Full Length Research Paper
ABSTRACT
Phenoloxidase (PO) is a key factor in insect immunity. On invasion of microorganisms and pathogens, prophenoloxidase (PPO) changes to its active form, PO. The present study has been conducted to purify and characterize the PO from the haemolymph of desert locust, Schistocerca gregaria (Forskal) following activation of immune system by invasion of bacteria, Bacillus thuringiensis kurstaki (Bt). PO is purified by a combination of ammonium sulfate precipitation, blue sepharose CL-6B and phenyl sepharose CL-4B chromatography yielded a 209.97-fold purity and 54.75% recovery of activity. Sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) reveals that the molecular weight of the purified PO is 70.154 kDa. The purified PO is characterized in terms of its biochemical and enzymatic properties by using L-DOPA as a specific substrate. Ca2+ and Cu2+ significantly stimulated PO activity when compared with other metals. The PO reaction was strongly inhibited by phenylthiourea and thiourea, moderately inhibited by ethylene diamine tetractic acid (EDTA) and poorly inhibited by ethylene glycol tetraacetic acid (EGTA) and diethyl dithiocarbamate (DTC). Inhibition of PO showed excellent recovery ability by addition of Ca2+ on EGTA-inhibited enzyme. Therefore, PO is most probably a kind of tyrosinase-type Ca2+-containing metalloenzyme. The content of Ca2+ is higher than other trace metal elements. The reactive intermediates yielded by PO with its specific substrate L-DOPA had a broad-spectrum bactericidal activity against Gram +ve bacteria (Bacillus cereus and Staphylococcus aureus) with a greater degree more than Gram-ve bacteria (Escherichia coli and Pseudomonas aeruginosa). From the present study, PO from S. gregaria is most probably a tyrosinase-type calcium-containing mono-phenoloxidase, which functions not only as a catalytic enzyme in melanin production in locusts, but perhaps also as a humoral factor in host defense via melaninization as in other insects.
Key words: Schistocerca gregaria, phenoloxidase, purification.
Abbreviation: EDTA, Ethylene diamine tetractic acid; EGTA, ethylene glycol tetraacetic acid; DTC, diethyl dithiocarbamate; AGERI, agricultural genetic engineering research institute; L-DOPA, L -dihydroxyphenylalanine; SCB, sodium cacodylate buffer.INTRODUCTION
The desert locust, Schistocerca gregaria (Forskal) (Orthoptera: Acrididae) represents a relatively important group of plant-feeding insects. They have strong immune responses against bacteria, as previously shown by (Meshrif and Barakat, 2002; Barakat et al., 2002; Mo'men et al., 2010). The high interest in biological control means of controlling insect pests intensifies the need for investigating the response of insect to disease organisms. The haemolymph offer a readily accessible criterion of this response. The last and more important line of defense is the internal defense system that is comprised of cellular components and humoral mechanisms that offer a very effective protection against invading microorganisms and co-operatively interact to destroy non-self-elements. The cellular defense by haemocytes takes place immediately after contact with the foreign invader (Carton and Nappi, 1997). Humoral mechanisms that are essentially concerned with the ability of insect to recognize and dispose self from non-self, and involve several physiologically active substances, normally present in the native haemolymph or synthesized after natural infections (Boman and Hultmark, 1987). These substances appear in the haemolymph within a few hours after infection and display a broad spectrum of antimicrobial activity. Phenoloxidase (PO) is one of the most important enzymes that involved in the innate immune system of invertebrates. PO is synthesized as an inactive zymogen, prophenoloxidase (PPO) which can be activated by specific proteolysis (Cerenius and Söderhäll, 2004). When insects are infected by microorganisms, PPO activation elicits by microbial cell surface components, such as, lipopolysaccharide (LPS), peptidoglycans, β-1,3-glucose (Mo'men et al., 2012), the activities of the haemocytic enzymes, including phenoloxidase are enhanced during the challenge course.
However, due to the instability and rapid loss of the activity of this enzyme during the purification, more attention is paid to the investigation of PPO. So far, PPO is purified and characterized from only a small number of insect species including Lepidopteran, Hyalophora cecropia (Andersson et al., 1989) and Ostrinia furnacalis (Feng et al., 2008). Dipteran, Sarcophaga bullata (Chase et al., 2000), and cockroaches (Durrant et al., 1993).PO activity is investigated in other insects; Eurygaster integriceps (Zibaee et al., 2011) and Hyphantria cunea (Ajamhassani et al., 2012). Our knowledge of this enzyme (PO) at the protein level is limited. For example, the exact site of synthesis, regulation of PPO, its activating enzymes and inhibitors are still controversial. Although, there have been a number of studies involving various functional aspects of insect PPO, one or more of these aspects as integral parts of cells could be liberated to act on bacteria upon destruction of the cells or a change in the cell’s natural environment. Accordingly, the amount of antibacterial activity in the blood of normal insect should be proportional to the amount of cell destruction or to the degree in which the environment was altered.
The present study aims to isolate, purify and characterize the components involved in the PO cascade system, and to clarify more information dealing with the physicochemical properties of phenoloxidase of S. gregaria.
MATERIALS AND METHODS
Maintenance of insects
The desert locust, S. gregaria (Forskal), was maintained and reared for ten generations at 30 ± 2ËšC, a photoperiod of 16:8 (light: dark) and relative humidity varied between 60 and 80%, according to methods of Huxham and Lackie (1989). Cages were illuminated with one electric bulb, 100 watt, per cage in winter, and 60 watt in summer. All experiments outlined below were carried out with adults (both sexes), all being within 2 to 4 days after ecdysis.
Source of the bacterial pathogens
The bacterium, Bacillus thuringiensis kurstaki (Bt) (3200 IU/mg, AGERIN- wettable powder) was chosen as the pathogen for this study because of its wide use as a biocontrol agent among insects. The bacterium, B. thuringiensis kurstaki (Bt) were produced by the Agricultural Genetic Engineering Research Institute (AGERI) at the Ministry of Agriculture, Giza, Egypt. Non-Pathogenic strains of Gram positive bacteria (Bacillus cereus and Staphylococcus aureus) and Gram negative bacteria (Escherichia coli and Pseudomonas aeruginosa), were obtained from Department of Microbiology at Ain Shams University.
Mass culturing of the bacterial pathogens
Subcultures from bacterial pathogens were grown aerobically at 28 ± 2ï‚°C in nutrient broth tubes for 48 h. To obtain solitary pure colonies; nutrient agar plates were prepared and cultured with inoculates of the grown bacteria in the nutrient broth, using the streaking dilution method. The plates were incubated at 28 ± 2°C for 48 h. After growth, only solitary colonies were selected, cultured on nutrient agar slants and incubated at 28 ± 2°C for 48 h, and then kept in the refrigerator at 4ï‚°C until used. These slants were regenerated monthly.
