ABSTRACT
Although intense research has gone into the exploration of various Combretaceae species towards the discovery of therapeutic relevant compounds, their endophytes have never been explored as potential repositories of alternative sources of novel and medically beneficial equivalents. In the present study, five bacterial endophytes (Lysinibacillus, Staphylococcus, Enterobacter, Pseudomonas and Bacillus species) were isolated from different parts (hard stem, leaves and soft stem) of Combretum molle and identified to species level using morphological data and sequencing of the 16S rRNA. Four of the five endophytes showed varying degrees of antimicrobial characteristics against Bacillus cereus, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa.
Key words: Bacterial endophytes, bioactive compounds, Combretum molle, phylogenetic analysis, medicinal plant.
Antibacterial resistance of microbial pathogens remains a threat to public health worldwide (Costelloe et al., 2010). Infections are increasingly becoming a challenge and established antibiotics have become less effective against some common bacterial infections (Bhalodia and Shukla, 2011). Such challenges are often due to inappropriate use of antibiotics, large and increasing numbers of immunocompromised patients, delays in diagnosis of infection and poor hygiene (Santos et al., 2015). As a result, there is need for the search of new, diverse and efficacious antimicrobial compounds. Until this is accomplished, naturally derived products remain an essential source for novel pharmaceuticals. A range of microorganisms termed endophytes have been shown to be a rich source of bioactive compounds that can be used in therapeutics (Ravnikar et al., 2015). Endophytes are microorganisms that reside within plant tissues without causing any substantive harm (Kumar et al., 2015). Endophytes can either be fungal or bacterial in nature and are capable of producing biologically active compounds, some of which are used by the plant as part of its arsenal in its defence against pathogens, while some promote plant growth (Gonzalez-Teuber et al., 2014; Strobel and Daisy, 2013).
Most of the bioactive compounds extracted from endophytes have shown a plethora of bioactivities including but not limited to antimicrobial, immunosuppressant and anticancer (Nair and Padmavathy, 2014). Combretum molle is used as a remedy throughout Africa to cure various diseases such as infertility in women, malaria and microbial infections (Ademola and Eloff, 2010). C. molle leaves have been reported to possess analgesic, anti-inflammatory cardiovascular, antibacterial, antifungal, anti-malarial, antitrypanosomal and anthelmintic effects (Morais-Lima et al., 2012; Ojewole, 2009). To date, no endophytes studies have been carried out from C. molle, thus the aim of the present study was to isolate and identify endophytic bacteria from C. molle and further test their crude extracts on pathogenic microorganisms.
Plant sample collection
The plant material was harvested from Lwamondo village in Venda (23°02'37.7"S 30°24'00.2"E), Limpopo province, South Africa. Healthy, disease free plant parts (stem and leaves) of C. molle were collected and placed in sterile polyethylene bags and transported to the laboratory at 4°C.
Identification of the plant
Plant material was identified at the University of Johannesburg herbarium (JRAU). The sample specimen was deposited in the herbarium and assigned voucher number Diale-Serepa-Dlamini 1 and species name C. molle.
Isolation of bacterial endophytes
Immediately after collection of the plant material in the laboratory, the endophytes were isolated from the plant (soft, hard stems and leaves) using a method described by Jasim et al. (2014). In brief, plant parts were thoroughly washed with tap water to remove dust and cut into small segments (1 to 3 cm long). Soil debris-free plant parts were subsequently treated with Tween 80 for 10 minutes with vigorous shaking followed by rinse with distilled water. The plant samples were further immersed in 70% ethanol for 1 min and then treated with 1% sodium hypochlorite (NaOCl) for 10 min. The samples were then rinsed five times with sterile distilled water and the final wash was spread on nutrient agar plates as controls. For isolation of bacterial endophytes, the outer surface of the sterile plant parts was trimmed; the pieces were then macerated in phosphate buffered saline (PBS). Serial dilutions of up to 10-3 were prepared and 0.1 mL of the dilution was spread on nutrient agar plates. Plates (including the controls) were incubated at 30°C for 2 days. The plates were observed daily for bacterial colony growth. Isolated colonies were re-cultured on sterile Nutrient agar plates until pure colonies were obtained. Glycerol (30%, glycerol diluted in sterile distilled water) stocks of each bacterial isolate were prepared and stored at -80°C for future use.
