ABSTRACT
The present study was aimed at identifying the functional groups and phyto-constituents present in Xylopia aethiopica (Dunal) A Rich fruit using Fourier Transform Infrared Spectrometry (FTIR) and Gas chromatography-mass spectroscopy (GC-MS), spectroscopy. FTIR method was performed using Perkin Elmer Spectrophotometer and the characteristic peaks were detected. The phytochemical constituents were screened by GC-MS method and the compound detection employed the NIST Ver. 2.0 year 2005 library. The results of the GC-MS analysis showed different peaks determining the presence of 15 phytochemical compounds in the fruit extract of A. aetiopica. The phyto-constituents with their percentage areas include β-Ylangene(2.85%), 1,6-Cyclodecadiene, 1-methyl-5-methylene-8- (1-methylene)-, [s-(E,E)]- (1.71%); (-)-Spathulenol (1.23%);Trans-Z-α-Bisabolene epoxide (1.68%); n-Hexadecanoic acid (2.90%); Manoyl oxide (2.51%); Linoleic acid (8.14%); Oleic acid (3.13%); Cis-Z-α-Bisabolene (1.34%); Pimara-7,15-diene-3-one (8.86%); 1-Heptatricotanol (2.07%); Kaur-1b-ene (6.59%); β-Pimaric acid (36.39%); Doconexent (1.66%) and Androstan-17-one, 3-ethyl-3-hydroxyl-, (5a)- (17.48%). The result of the FTIR spectroscopic studies revealed the presence of arenes, alcohols, phenols, carboxylic acids, ethers, aromatics, aryl ketone, alkenes, saturated aldehyde and phenols. The findings of the study revealed that the GC-MS and FTIR spectral analyses of Xylopia aethiopica (Dunal) A. Rich fruit extract composed of various bioactive compounds which have are used in ethnomedicine to treat and cure infections and diseases.
Key words: Fourier Transform Infrared Spectrometry (FTIR), Gas chromatography-mass spectroscopy (GC-MS), phytochemical, Xylopia aethiopica.
Over the years, man has been in constant combat with agents that cause diseases and infections and attempt to avert their associated consequences with conventional medicines have proved abortive. Recently, the tribes in developing countries, particularly Nigeria, rely primarily on herbal medicines to overcome health problems (Anand and Gokulakrishnan, 2012). Okwu and Josiah (2006) observed that medicinal plants have been variously employed by, traditional practitioners to treat diseases, heal wounds and infections. A large proportion of these plants are trees, shrubs and weeds (Ikeyi and Omeh, 2014), and they are consumed as foods (Faleye and Ogundaini, 2012). The efficacies of these medicinal plants in the treatment of diseases have been attributed to the presence of bioactive compounds, which have diverse physiological and pharmacological responses (Ekanem and Udo, 2009).
Phytochemicals are bioactive substances derived from plants and are associated with the protection of human health against chronic degenerative diseases (Florence et al., 2015) which do not act alone but most time, in a combination of complexes (Cowan, 1999). Determination of phytoconstituents is largely performed by relatively expensive and often laborious techniques such as Gas Chromatography (GC) combined with specific detections schemes (Uzer et al., 2005). The specific detection schemes can be Mass Spectrometry (MS) or Fourier Transform Infrared Spectrometry (FTIR). Gas chromatography combined with mass spectroscopy (GC-MS) can identify pure compounds present at less than 1ng biological specimen and quantification purpose (Florence and Jeeva, 2015). The unknown organic compounds in a complex mixture can be determined by the interpretation and also by matching the spectra with reference spectra (Hites, 1997). FT-IR Spectroscopy has demonstrated to be a reliable and sensitive method for finding out the functional groups, present in plant samples using IR region in the range of 400 to 4000 cm-1. For most common plant compounds, the spectrum of an unknown compound can be identified by comparison to library of known compounds (Griffiths and Haseth, 1986).
The plant, Xylopia aethiopica (Dunal) A. Rich belonging to the family Annonaceae is widely distributed in the West African forest from Senegal to Sudan in Eastern Africa, and down to Angola in Southern Africa (Burkhill, 1985). The plant is described by different tribes of Nigeria as Uda (Igbo), Sesdo (Yoruba), Kimba (Hausa) and Aghako (Edo) (Nnodim et al., 2013). X. aethiopicais is a perennial woody and evergreen aromatic tree or shrub with main trunk and branches, growing up to 20 m high (Enemchukwu et al., 2014). The fruits are green when matured and black with pungent aromatic scent when dried (Ikeyi and Omeh, 2014).