Injection technique and haemolymph collection
In order to induce activation of locust immune system, which leads to the conversion of prophenoloxidase cascade to its active form (phenoloxidase), a stock suspension of a sub-lethal concentration of Bt was prepared, 10 µl of this concentration was injected into the haemocoel of the locusts. Insects were injected with a 10 µl Hamilton micro-syringe fitted with a 26-gauge needle according to Miranpuri and Khachatourians (1993). Ten microlitres of the concentration 1.2 × 106 cells/ml of (Btk) were used as a sub-lethal concentration to investigate the subsequent experiments according to Mo'men et al. (2010). Injected locusts were removed from the rearing cages, submerged in hot water bath at 60°C for 2 to 5 min; they were allowed to dry on paper towel. The heat-killed insects were amputated at the hind coxa with fine scissors. The haemolymph was obtained with a fine-tipped calibrated glass capillary, which was kept at -20°C until further analyses.
Estimation of the total haemolymph proteins
The total protein concentration in the haemolymph was quantified according to the method described by Bradford (1976).
Phenoloxidase activity assay
In order to measure PO activity, a preliminary assay was set up, in which we recorded the formation of dopachrome from L-dihydroxyphenylalanine (L-DOPA) spectrophotometrically at 470 nm, according to Aso et al. (1985) with some modifications. A solution of L-DOPA (2 mg/ml) was made in a sodium phosphate buffer (SPB) (0.01 M, pH 5.9). Aliquots (20 µl) of haemolymph were diluted (v/v) in a sodium cacodylate buffer (SCB) (0.01 M sodium cacodylate, 0.25 M sucrose, 0.01 M trisodium citrate), were added to 2 ml of DOPA solution, after which the formation of dopachrome (reddish brown pigments) was recorded each minute for 5 min to make sure that we respected the linear increase of the optical density. In case of control, 20 µl SCB were used. The phenoloxidase activity was expressed as PO unit, where one unit is the amount of enzyme activity required to produce an increase in the absorbance by 0.001 min/mg protein.
Phenoloxidase purification
All purification steps were performed at 4°C in a sodium cacodylate buffer (SCB) unless otherwise noted. Haemolymph (8 ml) was first diluted into 2:1 with the CB. The saturated ammonium sulphate solution was added to haemolymph until reaching a saturation of 40%. The precipitate was spun down by centrifugation for 10 min at 12,000 rpm, and redissolved in 500 μl of 20 mmol/L sodium cacodylate solution (pH 6.5). The protein was dialyzed in 2000 ml CB overnight at 4°C, and then applied to a blue sepharose CL-6B column (1.0 cm × 10 cm) pre-equilibrated with the CB. The column was eluted with an elution buffer (100 mM/L CaCl2, 10 mM/L Na2CB and pH 6.5) at a flow rate of 1.5 ml/min. The fractions containing PO from three simultaneous blue sepharose CL-6B chromatography were pooled, and concentrated with sucrose, then applied to a phenyl sepharose CL-4B column (0.8 cm × 12 cm) that was equilibrated with the CB buffer. The column was washed with distilled water at a flow rate of 1.5 ml/min until the absorbance of fractions at 280 nm returned to zero. The fractions with PO activity were dried in a Heto FD3 Model Vacuum Cold Dryer. The purified enzyme was stored at − 80°C according to Feng et al. (2008).
Molecular weight estimation
To determine the success of purification scheme we monitor the procedure of each step by performing, one-dimensional gel electrophoresis in vertical polyacrylamide gel; Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) as described by Laemmli (1970). On 10% gels, using SDS-molecular standard mixture of proteins; 205 kDa: 29 kDa (from Sigma) with a 4% stacking gel, at 100 volts for 5 h at room temperature.
Isolation of PO from polyacrylamide gel
After electrophoresis, the protein band of interest must be located in the gel by Side-strip technique. Staining of strips of the gel cut from the side when isolating abundant proteins that are well separated from other bands, was achieved according to Harlow and David (1988).
Polyclonal antibody production
Specific polyclonal antisera against the isolated PO were raised in young rabbit; approximately 800 µg of the purified PO was emul-sified with Freund’s complete adjuvant and injected subcutaneously at multiple sites of the rabbit, at intervals of 4 weeks. Eight days after the final booster, blood was collected and serum prepared according to Fergusson (1996). The antisera were aliquot and stored at -70°C.
Conformation of antibody specificity
It was carried out by using Western blotting analysis. This technique, as described by Towbin et al. (1979), depends on placing a sheet of nitrocellulose against the surface of an SDS-PAGE protein fractionation gel, and then a current is applied across the gel, thus causing the protein to move out of the gel onto the nitrocellulose where they bind firmly.
Effect of metal ions on the PO activity
To verify if the activation of the purified PO is influenced by the presence and concentration of metal ions, the activity of PO in the presence of different metal ions; MgSO4, ZnSO4, MnCl2, CuSO4 and CaCl2, was measured, respectively. According to methods of Feng et al. (2008), the purified PO (10 µg) was added to solutions diluted, respectively, with 100 mmol/L MgSO4, 100 mmol/L ZnSO4, 100 mmol/L MnCl2, 100 mmol/L CuSO4, and 100 mmol/L CaCl2 to various concentrations. 40 μl of 0.1 mol/L sodium phosphate buffer (SPB) and 100 μl of 2 mmol/L L-DOPA were added. The mixtures in a final volume of 1 ml were incubated for 30 min at 30°C, and the increase in absorbance at 490 nm after 10 min was continuously monitored for calculation of the PO activity.
Inhibition assay of PO activity
In order to determine the effect of various inhibitors on the activity of the purified PO, different compounds including: thiourea, phenylthiourea, ethylene diamine tetraacetic acid (EDTA), diethyldithiocarbamate (DTC) and ethylene glycol tetraacetic acid (EGTA), were tested for their inhibitory effect according to the method described by Fan et al. (2009).
Recovery effect of PO activity
To verify the metalloenzyme property of the PO, the recovery effects of some metal ions on PO activity were investigated according to the method of Fan et al. (2009). Recovery effect of Ca2+ on the activity of ethylene glycol tetraacetic acid (EGTA) pretreated purified PO from S. gregaria haemolymph, measurement and comparison between the enzymatic activity of 10 µl purified PO only, purified PO + 20 mM EGTA, purified PO + 20 mM EGTA + 10 mM Ca2+, purified PO + 20 mM EGTA + 15 mM Ca2+ and purified PO + 20 mM EGTA + 20 mM Ca2+ were made; and the reaction mixture was measured spectrophotometrically under the same conditions as described above.