Morphological identification of endophytic bacteria
Gram staining
Pure colonies were subjected to Gram staining as described by Collins et al. (2004) to establish morphological characteristics such as shape and Gram stain reaction. Gram stain slides were observed using a compound bright-field microscope (OLYMPUS CH20BIMF200) with 100× magnification (Gupta et al., 2015).
Scanning electron microscope
Sample preparations for the Scanning Electron Microscope (SEM) were prepared using Golding et al. (2016) and Schadler et al. (2008) methods. In brief, bacterial strains were grown in 5 mL Luria broth overnight at 30°C, shaking at 150 rpm. The bacterial cultures were centrifuged at 1100 × g for 10 min and the supernatant discarded. This was followed by brief rinse with distilled water and fixing the pellet with (1:1v/v) 1% formaldehyde and 2% glutaraldehyde for 1 h at room temperature (25°C). Samples were centrifuged at 1100 × g for 10 min; the supernatant was removed and the pellet washed with 1000 µL of sterile distilled water. For dehydration, bacterial cells were treated with different concentrations of ethanol (30, 50, 70, 90, 95 and 100%) with 10 min intervals. Samples were stored open at 4°C overnight. The dehydrated samples were mounted on SEM stubs, and coated with gold using emscope SC 500 and viewed with TESCAN VEGA SEM (VEGA 3 LHM, AVG9731276ZA) connected to a monitor.
Molecular identification of the bacterial endophytes using the 16S rRNA
Extraction of genomic DNA
Pure colonies of each bacterial isolate obtained from nutrient agar were inoculated into nutrient broth and grown overnight at 30°C. Cultures were centrifuged at 13000 × g for 5 min and supernatants discarded. The DNA was extracted using ZR fungal or Bacterial DNA kit (Zymo Research, catalog No R2014) following manufacturer’s protocol. The extracted DNA was quantified using the NanoDrop ND-2000 UV-Vis spectrophotometer (Thermo Fisher scientific, USA).
Polymerase chain reaction (PCR) amplification and sequencing
The 16S rRNA gene of each bacterial isolate was amplified by PCR following protocol and primers described by Yeates et al. (1997). The PCR products were cleaned with ExoSAP-it™ following manufactures recommendations and sequenced at Inqaba Biotechnical Industries (Pty) Ltd, Pretoria, South Africa.
Phylogenetic analysis
The obtained sequences were screened for chimeras using DECIPHER (Wright et al., 2012) and subjected to BLAST (v.2.6.0) analysis at NCBI against rRNA sequence database of bacteria and archaea to identify closest bacterial species. Only bacterial species with 99 to 100% similarity were selected for phylogenetic analysis. Alignments of nucleotide sequences (isolate and species obtained through BLAST) were performed using MUSCLE with default options (Liu et al., 2016). Phylogenetic trees were constructed using a Neighbor-Joining (NJ) method based on the Tamura-Nei model (Tamura and Nei, 1993). The positions with gaps and missing nucleotide data were eliminated. All evolutionary analyses were conducted in MEGA 7 (Kumar et al., 2016). The 16S rRNA gene sequences of bacterial isolates identified in the study were deposited in GenBank (https://www.ncbi.nlm.nih.gov/genbank/) with the following accession numbers MF105747 Enterobacter species SSRP1, MF105748 Lysinibacillus species HSRN, MF105749 Pseudomonas species SSRN1, MF105750 Bacillus species LRP, and MF105751 Staphylococcus species LCP. The assigned names of the bacterial isolates were based on the BLAST homology percentages as well as phylogenetic results.
