ABSTRACT
Polypharmacological activities of the biflavonoid fraction of Garcinia kola seed justify its development as a nutraceutical, however, quality assurance of active nutraceutical ingredient (ANI) requires conformance to appropriate standards of composition and quality. It was hypothesized that variation in extraction protocols, as previously reported for the biflavonoid fraction, would lead to variation in extract composition and potency. Computational antioxidant capacity simulation (CAOCS) assay of G. kola extracts obtained by different extraction protocols tested the hypothesis, through incremental addition (250 and 100 µL) of standard antioxidant (AOX) extract solutions in a photometric titration. Preferred model fitting was then statistically selected between mono- and bi-exponential decay. Best - fit reaction constant (kptt) was integrated into a metric for ranking antioxidant capacity (AOC) of the extracts. The AOC metric is a molecular descriptor for kinetics of phenolic bond cleavage. Three AOX extracts, namely, ethyl acetate seed extract, kolaviron and acetone seed extract were found to vary in composition, and produced optimal AOC values of 1500/g, 1150/g, and 1050/g respectively. Our findings demonstrated that the composition and potency of biflavonoid fraction of G. kola seed are critically dependent on solvent extraction protocol, and hence, consistent with the hypothesis. CAOCS assay is a suitable analytical tool for ensuring batch–to–batch sameness of ANI prepared from G. kola seed.
Key words: Garcinia kola seed extracts, biflavonoids, active nutraceutical ingredient, antioxidant capacity, quality assurance.
Abbreviation:
AICc, Akaike's information criterion; ANI, active nutraceutical ingredient; AOC, antioxidant capacity; AOX, antioxidant; ASE, acetone seed extract; BED, bi-exponential decay; CAOCS, computational antioxidant capacity simulation; DSP, Digital signal processing; EASE, ethyl acetate seed extract; KV, kolaviron; MED, mono-exponential decay; PPA, photometric phenolphthalein assay; PPRA, photometric phenol red assay; PTKM, proton transfer kinetics modeling; TLC, thin layer chromatography.
The seed of Garcinia kola (Heckel, Fam: Guttiferae/ Clausiaceae) is a very popular adaptogen in West African countries, and Nigeria in particular. There are many folkloric usages, which include treatment of bronchitis, laryngitis, oral infections, colic, dysentery, bacterial and viral infections (Irvine, 1961; Farombi, 2003). The seed has been clinically evaluated as treatment for knee osteoarthritis (Adegbehingbe et al., 2008), while its potential as an aphrodisiac was also demonstrated in male Wistar rats (Sewani-Rusike et al., 2016; Farombi et al., 2013b).
The biflavonoid fraction, originally described as "kolaviron" (KV) by Iwu reportedly contain a mixture of structurally related biflavonoids (Iwu, 1985). KV was shown, mechanistically, to have significant hepatoprotective (Farombi, 2000; Farombi et al., 2009), antiinflammatory (Farombi et al., 2013a; Abarikwu, 2014; Onasanwo et al., 2016), antioxidant (Farombi et al., 2002; Farombi et al., 2004a, b) and immunomodulatory activities (Nworu et al., 2008). The bioactivity profile of KV therefore justifies its usage as dietary supplement for chemoprevention of diseases and health promotion.
Despite the well documented usefulness of G. kola seeds, its use as dietary supplement is still only prevalent among older adults (over age 50 years) in Nigeria, because the bitter taste precludes its adoption by a younger generation. This usage pattern is consistent with the pattern of supplement usage in general in the United States as revealed by National surveys conducted over three decades (Bailey et al., 2011; Block et al., 1988; Radimer et al., 2004; Koplan et al., 1986). It is therefore desirable to promote its usage among younger adults (under age 50 years) and wider adoption among older adults.
The argument for increased usage of proven supplements across adult age groups is supported by an emerging paradigm in healthcare: “integrative healthcare”, which aims to prevent disease in the first place, and emphasize attacking underlying causes of disease rather than symptoms, in treatment modalities (Mister, 2012).