Substrate specificity assay of PO
We investigated the substrate specificity of PO purified from the haemolymph of locusts using the method according to Andersen (1980).
Effect of reactive intermediates produced in PO-catalyzed reactions
In order to test the effect of PO-substrate-derived compounds on the growth and survival of bacterial cells: Bacillus cereus, S. aureus, E. coli and P. aeruginosa, (dependent on their growth rates), the experiment was carried out according to Zhao et al. (2007).
Statistical analysis
Results of susceptibility test were represented graphically as probit-logarithmic regression line. Statistical analysis of data was made by using software: Probit Analysis Program, Version 4.0. All data of the rest experiments were expressed as mean ± standard error (SE) and analyzed by using the SPSS11.5.0 software (SPSS Inc., 2012). The differences between means were analyzed by independent samples t-test and one-way ANOVA. The level of significance for each experiment was set at P < 0.05 or P < 0.01.
RESULTS
Total haemolymph protein concentration
The unpurified haemolymph of S. gregaria adult contained 80.537± 0.07 mg/ml protein. The total protein content of purified haemolymph after serial purification steps using ammonium sulphate precipitation (NH4)2SO4, 40% saturation followed by affinity chromatography (Blue Sepharose CL-6B chromatography and Phenyl Sepharose CL-4B chromatography) were decreased significantly compared with unpurified haemolymph. Data presented and graphically illustrated in Table (1).
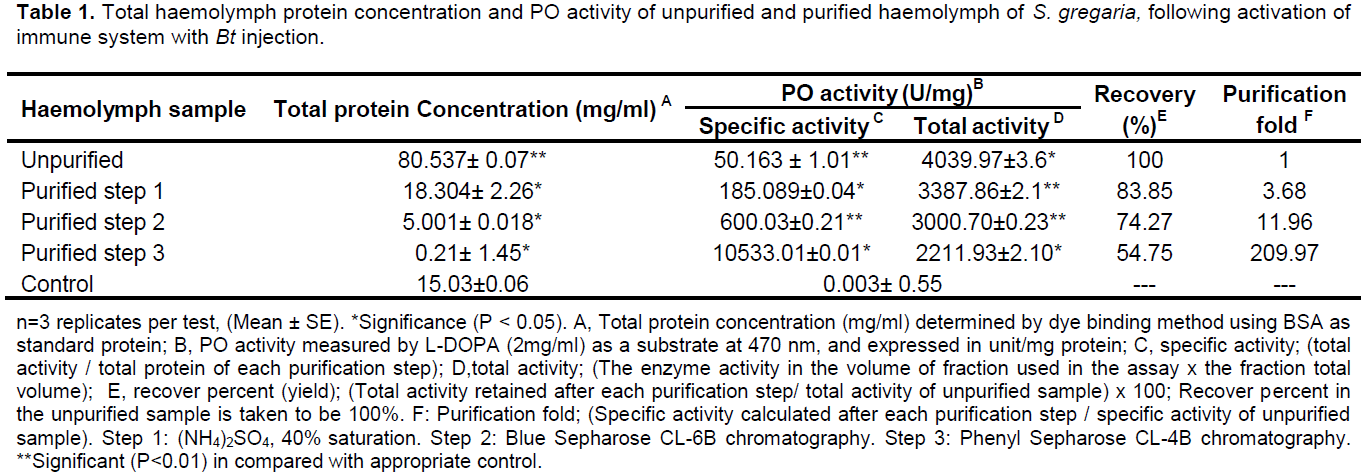
Enzyme purification
The PO purification results are shown in Table 1. The purification procedure yielded a total of 0.21 mg PO from a starting sample of 8 ml haemolymph containing about 80.537 mg total protein. The PO was purified 209.97-fold with a 54.75% total recovery of activity. SDS-PAGE of the purified protein revealed a single band with an estimated molecular mass of approximately 70.154 kDa (Figure 1).
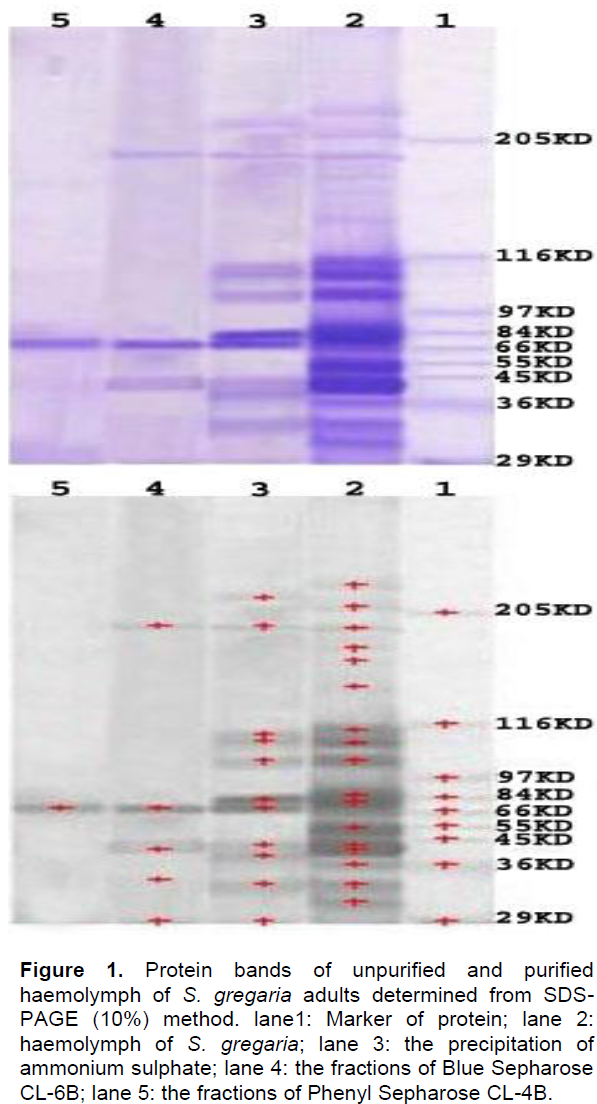
Confirmation of antibody specificity
Rabbit antisera against the purified PO were obtained. Specific polyclonal antisera against the isolated PO were capable of recognizing one band of approximately 70.154 kDa on SDS-PAGE gels corresponding to that of purified PO band detected from SDS-PAGE (Figure 2).