Phytochemical analysis
Sample preparation
C. molle plant parts (stem, leaves and bulk) were dried at 27°C for 7 days and then they were blended into a fine powder.
Qualitative analysis of phytochemicals on C. molle
Phytochemical screening was conducted using Trease and Evans (1983) and Harbourne (1983) methods.
Phytochemicals analysis of endophytes crude extract
Phytochemical screening of endophytes crude extracts was conducted using the same methods (Trease and Evans, 1983; Harbourne, 1983) with some modifications.
Production of secondary metabolites from bacterial endophytes
Nutrient broth (8 L) was prepared in 2 L Erlenmeyer flasks and autoclaved at 121°C for 15 min. Each 2 L flask was inoculated with one of each endophytic bacteria and incubated at 30°C for 7 days (Sandhu et al., 2014). After 7 days of cultivation, sterilized XAD-7-HP resin (20 g/L) (SIGMA, South Africa, BCBR6696V) was added to the culture for 2 h shaking at 200 rpm. The resin was filtered using cheese cloth and eluted with acetone three times. Acetone was removed using a Rota evaporator. The remaining water was extracted with ethyl acetate three times and concentrated using a rotary evaporator (Maloney et al., 2009).
Antibacterial activity of the crude extracts from bacterial endophytes
Antibacterial tests were carried out by using a modified disc diffusion method described by Bauer et al. (1966). All pathogenic strains (Gram-negative strains: Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and Klebsiella oxytoca ATCC 13182; Gram-positive: Staphylococcus aureus NCTC 6571 and Bacillus cereus ATCC 10876) were grown overnight at 37°C in Muller-Hinton broth and adjusted using 0.5 McFarland standards such that the concentration was 107 to 108 colony forming unit (CFU/mL). Under sterile conditions, 0.1 mL of each pathogenic strain was spread on Muller-Hinton agar. Sterile circular paper discs with a diameter of 6 mm were soaked in 10 µL of each bacterial endophyte crude. 10 µL of 1 mg/L streptomycin (SIGMA-ALDRICH, Switzerland, BCBP5897V) was used as positive control and loaded on the discs as described earlier. Six discs of different crude extracts including the control were placed on each spread plate inoculated with different pathogenic strains and incubated at 30°C for 72 h. Antimicrobial activity was observed daily by measuring the zone of inhibition (in mm). The antibacterial test was performed in triplicates.
Isolation and identification of bacterial endophytes
Morphological identification
The surface sterilization of plant material is important for isolation and studying endophytes. The stems and leaves of C. molle were surface sterilized for isolation of bacterial endophytes. The surface sterilization method was satisfactory as no growth emerged on control plates. Thus, the isolated bacterial colonies can be considered true endophytes. Five bacterial colonies were isolated (Table 1). The colonies were differentiated based on their Gram reaction, colony colour and morphology. The Gram stain reaction results showed three of the bacterial isolates to be Gram-positive and two Gram-negative bacterium. The morphological shapes observed from Gram stain reaction were further confirmed using SEM results (Figure 1A to E) which had uniform cells indicating that the bacterial cultures were pure.


Phylogenetic relationship
The BLAST search results of the 16S rRNA gene sequences resulted in varying bacterial genera. Bacterial endophyte HSRN had maximum identity to Lysinibacillus fusiformis (100%), LCP had maximum identity to Staphylococcus epidermis (100%), SSRP1 had maximum identity to Enterobacter cloacae (99%), SSRN1 had maximum identity to Pseudomonas fulva (100%) and LRP had maximum identity Bacillus subtilis (99%), thus the isolated putative bacterial endophytes can be considered bacterial strains of Lysinibacillus, Staphylococcus, Enterobacter, Pseudomonas and Bacillus spp. (Table 2). To our knowledge, this is the first study to report these bacterial endophytes from C. molle. The phylogenetic analysis showed that the endophytic bacterial isolates are grouped with various closely related bacterial species (Figure 2). From Figure 2, Enterobacter spp. SSRP1 MF105747 had a sister relationship with Kosakonia cowanni KP236256 a species isolated from a sea grass Thalassia hemprichii with a bootstrap of 100%.