A wider use of G. kola - based supplement is achievable through careful preparation of active nutraceutical ingredient (ANI) comprised of the biflavonoids, for use in appropriate and more palatable formulations. We observed that variations of solvent - solvent extraction protocol were reported for extraction of the biflavonoids described as KV, by different investigators. Iwu initially used chloroform fraction of defatted methanol extract of powdered G. kola seeds (Iwu, 1985). In a later report by Iwu et al. (1987), the combined chloroform and n-butanol fraction of defatted acetone extract, was described as KV. Olaleye et al. (2000) reported KV as the ethyl acetate fraction of defatted acetone extract of G. kola. Nwaneri-Chidozie et al. (2014) reported extraction with methanol and subsequent partitioning with ethyl acetate, while Farombi et al. (2005) reported chloroform fraction of defatted methanol extract. It is obvious from literature that the mixture of biflavonoids were assumed to be extracted by these varied protocols, albeit, without any validation of the assumption. Therefore, we hypothesize that the relative amounts of the individual polyphenols (Figure 1) and overall composition would be somewhat different in the various extracts obtained, due to variation in solubilization capacity of organic solvents for a given class of compounds. Furthermore, purity of the biflavonoid extracts would be dependent on relative amounts of other non-biflavonoid secondary metabolites extracted along with the polyphenols.

Quality assurance considerations for manufactured dietary supplements require that they conform to appropriate standards of purity, quality and composition (TABD - DSEG, 2002). It is therefore the aim of this study to quantitatively evaluate the quality of biflavonoid extracts of G. kola seed in addition to qualitative evaluation of purity and composition. This will serve as first step towards standardizing the extraction protocol, to ensure consistent purity and optimal potency. Quality - assured ANI is critical to successful commercial manufacturing of dietary supplements. Computational antioxidant capacity simulation (CAOCS) assay is a bespoke assay developed for antioxidant capacity profiling of polyphenols and phenol-like compounds (Idowu et al., 2009; Idowu, 2014). The systematic workflow and associated informatics required to implement the two photometric assays that constitute CAOCS assay is displayed in Figure 2.

In this paper, we report a quantitative study, using CAOCS assay, of the antioxidant capacity (AOC) of KV and two other G. kola extracts obtained by variants of the extraction protocol. A primary molecular descriptor for kinetics of phenolic bond cleavage was integrated into a metric for ranking antioxidant capacity (AOC), as a measure of extract potency and quality.
Chemicals and reagents
Sodium hydroxide pellets (LobaChemie, India, 98%), Phenolphthalein (Wardle Chemicals, UK, 98%), Methanol, Ethyl acetate, n-Hexane, Chloroform, and Acetone; all were from Sigma, USA Borax (LabTech, India, 98%).
Equipment
Analytical balance (Kern, Germany), Digital colorimeter (Jenway, Model 6051, U.K.), Magnetic stirrer (Gallenkamp, U.K.), Laboratory oven (Astell Hearson, U.K.), Rotary evaporator (Heidolph, Germany), pH meter (PHS-3C, China).
Plant material
The seeds of G. kola were purchased in a local market at Ibadan, Oyo State, Nigeria. The specimen was authenticated at the Forestry Research Institute of Nigeria, (FRIN), Ibadan, where a voucher specimen was deposited with the voucher specimen number; FHI 110593.
Preparation of reagent stock solutions
i) Preparation of 0.10% w/v Phenolphthalein solution: Phenolphthalein (0.10 g) was weighed and dissolved with little quantity of methanol in a beaker and upon complete dissolution, transferred into a 100 mL volumetric flask and made up to volume with methanol.
ii) Preparation of 0.025M sodium hydroxide solution: Sodium hydroxide pellets (0.10 g) was weighed and dissolved with little quantity of distilled water in a beaker, and upon complete dissolution, allowed to cool. The solution was then transferred to a 100 mL volumetric flask, and made up to volume with distilled water.