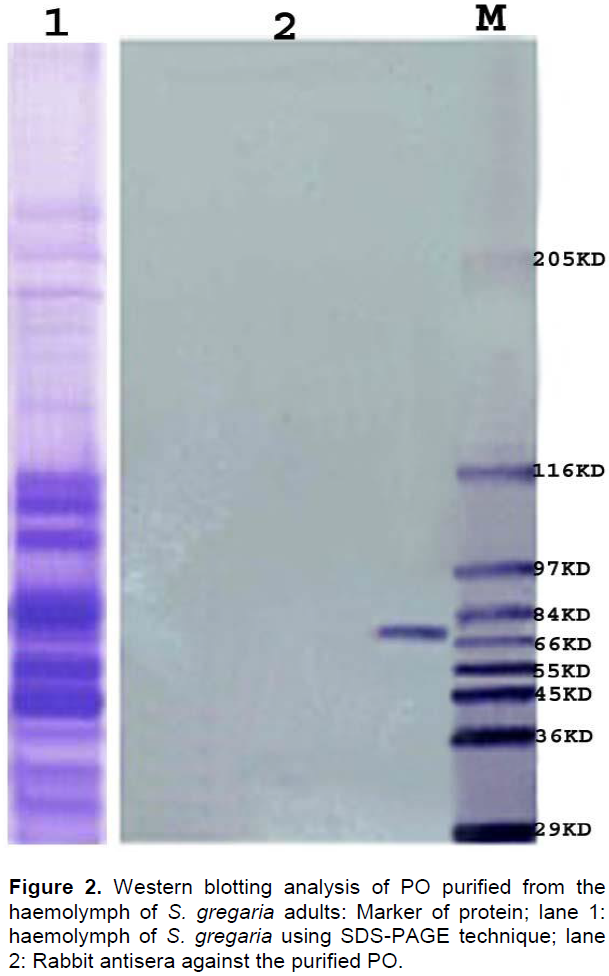
Effect of metal ions on PO activity
The PO activity was increased when the Ca2+, Mg 2+and Cu2+ concentration was increased to 15 mM/L. However, when the concentration was increased to 20 mM/L, it became inhibitory. In the presence of Mn2+ or Zn2+, the PO activity was increased only by a slight level (Figure 3).
Inhibition assay
The PO activity was completely inhibited by phenylthiourea and thiourea at (20 µM/L), moderately inhibited by ethylendiamine-tetracetic acid (EDTA) and triethylen\ tetraminehexaacetic acid (TTHA), poorly inhibited by diethyldithio-carbamate (DTC) (Figure 4).
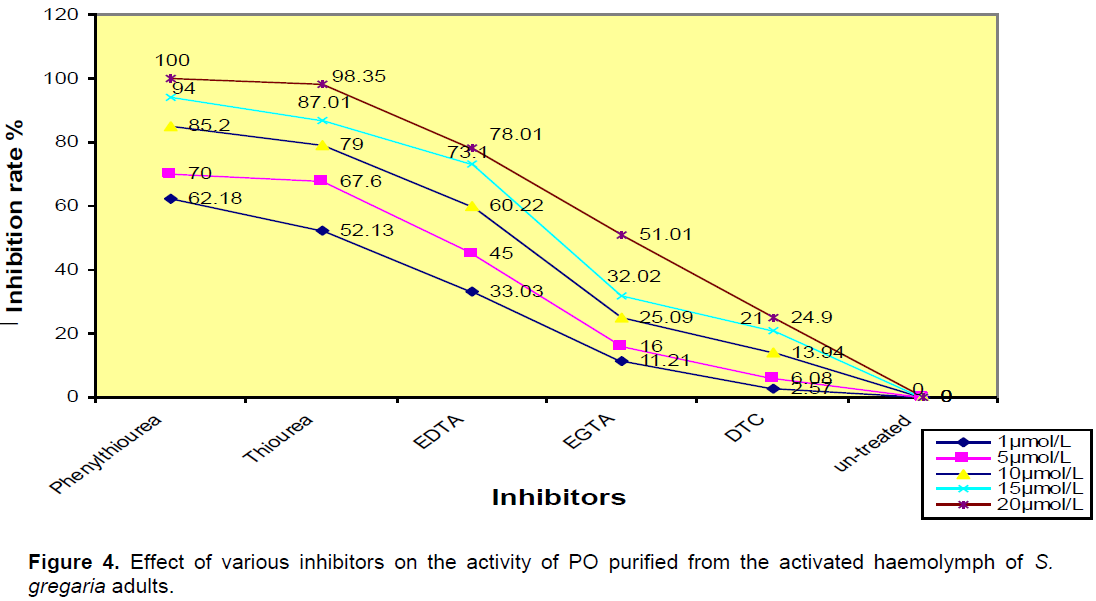
Recovery effect of Ca2+ on the activity of EGTA-pretreated PO purified from activated S. gregaria haemolymph
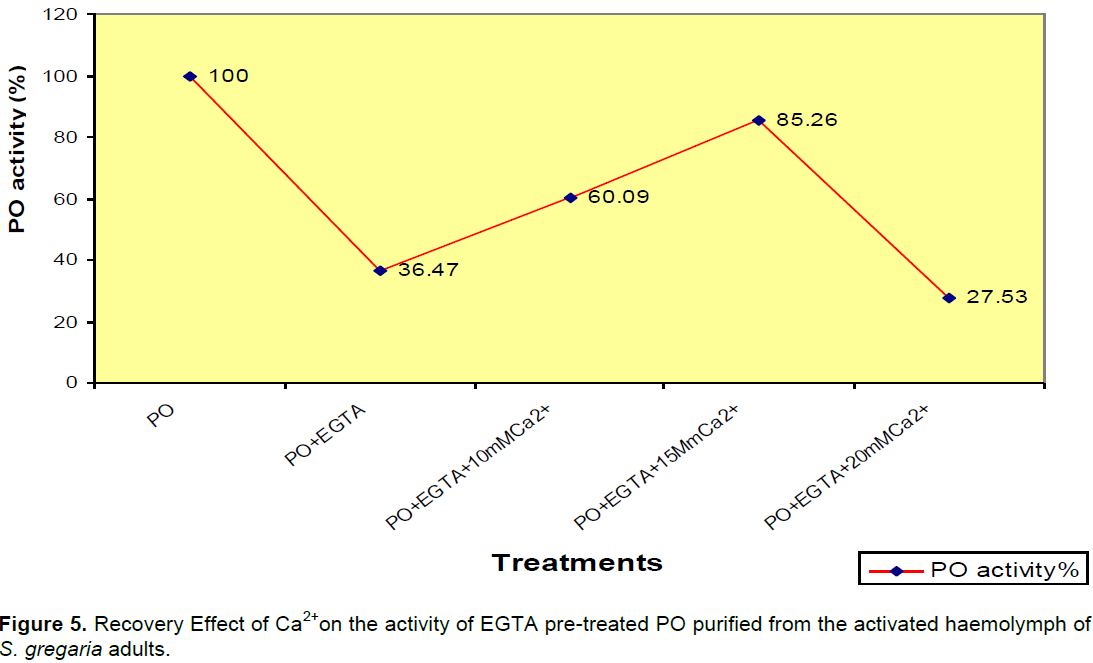
The enzymatic activity of the PO greatly inhibited by 20mM EGTA was restored to its original level by 15 mM Ca2+ .This results indicate that PO purified from S. gregaria probably is a calcium-containing metalloenzyme (Figure 5).
