsible for postharvest bio-deterioration of B. oleracea and possibly other vegetables. Better understanding of the chemical components of these natural extracts and more researchinto how they can be obtained in large quantities and packaged in a form that can be attractive to farmers is needed. This might also to some extent, solve the problem of chemical pollution and poisoning arising from the use of chemicals in disease management.
The authors have not declared any conflict of interests.