Burkhill (1985) reported that, seeds of X. aethiopica have both nutritional and medicinal values. In South Eastern Nigeria, the fruits are used as spices and aqueous decoction is used especially after birth, probably for its antiseptic properties, to arrest bleedings (Nnodim et al., 2013). Several parts of the plant (leaves, seeds and roots) are employed in folk medicine for managing various ailments such as skin infections, cough and fever (Mishana et al., 2000). The fruits are used as a postpartum tonics and remedy for many ailments like bronchitis, asthma, rheumatism, neuralgia and amenorrhea in women (Burkhill, 1985). The seeds extract of X. aethiopica has been identified by many researchers to possess anti-hypertensive, diuretic, antimicrobial, antioxidant and cytotoxic effects on a wide range of cancer cell lines (Somova et al., 2001; Asekun and Adeniyi, 2004; Ju et al., 2004).
Considering the relevance of the plant in folk medicine for the treatment of various ailments, the need to identify the bioactive compounds responsible for its therapeutic potentials is quint essential. The thrust of this study was aimed at evaluating the phyto-constituents of X. aethiopica (Dunal) A. Rich using the technique of GC-MS/FTIR.
Collection, Identification and processing of plant material
The fruits of Xylopia aethiopica were purchased from Eke Okigwe Market, Okigwe Local Government Area of Delta State. Eke Okigwe lies between latitude 5°5010.9IIN and 5°49155IIN and longitude 7°21132.6IIE and 7°23117IIE. The plants were identified in the department of Plant Science and Biotechnology of the Abia State University, Uturu (ABSUU). The fruits were properly sorted to remove dust and decayed materials and then washed with sterile distilled water. The fruits were cut, shade dried, ground into fine powder and stored in air tight polythene bags until use.
Plant sample extraction
2 g of air dried powder of fruit sample was extracted with 50 ml of methanol, with gentle stirring for 72 h. The sample was kept in dark for 72 h with intermittent shaking. After incubation, the solution was filtered through Whatmann No. 1 filter paper and the filtrate was collected (crude extracts). It was then transferred to glass vials and kept at 4°C before use.
Gas Chromatography-Mass Spectrometry analysis (GC-MS)
10 g of powdered fruit sample was soaked with 30 ml ethanol overnight and filtered through ash less filter paper with anhydrous sodium sulphate (2 g), to remove residual water from the sample before soxhlet extraction. The extract is concentrated to 1 ml by bubbling nitrogen into the solution. The extract contained both polar and non-polar phyto-components. 2 µl of the methanolic extract of X. aethiopica fruit was employed for GC-MS analysis.
The Clarus 500 GC used in the analysis, employed a fused silica column packed with Elite-1 (100% dimethyl poly siloxane, 30 nm × 0.25 nm ID × 1 µm df) and the components were separated using Helium as carrier gas at a constant flow of 1ml/min.
The 2 µl sample extract injected into the instrument was detected by, the turbo gold mass detector (Perkin Elmer) with the aid of the turbo mass 5.1 software. During the 36th min GC extraction process, the oven was maintained at a temperature of 110°C with 2 min holding. The injector temperature was set at 250°C (mass analyser). The different parameters involved in the operation of the Clarus 500 MS, were also standardized (Inlet line temperature: 200°C; Source temperature: 200°C). Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 45 to 450 Da. The MS detection was completed in 36 min.
Fourier Transform Infrared spectroscopic analysis (FT-IR)
Oven-dried fruit samples (60°C) were ground into fine powder, using a mortar and pestle. Two milligrams of the sample was mixed with 100 mg KBr (FT-IR grade) and then compressed to prepare a salt-disc (3 mm diameter). The disc was immediately kept in the sample holder and FT-IR spectra were recorded in the absorption range between 400 and 4000 cm–1. All investigations were carried out with a Shimadzu FT-IR spectrometer.