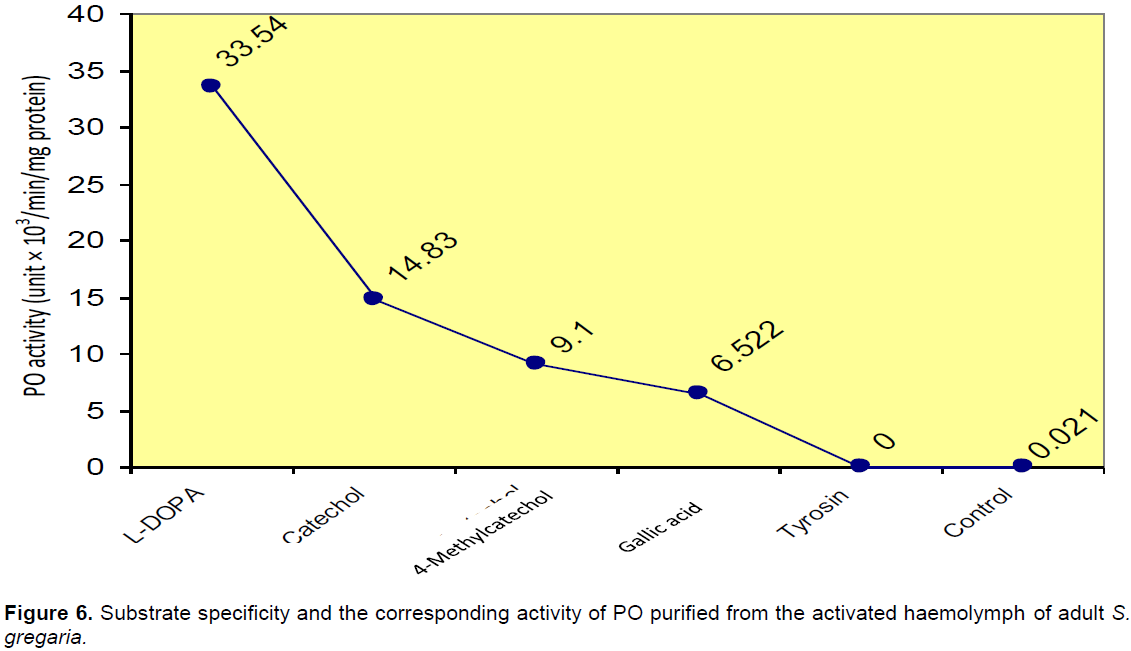
Enzyme activity and substrate specificity
In addition to L-DOPA, other O-phenols were suitable as substrates for the S. gregaria phenoloxidase, while no activity was detected towards the mono-phenol tyrosine (Figure 6).
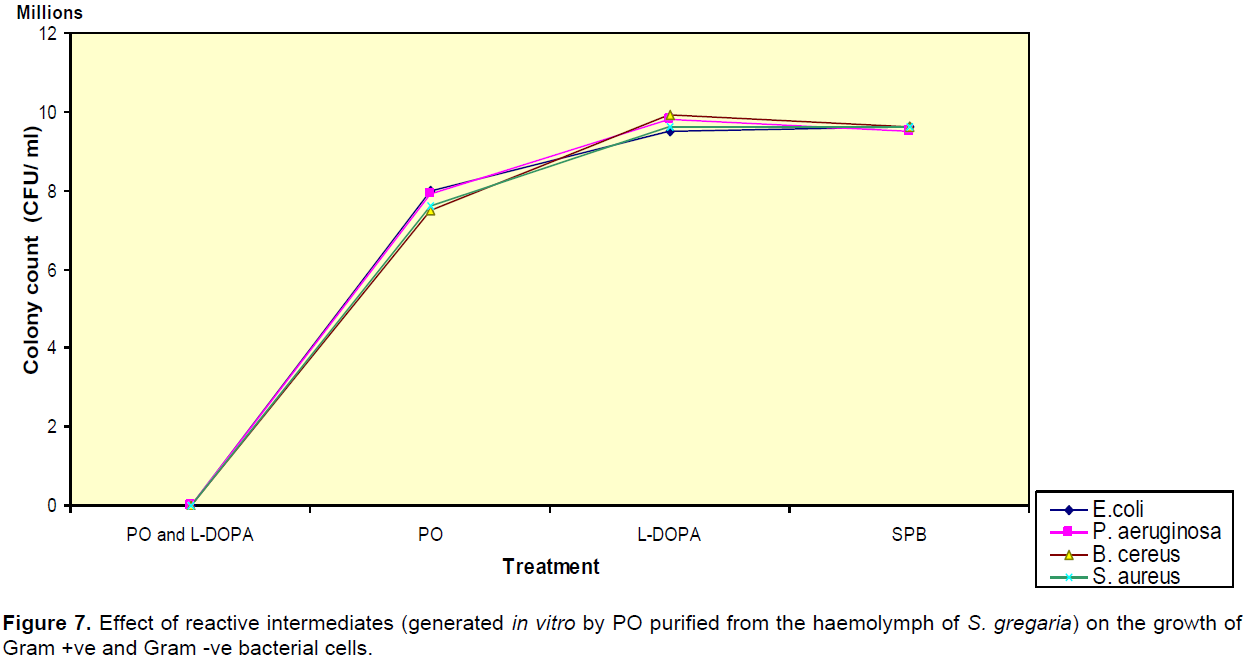
Effect of PO and L-DOPA derived compounds on the growth of bacterial cells
After PO and L-DOPA had been incubated with bacterial cells, we observed that the bacterial growth was significantly reduced (P < 0.05). Bacterial growth was not affected (P > 0.05) after the cells had been treated with the substrate or PO alone (Figure 7). The cell mortality was determined to be higher in the bacterial samples (B. cereus, S. aureus, E. coli and P. aeruginosa, respec-tively) treated with PO and L-DOPA. B. cereus was most susceptible, while P. aeruginosa was most resistant.