K. cowanni belongs to the Enterobacteriaceae family. Lysinibacillus spp. HSRN MF105748 and Bacillus spp. LRP MF105750 also had a sister relationship with 75% bootstrap value. These two species are from the same family name Bacillaceae. Staphylococcus spp. LCP MF105751 had a sister relationship with Staphylococcus epidermidis KY968734 a species isolated from sphenoid sinus biopsy with a 97% bootstrap value. Pseudomonas spp. SSRN1 MF105749 shared a common ancestor with Pseudomonas taiwanensis KM576802 isolated from rhizosphere soil with 91% bootstrap value. All current bacterial endophytes strains were reported as endophytes from various plant species (Chaudhry and Patil, 2013; Christina et al., 2013; Mahummad et al., 2014; Zhao et al., 2015). Similar studies on isolation of endophytes have been reported by other researchers, where Pseudomonas and Bacillus spp. were isolated from Echinacea medicinal plant (Christina et al., 2013).
L. fusiformis isolated from the medicinal plant Panicum virgatum and S. epidermis isolated from rice seeds have been reported to be endophytes (Ryan et al., 2008). The aforementioned indicates that these species are present as endophytes within a variety of plant species which makes them more interesting for further studies, such as plant growth promotion and their possible applications in drug discovery and agriculture. The five-isolated putative bacterial endophytes represent five different genera, which indicate diverse bacterial endophytes present within C. molle. Endophytes are known to vary in diversity based on seasonal collection or sampling time, plant age, plant tissue type and environment (Jasim et al., 2013). In this study, it was strongly believed that C. molle is likely to be associated with other different types of bacterial endophytes.
Phytochemical analysis
Secondary metabolites studies of the leaves, stem and bark of C. molle showed the presence of tannins, flavonoids and steroids (Table 3). These secondary metabolites possess properties which are of great importance in the drug development (Joseph et al., 2013). Flavonoids are known to have antimicrobial, anti-cancer, anti-inflammatory and anti-viral properties (Kabera et al., 2014) and tannins have stringent properties and can be utilized for antibacterial, anti-diarrheal, haemostatic and anti-haemorrhoidal drugs (Ashok and Upadhyaya, 2012). All five endophytes crude extracts revealed the presence of flavonoids and tannins, except Pseudomonas spp. SSRN1 showed absence of tannins as indicated in Table 4. Elof et al. (2008) reported that the family Combretaceae contains a wide variety of tannins and flavonoids. Presence of flavonoids was also detected in other plants of the same genera named Combretum erythrophyllum (Bhatnagar et al., 2012). Thus, presence of flavonoids and tannins in C. molle indicates therapeutic potential of the plant.

Antimicrobial activity
The crude extracts of the putative endophytic bacterial strains were assayed for antimicrobial activity against pathogenic strains (Gram-negative strains: E. coli ATCC 25922, P. aeruginosa ATCC 27853 and K. oxytoca ATCC 13182; Gram-positive: S. aureus, NCTC 6571 and B. cereus, ATCC 10876). Among the five endophytic bacteria, only four except Staphylococcus spp. LCP showed antimicrobial activity. Pseudomonas spp. SSRN1 and Enterobacter spp. SSRP1 were considered as the most active strains as they both had a moderate activity against S. aureus. High zone of inhibition was by Pseudomonas spp. SSRN1 and Enterobacter spp. SSRP1, followed by Lysinibacillus spp. HSRN, then lastly Bacillus spp. LRP (Table 5). Endophytic bacteria have potential to produce novel natural compounds with antibacterial and antifungal activity (Christina et al., 2013). Bacterial endophytes (Pseudomonas spp. and Bacillus spp.) isolated from Plectranthus tenuiflorus have shown great antimicrobial activity against some human pathogenic strains such as Salmonella typhi, S. aureus, E. coli, Klebsiella pneumoniae, Streptococcus agalactiae Proteus mirabilis, Candida albicans (EI-Deeb et al., 2013).