Identification of components
Interpretation of mass spectrum obtained from GC-MS was conducted using the database of National Institute Standard and Technology (NIST) having more than 82,000 patterns. The spectrum of the unknown component was compared with the spectra of the known components stored in the NIST library. The name, molecular weight, molecular formula and structure of the components of the test materials were ascertained.
Identification of functional groups
The FTIR spectrum was used to identify the functional groups of the active components, present in plant sample, based on the peaks values in the region of IR radiation. When the plant extract was passed into FTIR, the functional groups of the components were separated based on its peaks ratio.
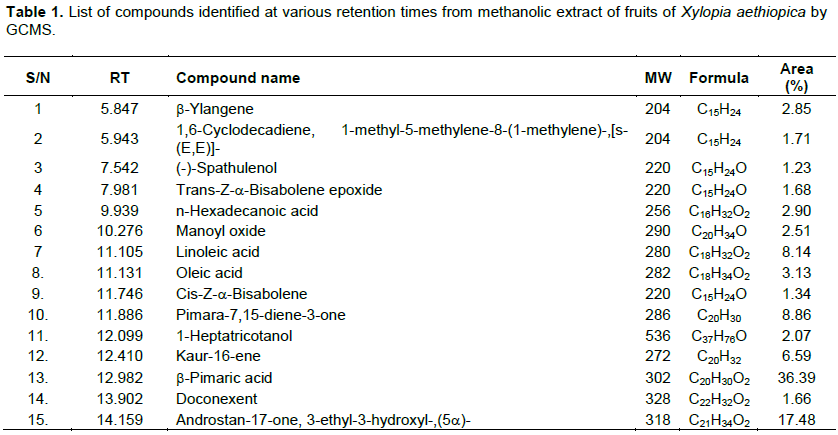
Gas Chromatography-Mass Spectrometry analysis (GC-MS) is one of the best methods to identify the bioactive compounds of, nonpolar components and volatile essential oil, fatty acids and lipids. Fifteen compounds were identified from the methanolic extract of X. aethiopica fruit. The identification of the phytochemical compounds was confirmed based on the peak area retention time and molecular formula was presented in Figure 1 and Table 1. The GC-MS analysis of X. aethiopican fruit extract revealed that, the presence of phytochemicals represented b-Ylangene (2.85%), 1,6-Cyclodecadiene, 1-methyl-5-methylene-8-(1-methylene)-, [s-(E,E)]-(1.71%);(-)-Spathulenol (1.23%); Trans-Z-a-Bisabolene epoxide (1.68%); n-Hexadecanoic acid (2.90%); Manoyl oxide (2.51%); Linoleic acid (8.14%); Oleic acid (3.13%); Cis-Z-a-Bisabolene (1.34%); Pimara-7,15-diene-3-one (8.86%); 1-Heptatricotanol (2.07%); Kaur-1b-ene (6.59%); b-Pimaric acid (36.39%); Doconexent (1.66%) and Androstan-17-one, 3-ethyl-3-hydroxyl-,(5a)-(17.48%).
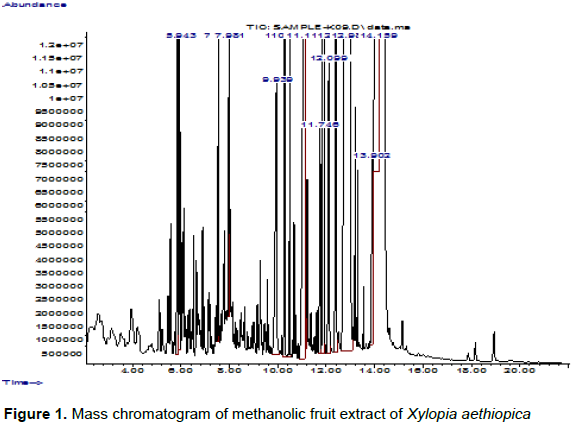
According to Duke’s ethnobotanical and phyto-chemistry database (Duke’s, 1998) the identified compounds possess many biological properties. Among the identified bioactive compounds, b-Ylangene is suggested to be a sesquiterpenoid and may be employed in folk medicine as cytotoxic and anti-inflammatory agents (Yun-Jie et al., 2013). The compound 1,6-Cyclodecadiene, 1-methyl-5-methylene-8-(1-methylene)-,[s-(E,E)]-, otherwise known as Germacrene D is a class of volatile organic hydrocarbons, which plays a role as a precursor of various sesquiterpenes such as cadinenes and selinenes (Telascrea et al., 2007). Plant terpenes according to Langenheim (1994) have anti-herbivore defenses.