DISCUSSION
Several investigators purified and characterized some different insect POs (Durrant et al., 1993; Chase et al., 2000; Fan et al., 2009; Zibaee et al., 2011; Ajamhassani et al., 2012). Three types of POs are reported in insects as follows: laccase type (E.C.1.10.3.2; p-diphenol: O2 oxidoreductase), catechol oxidase type (E.C.1.10.3.1; diphenol: O2 oxidoreductase), and tyrosinase type (E.C.1.14.18.1; monophenol, L-DOPA: O2 oxido-reductase) (Barrett, 1987). The major problem to analyze the PO is the lack of effective tools to identify and quantitate individual PO isoforms. Several investigators use conventional chromatographic method (Chase et al., 2000) instead of immunoaffinity chromatography which is ineffective for purifying PO (Kopácek et al., 1995). In the present study, a combination of ammonium sulfate precipitation, blue sepharose CL-6B chromatography and phenyl sepharose CL-4B chromatography was employed to purify the PO from the haemolymph of S. gregaria. The enzyme estimates as 70.154 KDa by gel filtration in Sepharose and SDS-PAGE. These data are compatible with the purified enzyme from S. bullata (Chase et al., 2000), H. cecropia (Anderson et al., 1989), Locusta migratoria (Cherqui et al., 1996), E. integriceps (Zibaee et al., 2011), H. cunea (Ajamhassani et al., 2012) and Helicoverpa armigera (Goudru et al., 2013), that a single isoform characterizes from them. Many reports detect different isoforms of PO in several insects, for example, there are two isoforms in Galleria mellonella (Kopácek et al., 1995) and Bombyx mori (Yasuhara et al., 1995), three isoforms in the fruit fly Drosophila melanogaster (Fujimoto et al., 1993), and Branchiostoma tsingtauense (Pang et al., 2005), six in the mosquito Anopheles gambiae (Müller et al., 1999), The physiological sig-nificance of PO isoforms in the above mentioned insects still remains to be studied (Feng et al., 2008). Different substrates can adopt the appropriate conformation to interact with the PO protein. There is no information at this time on the substrate binding pocket in PO. The differences in the substrate binding pockets between the different insects are probably the result of differences in substrate-protein contact points or differences in the size of the substrate binding pocket reference. In the present study, the PO from S. gregaria is capable of oxidizing L-DOPA effectively, but fails to oxidize tyrosine. These results implies that this enzyme is most probably a kind of monophenol, tyrosinase-type o-oxidoreductase, not a laccase-type or catechol oxidase-type enzyme, this is in agreement with the results of Cherqui et al. (1996), Pang et al. (2005) and Asano and Ashida (2001).
Phenoloxidase activity is almost entirely inhibited by phenylthiourea and thiourea. This complete inhibition may be attributed to the influence of phenylthiourea on this process which is caused by its interaction with active sites of PO rather than with intermediate products of DOPA oxidation preventing the subsequent melanin formation. This explanation is in agreement with the results of Ryazanova et al. (2012). Results also indicate that treatment with EGTA nearly showed a similar pattern of inhibition as EDTA, since EDTA is a divalent cation scavenger and EGTA is a specific calcium chelator. Inhibition of PO activity with EDTA, indicates the involvement of divalent cations in the melanin-synthesis pathway, it may resemble other invertebrate POs that contain multiple copper atoms and/or copper binding sites (Aspan and Söderhäll, 1991; Aspan et al., 1995; Nellaiappan and Sugumaran, 1996). In fact, the effects of both EDTA and EGTA may be due to calcium dependency of the POs, as calcium is known to increase activity of several invertebrate POs (Perdomo-Morales et al., 2007), suggesting that the binding of some calcium atoms is necessary in the activating center of S. gregaria PO. The result of recovery test show that the enzymatic activity of the purified PO greatly inhibits by 20 mM EGTA, restores to its original level by 15 mM Ca2+. It was concluded that S. gregaria PO is most probably a kind of calcium-containing metalloenzyme and different from other insects such as Heliothis virescens (Lockey and Ourth (1992). DTC is a specific chelator for cupper presence in PPO which may explain the poor inhibition of S. gregaria PO activity that indicates few cupper atoms exist in the S. gregaria PO. This explanation is in accordance with those of Feng et al. (2008) on O. furnacalis larvae. Some metal ions can significantly modify the structure of PO (Li et al., 2000) that leads to increase or decrease in the activity of the enzyme, this ability to change conformation in solution might explain how the enzyme enhances its activity. Several metal ions tested with PO of S. gregaria showed that PO activity increases significantly when the Ca2+ concentration increases to 15 mM/L. However, when the concentration increases to 20 mM/L, it became inhibitory. In the presence of Cu2+ the PO activity increases only by a moderate level, while Mg2+, Zn2+ and Mn2+ show non-significant increase in the PO activity. So, it was concluded that the content of calcium is higher than other trace metal elements. Calcium-mediated PO activity enhancement has been reported for a large number of insects: for example B. mori (Ashida et al., 1983), S. gregaria (Dularay and Lackie, 1985), Blaberus craniifer (Leonard et al., 1985), L. migratoria (Brehelin et al., 1989), Lymantria dispar and Galleria mellonella (Dunphy, 1991). Thus, the knowledge of the binding of trace metal elements to the PO is required to be investigated extensively.
Insect POs, or tyrosinase-type POs, are similar to mammalian tyrosinases with two catalytic activities: the oxygenase activity which hydroxylates monophenols to o-diphenols and the oxidase activity which converts o- diphenols to quinones (Sugumaran, 2002; Nappi and Christensen, 2005). It has long been known that insects rely heavily on tyrosine metabolism for cuticle hardening and for innate immune responses (Vavricka et al., 2014). It was found that the roles of melanization include anti-bacterial, anti-fungal, anti-viral and anti-parasitic responses. This reaction has a broad-spectrum for all possible agents that can invade insects. This kind of universal killing power seems to stem from its basic mechanism of toxicity (Zhao et al., 2007). Several comprehensive reviews covering the humoral immunity discussed a number of immune proteins that were induced by the injection with bacteria (GÙtz and Boman, 1985; Boman and Hultmark, 1987; and Hultmark, 1993). Barakat (1997) indicated that the humoral defense reactions needed to some extent for newly synthesis and release of the antibacterial proteins. Meshrif and Barakat (2002) investigated the appearance of antibacterial substances in the haemolymph of the bacterial-injected insects as well as the uninjected insects, the antibacterial activity needs certain time to appear and integrate with the cellular reactions to produce an effective immune response in this species. Therefore, we decided to test the controversial function of PO directly by measuring possible antimicrobial activity of the reactive compounds produced in-vitro by this enzyme. After treating bacteria with the reaction mixtures containing purified S. gregaria PO with its specific substrate L-DOPA, the antibacterial effect (growth inhibition of bacterial cells) was observed. These findings established that the reactive intermediates yielded by PO had a broad-spectrum bactericidal activity against bacteria. Gram +ve bacteria (B. cereus and S. aureus) are more susceptible than Gram-ve bacteria (E. coli and P. aeruginosa). These results are similar to those of Zhao et al. (2007) who reported the antimicrobial effect of reactive intermediates produced in phenoloxidase-catalyzed reactions after being treated with Manduca sexta PO and dopamine, Bacillus subtilis ceased to grow.