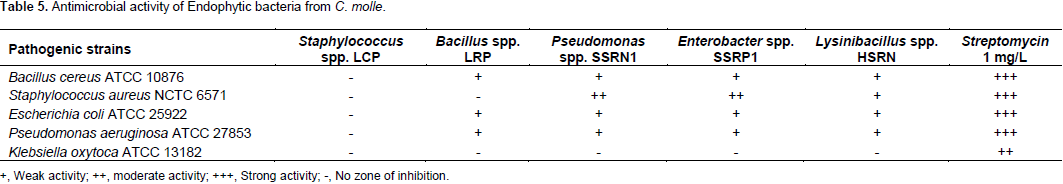
Furthermore, Enterobacter spp. isolated from Raphanus sativus L. also showed antibacterial activity against a few human pathogenic bacteria including E. coli, Salmonella enteritidis, Shigella sonnei, Salmonella typhimurium, P. aeruginosa, Shigella flexneri and B. cereus (Seo et al., 2010). Pseudomonas spp. have proven to possess antimicrobial compounds called ecomycins and pseudomycins (Christina et al., 2013). Secondary metabolites from C. molle were also reported to possess antimicrobial activity (Fankam et al., 2015; Kaleab et al., 2006). It is evident from the current study that the isolated bacterial endophytes also have antibacterial activity with a broad antibacterial spectrum. Thus, the bacterial endophytes with antibacterial activity from the current study can play a part in inhibiting plant pathogens growth. In addition, potential applications such as drug discovery and biocontrol use in agriculture can arise from these bacterial endophytes and necessitates further investigations.
This is the first study to report on bacterial endophytes occurrence in C. molle. The reported bacterial endophytes isolated have been shown to be a rich source of diverse bioactive compounds with potential applications in drug discovery and agriculture. Studies are currently underway to ascertain if the bacterial endophytes produce the same or similar secondary metabolites as their plant host C. molle. Further studies can lead to the development of novel therapeutic drugs from secondary metabolites produced by these bacterial endophytes. Thus, in addition to the well-established photochemistry and bioactivity of C. molle, there is now evidence of an extended and potential alternative source of antimicrobials.
The authors have not declared any conflict of interests.
This work was funded by the National Research Foundation of South Africa and University of Johannesburg merit bursary for postgraduate students.
REFERENCES
|
Ademola IO, Eloff JN (2010). Veterinary parasitology in vitro anthelmintic activity of Combretum molle (R. Br. ex G. Don) (Combretaceae) against Haemonchus contortus ova and larvae. Vet. Parasitol. 169:198-203.
Crossref
|
|
|
|
Ashok PK, Upadhyaya K (2012). Tannins are Astringent. J. Pharmaco. Phytochem. 1:45-50.
|
|
|
|
|
Bauer AW, Kirby WMM, Serris JC, Turck M (1966). Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 45:493-496.
Crossref
|
|
|
|
|
Bhalodia NR, Shukla VJ (2011). Antibacterial and antifungal activities from leaf extracts of Cassia fistula L.: An ethnomedicinal plant. J. Adv. Pharm. Technol. Res. 2:104-109.
Crossref
|
|
|
|
|
Bhatnagar S, Sahoo S, Mohapatra AK, Behera DR (2012). Phytochemical analysis, antioxidant and cytotoxic activity of medicinal plant Combretum roxburghii (Family: Combretaceae). Inter. J. Drug. Dev. Res. 4:193-202.
|
|
|
|
|
Chaudhry V, Patil PB (2016). Genomic investigation reveals evolution and lifestyle adaptation of endophytic Staphylococcus epidermidis. Sci. Rep. 6:1-11.