Several researches on the bioactivity of phytochemicals found in natural products have revealed that, germacrene D has deterrent effects against herbivores and it has been reported to have insecticidal activity against mosquitoes (Kiran and Devi, 2007), antibacterial activity against gram negative and positive bacteria (Hamadan et al., 2013), as well as repellent activity against aphids (Bruce et al., 2005) and ticks (Birkett et al., 2008). Spathulenol is an oxygenated sesquiterpene and has been reported to have immunomodulatory effects, mosquito repellant activity, antimicrobial and anti-inflammatory activities (Prakasia and Nair, 2015).
Trans-Z-a-Bisabolene epoxide is an oxygenated sesquiterpene and has been observed to have antitumor and anti-inflammatory properties (Srinivasan et al., 2014). Manoyloxide is a diterpene and have been reported to have antimicrobial effects against Borrelia burgdorferi sensu stricto (Bbss) in vitro (Hutschenreuther et al., 2010). Doconexent otherwise known as Cis-4,7,10,13,16,19-Docosahexanoic acid has been reported to have anticardiovascular, antitumor and neuroprotective effects (Guesnet and Alessandri, 2011). Kalaivani et al., (2012) reported that Androstan-17-one, 3-ethyl-3-hydroxy-(5a) has neuroactive, analgesic and anesthetic effects. n-Hexadecanoic acid (palmitic acid) and 9,12-Octadecadienoic acid (Z,Z)- (linoelic acid) are fatty acids and have been reported to possess antioxidant, hypo-cholesterolemic, hemolytic 5-Alpha reductase inhibitor, anti-androgenic, lubricant, anti-histaminic, insectifuge, anti-eczemic and antiacne activities (Kalaivani et al., 2012). Oleic acid is a monounsaturated fatty acid and has been reported by Devi et al. (2014) to possess anti-inflammatory, anti-androgenic, cancer preventive, antimicrobial, dermatitigenic, hypocholesterolemic, anemiagenic, flavor, 5-alpha reductase inhibitor, insectifuge and larvicidal activities.
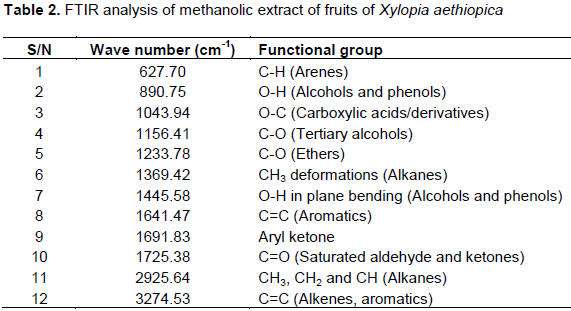
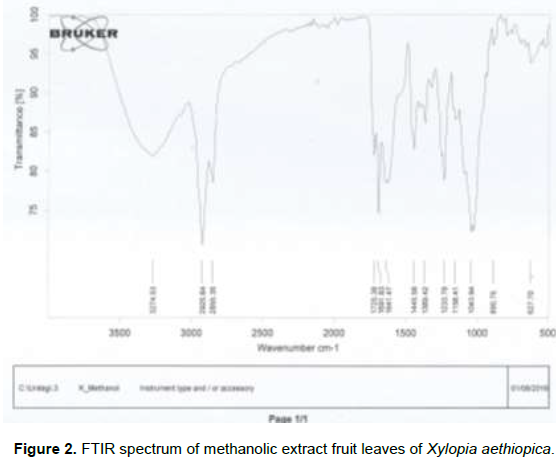
The results of FT-IR spectroscopic analysis of methanolic fruit extract revealed the presence of arenes, alcohols, phenols, carboxylic acids, ethers, aromatics, aryl ketone, saturated aldehyde and phenols and alkenes (Figure 2 and Table 2). The absorption at 3274.53 cm-1 is due to C=C group that is present in the extract. The band at 2925.64 cm-1 is due to C-H3, C-H2 and C-H; the band at 1725.38 cm-1 showed saturated aldehyde and ketones; the band at 1691.83 cm1 showed aryl ketone bend; the band at 1641.47 cm-1 showed Aromatic C=C bend; the band at 1445.58 cm-1 showed Alcohol and Phenol O-H in-plane bend, the band at 1369.42 cm-1 showed CH3 deformation, the band at 1233.78 cm-1 showed ether C-O bend; the band at 1156.41 cm-1 showed tertiary alcohol C-O bend, the band at 1043.94 cm-1 showed carboxylic acid O-C bend; the band at 890.75 cm-1 showed Alcohol and phenol O-H stretch; and 627.70 cm-1 showed Arenes C-H bend. Fourier Transform Infrared Spectroscopy (FT-IR) is a reliable and sensitive technique, employed for the detection of bimolecular composition (Kumar and Prasad, 2011). The results of the present study further revealed that, the FT-IR analysis of the methanolic extract of X. aethiopica fruit separated the functional groups of the component, based on its peak ratio which identified the chemical compounds. The presence of arenes, alcohols, phenols, carboxylic acids, ethers, aromatics, aryl ketone, alkenes, saturated aldehyde and phenols might be responsible for various medicinal properties of the plant.