Preparation of G. kola extracts
Seeds of G. kola were peeled, cut into thin slices and dried in the oven at 50°C for 24 h. The dried seed were afterwards ground into powder with a milling machine. Various extracts were prepared from the powdered seed by following various extraction protocols.
i) Kolaviron (KV): KV was prepared by adapting the method of Iwu (1985) and Olaleye et al. (2000). Powdered seed (1 kg) was defatted exhaustively by cold maceration in n-hexane. This was followed by exhaustive extraction by cold maceration in methanol (2 L per cycle). The methanol extract was concentrated (300 mL) and diluted with twice its volume of water. The alcoholic-aqueous mixture was then partitioned into ethyl acetate (6 x 250 mL). The combined ethyl acetate fraction was concentrated by rotary evaporation and dried in vacuo at 40°C. The dried extract was stored in a clean beaker.
ii) Acetone seed extract (ASE): The crude extract from which
kolaviron was prepared by Olaleye et al. (2000) is the acetone seed extract. Powdered seed (1 kg) was defatted exhaustively by cold maceration in n-hexane. The dried defatted marc was exhaustively extracted by cold maceration in acetone (2 L per cycle). The combined acetone extraction was concentrated to dryness by using a rotary evaporator and dried in vacuo at 40°C. The dried extract was stored in a clean beaker (ASE).
iii) Ethyl acetate seed extract (EASE): An alternative to solvent-solvent extraction procedure was devised to prevent loss of the biflavonoids to the aqueous phase. Powdered seed (1 kg) was defatted exhaustively by cold maceration in n-hexane. This was followed by exhaustive extraction of dried marc by cold maceration in Chloroform (2 L per cycle), which was followed by exhaustive extraction of dried marc by cold maceration in ethyl acetate (2 L per cycle). The combined ethyl acetate extract was concentrated to dryness by using a rotary evaporator and dried in vacuo at 40°C. The dried extract was stored in a clean beaker.
CAOCS assay - Photometric Phenolphthalein Assay (PPA)
250 µL increment
Phenolphthalein solution in methanol (0.50 mL, 0.10% w/v) was pipetted into a volumetric flask (5.0 mL). To this was added sodium hydroxide solution in water (1.0 mL, 0.025 M) in order to generate the oxidized specie of the probe molecule. This solution was titrated by incremental addition of the test antioxidant (AOX) solution (250 µL increment), up to a maximum of 1.75 mL), and the volume was made to mark with methanol. The absorbance of the solution was measured at 540 nm on a digital colorimeter after each increment. All measurements were performed in duplicate. A plot of absorbance against volume of antioxidant was made and both bi-exponential decay (BED) and mono-exponential decay (MED) model were fitted to the data. The preferred model was statistically selected after fit-comparison, by using Akaike's Information Criterion. This assay is based on proton transfer kinetics modeling (PTKM) (Idowu, 2014).
100 µL increment
Phenophthalein solution in methanol (0.20 mL, 0.10% w/v) was pipetted into a volumetric flask (2.0 mL). To this was added sodium hydroxide solution in water (0.40 mL, 0.025 M) in order to generate the oxidized specie of the probe molecule. The solution was then titrated by incremental addition of the test antioxidant (AOX) solution (100 µL increment) up to a maximum of 0.70 mL (50 µL increment was adopted in some cases for 0.80 and 1.00% w/v standard solutions, when plateau was reached after addition of 0.40 mL, with 100 µL increment), and the volume was made to mark with methanol. The absorbance of the solution was measured at 540 nm on a digital colorimeter after each increment, using a micro-cuvette. All measurements were performed in duplicate. A plot of absorbance against volume of antioxidant was made as a form of PTKM. Preferred mathematical model was statistically selected between BED and MED, after fit comparison, using AICc.
CAOCS assay of G. kola extracts as AOX
i) KV: The assay was performed as described above, by using test solutions of KV in methanol (0.20, 0.30, 0.40, 0.60, 0.80 and 1.00% w/v).
ii) ASE: The assay was performed as described above, by using test solutions of ASE in methanol (0.20, 0.30, 0.40, 0.60, 0.80 and 1.00% w/v).
iii) EASE: The assay was performed as described above, by using test solutions of EASE in methanol (0.20, 0.30, 0.40, 0.60, 0.80 and 1.00% w/v).
Mathematical modeling and statistical analysis
Mono-exponential decay (MED) model
Akaike's information criterion
Where there are several competing models, the Akaike’s information criterion (AICc) is defined by the model and the maximum likelihood estimates of the parameters, which give the minimum of AICc defined by:
AIC = (-2) Log10 (maximum likelihood) + 2 (number of independently adjusted parameters)
The model with the lowest AIC value is the one that fits the data with minimum loss of information, and hence with the highest “probability it is correct”, which is thus selected as the preferred model (Akaike, 1974).