Crossref
|
|
|
|
|
Christina A, Christopher V, Bhore SJ (2013). Endophytic bacteria as a source of antibiotic: An overview. Pharmacogn. Rev. 7:11-16.
Crossref
|
|
|
|
|
Collins MD, Falsen E, Brownlee K, Lawson PA (2004). Helcococcus sueciensis sp. nov., isolated from a human wound. Int. J. Syst. Evol. Microbiol. 54:1557-1560.
Crossref
|
|
|
|
|
Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD (2010). Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 340:1-10.
Crossref
|
|
|
|
|
EI-Deeb B, Fayez K, Gherbawy Y (2013). Isolation and characterization of endophytic bacteria from Plectranthus tenuiflorus medicinal plant in Saudi Arabia desert and their antimicrobial activities. J. Plant. Interact. 8:56-64.
Crossref
|
|
|
|
|
Eloff JN, Katerere DR, Mcgaw LJ (2008). The biological activity and chemistry of the southern African Combretaceae. J. Ethnopharm. 119:686-699.
Crossref
|
|
|
|
|
Fankam AG, Kuiate JR, Kuete V (2015). Antibacterial andantibiotic resistance modifying activity of the extracts from Allanblackia gabonensis, Combretum molle and Gladiolus quartinianus against Gram-negative bacteria including multi-drug resistant phenotypes. BMC Complement. Altern. Med. 15:1-12.
Crossref
|
|
|
|
|
Golding CG, Lindsey L, Lamboo GC, Beniac DR, Booth TF(2016). The scanning electron microscope in microbiology and diagnosis of infectious disease. Sci. Rep. 4:1-8.
Crossref
|
|
|
|
|
Gonzalez-Teuber M, Jimenez-Aleman GH, Boland W (2014). Foliar endophytic fungi as potential protectors from pathogens in myrmecophytic Acacia plants. Commun. Integr. Biol. 7:1-4.
Crossref
|
|
|
|
|
Gupta RM, Kale PS, Rathi ML, Jadhav NN (2015). Isolation, characterization and identification of endophytic bacteria by 16S rRNA partial sequencing technique from roots and leaves of Prosopis cineraria plant. Asian. J. Plant. Sci. Res. 5:36-43.
|
|
|
|
|
Harbourne JB (1983). Phytochemical screening methods: A guide to modern techniques of plant analysis. Chapman and Hall, London.
|
|
|
|
|
Jasim B, Joseph, AA, John C, Mathew J, Radhakrishnan EK (2014). Isolation and characterization of plant growth promoting endophytic bacteria from the rhizome of Zingiberofficinale. 3 Biotech. 4:197-204.
|
|
|
|
|
Joseph BS, Kumbhare PH, Kale MC (2013). Preliminary phytochemical screening of selected Medicinal Plants. Int. Res. J. Sci. Eng. 1:55-62.
|
|
|
|
|
Kabera JN, Semana E, Mussa AR, He X (2014). Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2:377-392.
|
|
|
|
|
Kaleab A, Avijit M, Franz B (2006). Antibacterial and antifungal activities of extracts of Combretum molle. Ethiop. Med. J. 44:269-77.
|
|
|
|
|
Kumar GA, Antony RA, Kannan VR (2015). Exploration of endophytic microorganisms from selected medicinal plants and their control potential to multi drug resistant pathogens. J. Med. Plants. Stud. 3:49-57.
|
|
|
|
|
Kumar S, Stecher G, Tamura K (2016). MEGA7: Molecular evolutionary genetics analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 33:1870-1874
Crossref
|
|
|
|
|
Liu H, He X, Song X, Xu L, Zhang Y, Zhou G, Zhu W, Chang C, Yin Z, Shi Y, Wang C, Chang H (2016). Infection, genetics and evolution isolation and molecular and phylogenetic analyses of encephalomyocarditis virus from wild boar in central China. Int. J. Mol. Epidemiol. Genet. 40:67-72.
|
|
|
|
|
Maloney KN, MacMillan JB, Kauffman, CA, Jensen, PR, DiPasquale AG, Rheingold AL, Fenical W (2009). Lodopyridone, a structurally unprecedented alkaloid from a marine actinomycete. Org. Lett. 11:5422-5424.