Cerenius et al. (2010) revealed that an active PO isolated from the freshwater crayfish Pacifistic leniusculus exhibited a strong antibacterial effect in-vitro on the bacteria Gram -ve whereas, a weaker but still significant effect against Gram +ve. Rowley et al. (2011) investigated the possible role of the PPO system of L. migratoria in the killing/inhibition of growth of several species of bacteria, and suggesting that the antimicrobial factor(s) may have been generated by either the PPO cascade or a related enzyme system. The limited data gathered so far seem to indicate that certain bacterial species are more sensitive than other to quinone inter-mediates produced in the melanization cascade. These intermediates may have developed a tolerance to the presence of some bacteria. These results established that PPO activation is an integral component of the insect defense system involving a multitude of enzymes (e.g. proteinases, oxidases, and dopachrome conversion enzyme (DCE), which immobilize and kill invading microorganisms. The nature of these bioactive molecules requires detailed study to characterize the significance of these compounds. From the present study, PO from S. gregaria is most probably a tyrosinase-type calcium-containing mono-phenoloxidase, which functions not only as a catalytic enzyme in melanin production in locusts, but perhaps also as a humoral factor in host defense via melaninization as in other insects. To understand the similarities as well as differences in molecular characterization and physiological function among these arthropod POs, it is necessary to conduct more accurate, qualitative and quantitative analyses by cloning and transcriptional or translational detection of PO.
CONFLICT OF INTERESTS
The author(s) did not declare any conflict of interest.
ABBREVIATIONS
EDTA, Ethylene diamine tetractic acid; EGTA, ethylene glycol tetraacetic acid; DTC, diethyl dithiocarbamate; AGERI, agricultural genetic engineering research institute; L-DOPA, L -dihydroxyphenylalanine; SCB, sodium cacodylate buffer.
REFERENCES
| Ajamhassani M, Sendi JJ, Farsi MJ, Zibaee A (2012). Purification and characterization of phenoloxidase from the hemolymph of Hyphantria cunea (Lepidoptera: Arctiidae). Department of Plant Protection, College of Agriculture, University of Guilan-Rasht, 41635â1314, Iran. | ||||
|
Andersen SO (1980). Cuticular Sclerotization. In: Miller, TA (eds.), Cuticle techniques in Arthropods. Springer Verlag, New York. pp. 185-215. Crossref |
||||
|
Andersson K, Sun SC, Boman HG, Steiner H (1989). Purification of the prophenoloxidase from Hyalophora cecropia and four proteins involved in its activation. Insect Biochem. 19:629 - 637. Crossref |
||||
|
Asano T, Ashida M (2001). Cuticular pro-phenoloxidase of silkworm, Bombyx mori. J. Biol. Chem. 276:11100-11112. Crossref |
||||
|
Ashida M, Ishizaki Y, Iwahana H (1983). Activation of prophenoloxidase by bacterial cell walls or beta-1, 3-glucans in plasma of the silkworm, Bombyx mori. Biochem. Biophys. Res. Community 113: 562 - 568. Crossref |
||||
|
Aso Y, Karmer K, Hopkins T, Lookhart GL (1985). Characterization of haemolymph protyrosinase and cuticular activator from Manduca sexta. Insect Biochem. 15: 9 - 17. Crossref |
||||
|
Aspan A, Söderhäll K (1991). Purification of prophenoloxidase from crayfish cells, and its activation by an endogenous serine proteinase. Insect Biochem. 21: 363 - 373. Crossref |
||||
| Aspan A, Huang TS, Cerenius L, SÙderhall K (1995). cDNA cloning of prophenoloxidase from the freshwater crayfish Pacifartacus leniuscufus and its activation. Proc. Natl. Acad. Sci. USA 92:939 - 943. | ||||
| Barakat EMS. (1997). A comparative study on the immune response of the wax moth, Galleria mellonella (L.) to some biotic and abiotic materials. Ph. D Thesis. Ain Shams University. | ||||
| Barakat EMS, Meshrif WS, Shehata MG (2002). Changes in the haemolymph of the desert locust, Schistocerca gregaria after injection of Bacillus thuringiensis. J. Egypt. Acad. Soc. Environ. Dev. 2: 95 - 115. | ||||
|
Barrett FM (1987). Phenoloxidases from larval cuticle of the sheep blowfly, Lucilia cuprina: characterization developmental changes and inhibition by antiphenoloxidase antibodies. Arch. Insect Biochem. Physiol. 5:99-118. Crossref |
||||
|
Boman HG, Hultmark D (1987). Cell-free immunity in insects. Ann. Rev. Microbiol. 41:103-114 Crossref |
||||
|
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72:248-254. Crossref |
||||
|
Brehelin M, Drif L, Baud L, Boemare N (1989). Insect haemolymph: co-operation between humoral and cellular factors in Locusta migratoria. Insect Biochem. 19:301-307. Crossref |
||||
|
Carton Y, Nappi AJ (1997). Drosophila cellular immunity against parasitoids. Parasitol. Today 13(6):218-227. Crossref |
||||
|
Cerenius L, Söderhäll K (2004). The prophenoloxidase-activating system in invertebrates. Rev. Immunol. 198:116-126. Crossref |
||||
|
Cerenius L, Babu R, Söderhäll K, Jiravanichpaisal AP (2010). In vitro effects on bacterial growth of phenoloxidase reaction products. Inverteb. Pathol. 103:21-23. Crossref |
||||
|
Chase MR, Raina K, Bruno J, Sugumaran M (2000). Purification, characterization and molecular cloning of prophenoloxidase from Sarcophaga bullata. Insect Biochem. Mol. Biol. 30:953-967. Crossref |
||||
|
Cherqui A, Duvic B, Brehelin M (1996). Purification and Characterization of prpphenoloxidase from the hemolymph of Locusta migratoria. Arch. Insect Biochem. Physiol. 32:225-235. Crossref |
||||
|
Dularay B, Lackie AM (1985). Haemocytic encapsulation and the prophenoloxidase activation pathway in the locust Schistocerca gregaria Forsk. Insect Biochem.15:827-834. Crossref |
||||
|
Dunphy GB (1991). Phenoloxidase activity in the serum of two species of insects, the gypsy moth, Lymantria dispar (Lymantriidae) and the greater wax moth, Galleria mellonella (Pyralidae). Comp. Biochem. Physiol. 98B:535-538. Crossref |