All mathematical and statistical analyses were performed by GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com, 2005).
Preferred model selection by fit comparison
The fit comparison was implemented for the assay carried out with 250 µL increment by using global curve - fitting method. For the BED model, plateau was shared and must be > 0, k1 and k2 were constrained and must be > 0. For the MED model, plateau was shared and must be > 0, and k was constrained and must be > 0. For the assay carried out with 100 µL increment, global curve-fitting was not adopted, and the difference was that plateau was not shared, though constrained as > 0, while all other constraints were maintained as described for the 250 µL increment assay.
The curve -fitting exercise started with absorbance signal of the first increment, excluding the initial absorbance signal. The model with the highest "probability that it is correct" was selected as the preferred model. Regression coefficient, R2 was computed for each standard solution and a global shared R2 was computed (where relevant) to reveal goodness -of- fit. In addition, best - fit reaction constant was computed for each standard solution. A linear regression of the reaction constant (kptt) with concentration of standard solutions produced a slope which was used for computation of the antioxidant capacity (AOC) for the extracts. AOC on PPA platform was converted to the AOC on Photometric Phenol Red Assay (PPRA) by a specific conversion factor.
Thin layer chromatographic analysis of G. kola extracts
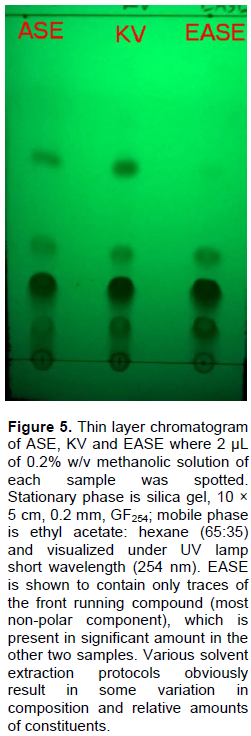
The composition of the three extracts was examined by performing thin layer chromatographic analysis on the extracts. Methanolic solution (2 µL, 0.2% w/v) of each sample was spotted on thin layer chromatographic plates, stationary phase is silica gel, (10 × 5 cm, 0.2 mm, GF254), and mobile phase is ethyl acetate: n-hexane (65:35). The plates were spotted 1 cm apart at the origin, which is 1 cm from the base and allowed to run a 7 cm path. The developed plates were air dried, and visualized under UV lamp short wavelength, 254 nm.
PTKM of data obtained from 250 µL increments, and computation of AOC for KV is as displayed in Figure 3A, and the corresponding model parameters are shown in Table 1a. MED was shown to be the preferred model, with a global R2 value of 0.90, computed AOC value of 597.15/g, reported to the nearest fifty as 600/g. PTKM and computation of AOC for EASE are displayed in Figure 3B, and the model parameters are shown in Table 3a. MED was shown to be the preferred model, with a global R2 value of 0.92, computed AOC value of 557.34/g, reported to the nearest fifty as 550/g. Also, the PTKM and computation of AOC for ASE are displayed in Figure 3C, and the model parameters are shown in Table 2a. MED was found to be the preferred model, with a global R2 of 0.88, computed AOC value of 636.96/g, reported to the nearest fifty as 650/g.

Figure 3. Graph of kinetic data from standard solutions of A) KV, B) EASE, and C) ASE on PPA showing concentration-dependent response obtained from 250 μL incremental addition of antioxidant solution preferably fitted by mono-exponential decay (MED) and the associated computation of antioxidant capacity. Assay sensitivity is diminished by similarity of the best-fit reaction constant for 0.8 and 1.0% AOX solutions across the three seed extracts. Potency ranking follows the sequence: ASE > KV > EASE.
PTKM, with preferred MED fit and AOC computation for EASE is displayed in Figure 4B, the model parameters are shown in Table 3b, computed AOC value is 1512.78 /g reported to the nearest fifty as 1500 /g. Similarly, PTKM, with preferred MED fit and AOC computation for ASE is displayed in Figure 4C, the model parameters are shown in Table 2b, computed AOC value is 1035.06 /g, reported to the nearest fifty as 1050 /g.