Crossref
|
|
|
|
|
Morais-Lima GR, Praxedes de Sales IR, Filho MRDC, Taveira de Jesus NZ, Falcao HS, Barbosa-filho JM, Cabral AGS, Souto AL, Tavares JF, Batista, LM (2012). Bioactivities of the genus Combretum (Combretaceae): A review. Molecules 17: 9142-9206.
Crossref
|
|
|
|
|
Nair DN, Padmavathy S (2014). Impact of endophytic microorganisms on plants, environment and humans. Sci. World. J. 1-11.
Crossref
|
|
|
|
|
Ojewole JA (2009). Cardiovascular effects of mollic acid glucoside, a1 alpha hydroxycycloartenoid saponin extractive from Combretum molle R Br ex G Don (Combretaceae) leaf. J. Nat. Med. 63:117-123.
Crossref
|
|
|
|
|
Ravnikar M, Tercelj M, Janes D, Strukelj B, Kreft S (2015). Antibacterial activity of endophytic fungi isolated from Conifer needles. Afr. J. Biotechnol. 14: 867-871.
Crossref
|
|
|
|
|
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008). Bacterial endophytes: Recent developments and applications. FEMS. Microbiol. Lett. 278:1-9.
Crossref
|
|
|
|
|
Sandhu SS, Kumar S, Aharwal RP (2014). Isolation and identification of endophytic fungi from Ricinus communis Linn. and their antibacterial activity. Int. J. Res. Pharm. Chem. 4:611-618.
|
|
|
|
|
Santos IP, Silva LCN, Silva MV, Araújo JM, Cavalcanti MS, Lima VLM (2015). Antibacterial activity of endophytic fungi from leaves of Indigofera suffruticosa Miller (Fabaceae). Front. Microbiol. 6:1-7.
Crossref
|
|
|
|
|
Schadler S, Burkhardt C, Kappler A (2008). Evaluation of electron microscopic sample preparation methods and imaging techniques for characterization of cell-mineral aggregates. Geom. J. 25:228-239.
Crossref
|
|
|
|
|
Seo WT, Lim WJ, Kim EJ, Yun HD, Lee YH, Cho KM (2010). Endophytic bacterial diversity in the young Radish and their antimicrobial activity against pathogens. J. Korean. Soc. Appl. Biol. Chem. 53:493-503.
Crossref
|
|
|
|
|
Strobel G, Daisy B (2003). Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 67:491-502.
Crossref
|
|
|
|
|
Tamura K, Nei M (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10:512-526.
|
|
|
|
|
Trease GE, Evans WC (1983). Pharmacognosy. 14th edtition, Publ. Brown Publications.
|
|
|
|
|
Wright ES, Yilmaz LS, Noguera DR (2012). DECIPHER, a Search-based approach to chimera identification for 16S rRNA Sequences. Appl. Environ. Microbiol. 83:717-725.
Crossref
|
|
|
|
|
Yeates C, Gillings MR, Davison AD, Altavilla N, Veal DA (1997). PCR amplification of crude microbial DNA extracted from soil. Lett. Appl. Microbiol. 25:303-307.
Crossref
|
|
|
|
|
Zhao L, Xu Y, Lai XH, Shan C, Deng Z, Ji Y (2015). Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Brazil. J. Microbiol. 46:977-989.
Crossref
|
|