||||
|
Durrant HJ, Ratcliffe NA, Hipkin CR, Aspan A, Söderhäll K (1993). Purification of the prophenoloxidase enzyme from haemocytes of the cockroach Blaberus discoidalis. Biochem. J. 289:87-91. Crossref |
||||
|
Fan T, Mingyu L, Jing W, Lingling Y, Rishan C (2009). Purification and characterization of phenoloxidase from Octopus ocellatus. Acta Biochim. Biophys. Sin. 41(10):865-872 Crossref |
||||
|
Feng C, Song Q, Lü W, Lu J (2008). Purification and characterization of hemolymph prophenoloxidase from Ostrinia furnacalis (Lepidoptera: Pyralidae) larvae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 151(2):139-46. Crossref |
||||
| Fergusson J (1996). Serum Separation from Whole Blood, protocol on line. Department of Biochemistry, University of Nottingham. The Medical School, Q.M.C., Clifton Boulevard, NG7 2UH, U.K. | ||||
|
Fujimoto K, Masuda K, Asada N, Ohnishi E (1993). Purification and characterization of prophenoloxidase from the pupae of Drosophila melanogaster. J. Biochem. 113:285-291. Pubmed |
||||
|
Goudru G, Sathish K, Senigala K, Chandish R, Hari C, Kuruba S (2013). Purification and characterization of prophenoloxidase from cotton bollworm, Helicoverpa armigera. Entomol. Res. 43:55-62. Crossref |
||||
| GÙtz P, Boman HG (1985). Insect immunity. In: Kertkut GA, Gilbert I (eds.), Comprehensive insect Physiology, Biochem. Pharmacol. Pergmon Press, Oxford. pp. 453-485. | ||||
| Harlow E, David P (1988). Antibodies: A Laboratory Manual. pp. 92 -117 | ||||
|
Hultmark D (1993). Immune reaction in Drosophila and other insects: a model for innate immunity. Trends Genet. 9:178-183. Crossref |
||||
|
Huxham IM, Lackie AM (1989). Behavior in vitro of separated haemocytes from the locust, Schistocerca gregaria. Cell Tissue Res. 251:677-668. Crossref |
||||
|
Kopácek P, Weise C, GÙtz P (1995). The prophenoloxidase from the wax moth Galleria mellonella: purification and characterization of the proenzyme. Insect Biochem. Mol. Biolol. 25:1081-1091. Crossref |
||||
|
Laemmli UK (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. Crossref |
||||
|
Leonard C, Kenneth S, Ratcliffe NA (1985). Studies on prophenoloxidase and protease activity of Blaberus craniifer hemocytes. Insect Biochem. 15:803-810. Crossref |
||||
|
Li X, Suzuki K, Kanaori K, Tajima K, Kashiwada A, Hiroaki H (2000). Soft metal ions, Cd(II) and Hg(II), induce triple-stranded alpha-helical assembly and folding of a de novo designed peptide in their trigonal geometries. Protein Sci. 9:1327-1333. Crossref |
||||
| Lockey TD, Ourth DD (1992). Isolation and characterization of haemolymph phenoloxidase from Heliothis virescens larvae. Comp. Biochem. Physiol. 102:891-896. | ||||
| Meshrif WS, Barakat ES (2002). Cell mediated immunity in the Locust, Shistocerca gregaria against the bacterium, Bacillus thuringiensis. J. Egypt. Acad. Soc. Environ. Dev. 2(1):117-130. | ||||
|
Miranpuri S, Khachatourians GG (1993). Haemocyte surface changes in the migratory grasshopper, Melanoplus sanguinipes in response to wounding and infection with Beuveria bassiana. J. Entomol. Exp. Appl. 68:157-164. Crossref |
||||
| Mo'men SHA, Salama MS, Barakat EMS, Salem DAM (2010). The Role of Prophenoloxidase Activation System in Cellular Defense Mechanisms in the Haemolymph of the Desert Locust, Schistocerca gregaria (Forskal) M.Sc. Thesis, Science Faculty, Ain Shams University. | ||||
| Mo'men SHA, Salem DAM, Barakat EMS, Salama MS (2012). Activation of Prophenoloxidase during Bacterial Injection into the Desert Locust, Schistocerca gregaria. Int. J. Med. Biol. Sci. 6:291-300. | ||||
|
Müller HM, Dimopoulos G, Blass C, Kafatos FC (1999). A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J. Biol. Chem. 274:11727-11735. Crossref |
||||
|
Nappi AJ, Christensen BM (2005). Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem. Mol. 35:443-459. Crossref |
||||
|
Nellaiappan K, Sugumaran M (1996). On the presence of prophenoloxidase in the hemolymph of the horseshoe crab, Limulus. Comp. Biochem. Physiol. 113B: 163 - 168. Crossref |
||||
|
Pang QX, Zhanga SC, Shi X, Sua F, Wua D (2005). Purification and characterization of phenoloxidase from amphioxus Branchiostoma belcheri tsingtauense. Fish Shellfish Immunol. 19:139-148. Crossref |
||||
|
Perdomo MR, Montero A, Perera VE, Pardo RZ, Alonso JE (2007). Phenoloxidase o activity in the hemolymph of the spiny lobster Panulirus argus. Fish Shellfish Immunol. 23:1187-1195. Crossref |
||||
| Rowley AF, Brookman JL, Ratcliffe NA (2011). Possible involvement of the prophenol-oxidase system of the locust, Locusta migratoria, in antimicrobial activity. Inverteb. Pathol. 60(2):31-38. | ||||
|
Ryazanova AD, Alekseev AA, Slephneva IA (2012). The phenylthiourea is a competitive inhibitor of the enzymatic oxidation of DOPA by phenoloxidase. J. Enzyme Inhib. Med. Chem. 27(1):78-83. Crossref |
||||
|
Towbin H, Staehelint T, Gordon J (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. 76 (9):4350-4354. Crossref |
||||
|
Vavricka CJ, Qian H, Prajwalini M, Haizhen D, Bruce MC, Li J (2014). Tyrosine metabolic enzymes from insects and mammals: A comparative perspective. Insect Sci. 21:13-19. Crossref |
||||
|
Yasuhara Y, Koizumi Y, Katagiri C, Ashida M (1995). Reexamination of properties of prophenoloxidase isolated from larval haemolymph of the silk worm, Bombyx mori. Arch. Biochem. Biophys. 320:14-23. Crossref |
||||
|
Zhao P, Li J, Wang Y, Jiang H (2007). Broad-spectrum antimicrobial activity of the reactive compounds generated in vitro by Manduca sexta phenoloxidase. Insect Biochem. 37(9):952-959. Crossref |
||||
|
Zibaee A, Bandani A, Malagoli D (2011). Purification and characterization of phenoloxidase from the hemocytes of Eurygaster integriceps (Hemiptera: Scutelleridae) Compar. Biochem. Physiol. 158B:117-123. Crossref |
||||
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0