Thin layer chromatogram for the three G. kola extracts is as displayed in Figure 5. Spotting equal volume of identical solution concentration for each extract allowed a rough estimate of the relative composition of the extracts with respect to individual compounds that constitute the biflavonoid fraction. EASE was shown to contain trace amounts of the front - running compound, which is present in significant amounts in the other two extracts. It is apparent that this compound, being the most lipophilic, has been largely removed by chloroform which was used to extract the defatted powdered seed sample and discarded, before obtaining the ethyl acetate fraction that was labelled EASE (Figure 2).
Polypharmacological effect profile of G. kola biflavonoid extracts
Polypharmacology is a concept that reflects the high complexity in the mechanisms of action of drugs. It underscores the multitarget nature of interactions that describes the effect profile of many drugs. Correlation between interaction profiles of drugs with target proteins and clinical effect profile of drugs was shown to validate this concept (Simon et al., 2012). Furthermore, it is an emerging paradigm in drug discovery that suggests that more effective drugs can be developed by specific modulation of multiple targets (Anighoro et al., 2014). The various bioactivity profile exhibited by G. kola extract, and the various underlying mechanisms corroborates multitarget interactions. In addition, the extracts consist of at least 4 structurally similar polyphenol compounds (Figures 1 and 5). Good TLC resolution of the constituents, however, underscores the differences that yet exist in their overall physicochemical properties. This would imply variations in their binding affinities with target proteins and contribute to synergism in the effect profile. This broad effect profile justifies the development of the extract as a nutraceutical, which is intended for disease prevention and health promotion. It is thus well documented that the overall effect profile of Garcinia biflavonoids (KV and its variants) comprises both antioxidant and non - antioxidant mechanisms.
Quality assurance requirements for nutraceuticals
Manufacturing of ANI requires application of quality assurance principles as enunciated by Good Manufacturing Practice:
"To assure batch uniformity and integrity of dietary products, written procedures shall be established and followed that describe the in-process controls, and tests, or examinations to be conducted on appropriate samples of in-process materials of each batch" (TABD-DSEG, 2002).
Several techniques have been applied to the analysis of nutraceuticals including, chromatographic, spectroscopic and hyphenated techniques (Bernal et al., 2011). Optimization of pressurized liquid extraction has been reported for the extraction of zeaxanthin from Chlorella ellipsoidea, (Koo et al., 2012), and the extraction of anthocyanins and biflavonoids from Schinus terebinthifolius Raddi (Feuereisen et al., 2017). The optimized variables that influence the yield are; extraction time, extraction temperature, and solvent.
The individual Garcinia biflavonoids have been isolated by counter-current chromatography (Okunji et al., 2007) for investigation as tyrosinase inhibitor, but there was no quantitative estimation of the extract as a whole. The individual biflavonoids were also isolated in order to investigate their relative antimalarial potency against strains of Plasmodium berghei infected mice and Plasmodium falciparum, in vitro (Konziase, 2015), but there was no quantitation of the whole extract in this study, either. Derbre et al. (2014) reported a total polyphenol assay of an ethanol extract of Garcinia biflavonoids by high performance liquid chromatography (HPLC) method with UV detection. The extract reportedly contains 48% biflavonoids (reported as equivalent of the pure GB 2, used as an external standard). It was shown that the biflavonoids are the predominant components of the ethanol extract. Other compounds present in trace amounts relative to the biflavonoids were detected by HPLC – MS. These include; alpha acids, humulinone, garcinoic acid and polar compounds identified as polyols. The biflavonoid extract was used to formulate cosmetic cream demonstrated to have anti-glycation effect. To the best of our knowledge, there is no previous report addressing the quantitative evaluation of the biflavonoid extract, using the “total chemistry” of the whole extract, for the purpose of standardizing the extraction protocol, towards preparation of a quality-assured nutraceutical.
In addition to thin layer chromatography fingerprinting which reveals the composition of the biflavonoid constituents, quantitative assay is valuable to confirm batch-to-batch sameness. CAOCS assay, which is based on the total (phenolic bond cleavage) chemistry of the biflavaonoid extracts, is a suitable quality assurance procedure. The relative potency of the three extracts was evaluated to guide extraction protocol optimization, and development of standard operating procedure (SOP) for manufacturing ANI. MED was found as the preferred model for fitting the absorbance decay data for the three extracts (Tables 1 to 3). The potency of G. kola seed extracts determined by CAOCS assay and AOC reported to the nearest fifty was shown to be inversely related to the size of the photometric titration increment.
Assay sensitivity and result accuracy
When 250 µL increment was used for the assay, the ranking obtained was ASE > KV > EASE, with AOC of 650, 600, and 550/g respectively. The values are generally below 1000/g, although the 650/g obtained for ASE is a significant increase from 300/g obtained for the same extract, when 1000 µL increment was used for the assay in a previous study (Idowu et al., 2009). The sensitivity of the assay was diminished by the fact that the best-fit reaction constant (kptt) of 0.80 and 1.00% standard solutions was similar for the three extracts (Tables 1 to 3a).
On the other hand, when a smaller increment (100 µL) was used for the assay, the ranking obtained was EASE > KV > ASE with AOC of 1500, 1150, and 1050/g respectively. The assay sensitivity was enhanced by a significant difference between the kptt of 0.80 and 1.00% standard solutions for the three extracts (Tables 1 to 3b) and a higher slope of the linear regression of kptt and concentration of standard solutions, across the board (Figure 4A-C).
In summary, the use of 100 µL increments gave the optimal, more sensitive and more accurate results. The ranking obtained; EASE > KV > ASE is corroborated by domain knowledge about the preparation of the extracts. ASE is a crude acetone extract of the whole seed hence it has the least biflavonoid concentration. KV is ethyl acetate fraction of a crude extract like the ASE and is therefore more concentrated in biflavonoids, especially because of the medium polarity of ethyl acetate that favours solubility of the polyphenols. In contrast to these two extracts, the preparation of EASE avoided solvent - solvent extraction protocol involving an aqueous phase altogether. The highest yield of biflavonoids in this extract is partly due to the fact that loss of polyphenols through their partial solubility in water was completely avoided, and the relative amount of lipophilic non-biflavonoid constituents are much lower, having been removed by chloroform. Our findings are thus shown to be consistent with our hypothesis.
Qualitative evaluation of extract purity and composition suggested that some variations exist in the relative amounts of polyphenols in the biflavonoid fraction of G. kola seed obtained by different solvent extraction protocols. The difference in extraction efficiency and composition of extracts was corroborated by quantitative estimate of potency, which evaluated the “total chemistry” of biflavonoids, with respect to kinetics of phenolic bond cleavage. The use of a smaller increment (100 µL rather than 250 µL), unambiguously, led to enhanced assay sensitivity and accuracy of results. A multivariate optimization studies guided by CAOCS assay, to maxi mize biflavonoid extraction yield is ongoing in our laboratory.
The authors have not declared any conflict of interest.
REFERENCES
|
Abarikwu SO (2014). Kolaviron a natural flavonoid from the seeds of Garcinia kola reduces LPS-induced inflammation in macrophages by combined inhibition of IL-6 secretion, and inflammatory transcription factors, ERK1/2, NF-KB, p38, Akt,p-cJUN and JNK. Biophys. Acta 1840:2373-2381.
Crossref
|
|
|
|
Adegbehingbe OO, Adesanya SA, Idowu TO, Okimi OC, Oyelami OA, Iwalewa EO (2008). Clinical effects of Garcinia kola in knee osteoarthritis. J. Orthopaed. Surg. Res. 3(1):34.
Crossref
|
|
|
|
|
Akaike H (1974). A New Look at the Statistical Model Identification. IEEE Trans. Autom. Control 19(6):716-723.
Crossref
|
|
|
|
|
Anighoro A, Bajorath J, Rastelli G (2014). Polypharmacology: Challenges and opportunities in drug discovery. J. Med. Chem. 57:7874-7887.
Crossref
|
|
|
|
|
Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas, PR, Betz JM, Sempos CT, Picciano MF (2011). Dietary supplement use in the United States, 2003–2006. J. Nutr. 141(2):261-266.
Crossref
|
|
|
|
|
Bernal J, Mendiola JA, Ibá-ez E, Cifuentes A (2011). Advanced analysis of nutraceuticals. J. Pharm. Biomed. Anal. 55(4):758-774.
Crossref
|
|
|
|
|
Block G, Cox C, Madans J, Schreiber GB, Licitra L, Melia N (1988). Vitamin supplement use, by demographic characteristics. Am. J. Epidemiol. 127(2):297-309.
Crossref
|
|
|
|
|
Derbre S, Morel S, Richomme P, Toure AK (2014). Anti-glycation agent comprising a Garcinia kola extract or fraction. United States Patent Application Publication, US 9107849, 1–9.
|
|
|
|
|
Farombi EO (2000). Mechanisms for the hepatoprotective action of kolaviron: studies on hepatic enzymes, microsomal lipids and lipid peroxidation in carbontetrachloride-treated rats. Pharmacol. Res. 42(1):75-80.
Crossref
|
|
|
|
|
Farombi EO (2003). African indigenous plants with chemotherapeutic potentials and biotechnological approach to the production of bioactive prophylactic agents. Afr. J. Biotechnol. 2(12):662-671.
Crossref
|
|
|
|
|
Farombi EO, Adedara IA, Ajayi BO, Ayepola OR, Egbeme EE (2013a). Kolaviron, a natural antioxidant and anti-inflammatory phytochemical prevents dextran sulphate sodium-induced colitis in rats. Basic Clin. Pharmacol. Toxicol. 113(1):49-55.
Crossref
|
|
|
|
|
Farombi EO, Adedara IA, Oyenihi AB, Ekakitie E, Kehinde S (2013b). Hepatic, testicular and spermatozoa antioxidant status in rats chronically treated with Garcinia kola seed. J. Ethnopharmacol. 146(2):536-542.
Crossref
|
|
|
|
|
Farombi EO, Adepoju BF, Ola-Davies OE, Emerole GO (2005). Chemoprevention of aflatoxin B1-induced genotoxicity and hepatic oxidative damage in rats by kolaviron, a natural bioflavonoid of Garcinia kola seeds. Eur. J. Cancer Prev. 14(3):207-214.
Crossref
|
|
|
|
|
Farombi EO, Akanni OO, Emerole GO (2002). Antioxidant and Scavenging Activities of Flavonoid Extract (Kolaviron) of Garcinia kola Seeds. Pharm. Biol. 40(2):107-116.
Crossref
|
|
|
|
|
Farombi EO, Hansen M, Ravn-Haren G, Moller P, Dragsted LO (2004a). Commonly consumed and naturally occuring dietary substances affect biomarkers of oxidative stress and DNA damage in healthy rats. Food Chem. Toxicol. 42(8):1315-1322.
Crossref
|
|
|
|
|
Farombi EO, Moller P, Dragsted LO (2004b). Ex-vivo and in vitro protective effects of kolaviron against oxygen-derived radical-induced DNA damage and oxidative stress in human lymphocytes and rat liver cells. Cell Biol. Toxicol. 20(2):71-82.
Crossref
|
|
|
|
|
Farombi EO, Shrotriya S, Surh YJ (2009). Kolaviron inhibits dimethyl nitrosamine-induced liver injury by suppressing COX-2 and iNOS expression via NF-κB and AP-1. Life Sci. 84(5-6):149-155.
Crossref
|
|
|
|
|
Feuereisen MM, Barraza MG, Zimmermann BF, Schieber A, Schulze-Kaysers N (2017). Pressurized liquid extraction of anthocyanins and biflavonoids from Schinus terebinthifolius Raddi: A multivariate optimization. Food Chem. 214:564-571.
Crossref
|
|
|
|
|
Idowu SO (2014). Computational antioxidant capacity simulation (CAOCS): A novel framework of antioxidant capacity profiling. Chem. Prod. Proc. Model. 9(1):25-43.
Crossref
|
|
|
|
|
Idowu SO, Adeyemo MA, Itiola AJ (2009). Computational models for the determination of antioxidant capacity and phenolics in dietary supplements using real-time proton transfer kinetics data. Chem. Prod. Proc. Model. 4(1):41.
Crossref
|
|
|
|
|
Irvine FR (1961). Woody plants of Ghana. Oxford University Press, Oxford.
|
|
|
|
|
Iwu MM (1985). Antihepatoxic constituents of Garcinia kola seeds. Experientia 41(5):699-700.
Crossref
|
|
|
|
|
Iwu MM, Igboko AA, Onwuchekwa UA Okunji CO (1987). Evaluation of the antihepatotoxic activity of the biflavonoids of Garcinia kola seed. J. Ethnopharmacol. 21:127-138.
Crossref
|
|
|
|
|
Konziase B (2015). Protective activity of biflavanones from Garcinia kola against Plasmodium infection. J. Ethnopharmacol. 172:214-218.
Crossref
|
|
|
|
|
Koo SY, Cha KH, Song DG, Chung D, Pan CH (2012). Optimization of pressurized liquid extraction of zeaxanthin from Chlorella ellipsoidea. J. Appl. Phycol. 24(4):725-730.
Crossref
|
|
|
|
|
Koplan JP, Annest JL, Layde PM, Rubin GL (1986). Nutrient intake and supplementation in the United States (NHANES II). Am. J. Public Health 76(3):287-289.
Crossref
|
|
|
|
|
Mister S (2012). Introduction. In. A. Dickinson (Ed.), The benefits of nutritional supplements (4th ed.). Washington D.C.: Council for Responsible Nutrition.
|
|
|
|
|
Nwaneri-Chidozie VO, Anyanwu KC, Adaramoye OA Emerole GO. (2014). Cardioprotective effect of kolaviron (Garcinia kola seed extract) in cholesterol-fed rats. Int. J. Pharm. Sci. Res. 5(3):96-99.
|
|
|
|
|
Nworu CS, Akah PA, Esimone CO, Okoli CO, Okoye FBC (2008). Immunomodulatory activities of kolaviron, a mixture of three related biflavonoids of Garcinia kola Heckel. Immunopharmacol. Immunotoxicol. 30(2):317-332.
Crossref
|
|
|
|
|
Okunji C, Komarnytsky S, Fear G, Poulev A, Ribnicky DM, Awachie PI, Ito Y, Raskin I (2007). Preparative isolation and identification of tyrosinase inhibitors from the seeds of Garcinia kola by high-speed counter-current chromatography. J. Chromatogr. A 1151(1-2):45-50.
Crossref
|
|
|
|
|
Olaleye SB, Farombi EO, Owoeye BV, Onasanwo SA, Elegbe RA (2000). Analgesic and anti-inflammatory effects of kolaviron (a Garcinia kola seed extract). Afr. J. Biomed. Res. 3:171-174.
|
|
|
|
|
Onasanwo SA, Velagapudi R Ol-Bakoush A, Olajide OA (2016). Inhibition of neruoinflammation in BV2 microglia by the biflavonoid kolaviron is dependent on the Nrf2/ARE antioxidant protoective mechanism. Mol. Cell. Biochem. 414(1):23-36.
Crossref
|
|
|
|
|
Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF (2004). Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999-2000. Am. J. Epidemiol. 160(4):339-349.
Crossref
|
|
|
|
|
Sewani-Rusike CR, Ralebona N, Nkeh-Chungag BN (2016). Dose-and time-dependent effects of Garcinia kola seed extract on sexual behaviour and reproductive parameters in male Wistar rats. Andrologia 48(3):300-307.
Crossref
|
|
|
|
|
Simon Z, Peragovics A, Vigh-Smeller M, Csukly G, Tombor L, Yang Z, Zahoranszky-Kohalmi G, Vegner L, Jelinek B, Hari P, Hetenyi C, Bitter I, Czobor P, Malnasi-Csizmadia A (2012). Drug effect prediction by polypharmacology-based interaction profiling. J. Chem. Inf. Model. 52(1):134-145.
Crossref
|
|
|
|
|
Trans-Atlantic Business Dialogue (TABD) Dietary Supplement Expert Group (2002). Good Manufacturing Practices (GMP's) for Dietary Supplements, 1–8. Retrieved from
View , Accessed on 18 March, 2017.
|
|