This is the first report on the chemical composition of phyto-constituents fruit of X. aethiopica (Dunal) A. Rich. The result reveals that the GC-MS and FTIR spectral analysis of X. aethiopica (Dunal) A. Rich methanolic fruit extract is composed of, various functional groups and variety of fatty acids which are responsible for many biological activities. It is strongly recommended that, this medicinal plant needs further research in many-sided field of natural products to isolate, typify and explicate the structure of bioactive molecules, which ensure the clinical trials and develop an effectual plant-based natural drug for various ailments in the point of health security.
The authors have not declared any conflict of interests.
REFERENCES
|
Anand T, Gokulakrishnan K (2012). Phytochemical analysis of Hybanthus enneaspermus using UV, FTIR and GC- MS.
Crossref
|
|
|
|
Asekun OT, Adeniyi BA (2004). Antioxidant and Cytotoxic Activities of the Fruit Essential Oils of Xylopia aetiopica from Nigeria. Fitoterapia 75:368-370.
Crossref
|
|
|
|
|
Birkett MA, Al Abassi S, Krober T, Chamberlain K, Hooper AM, Guerin PM, Pettersson J, Pickett JA, Slade R, Wadhams LJ (2008). Antiectoparasitic activity of the gum resin, gum haggar, from the East Africa plant, Commiphor aholtziana. Phytochemical 69:1710-1715.
Crossref
|
|
|
|
|
Bruce TJA, Birkett MA, Blande J, Hooper AM, Martin JL, Khambay B, Prosser I, Smart LE, Wadhams LJ (2005). Response of economically important aphids to components of Hemizygia petiolata essential oil. Pest Manage. Sci. 61:1115-1121.
Crossref
|
|
|
|
|
Burkhill HM (1985). The useful plants of West Africa.2nd Edn. Royal Botanic Gardens, 1 (A-D). pp. 130-132.
|
|
|
|
|
Devi I, Amutha J, Muthu AK (2014). Gas chromatography-mass spectrometry analysis of bioactive constituents in the ethanolic extract of Saccharum spontaneum Linn. Int. J. Pharm. Pharm. Sci. 6(2):755-759.
|
|
|
|
|
Duke J (1998). Duke's phytochmical and ethnobotanical databases. Available at: www.ars-grin.gov/duke/. Accessed 12/09/2016.
|
|
|
|
|
Ekanem AP, Udo FV (2009). African natural plant products: New discoveries and challenges in chemistry and quality. ACS publications. pp. 135-147.
|
|
|
|
|
Enemchukwu BN, Erimujor SO, Ubaoji KI (2014). Phytochemical screening and biochemical effects of aqueous seed extract of Xylopia aethiopica (Uda) on selected haematological indices in male wistar albino rats. The Bioscientist 2(1):103-109.
|
|
|
|
|
Faleye FJ, Ogundaini OA (2012). Evaluation of anti-oxidant and antimicrobial activities of two isolates from Aspilia Africana. Int. Res. J. Pharm. 3(7):135-138.
|
|
|
|
|
Florence AR, Jeeva S (2015). FTIR and GC-MS spectral analysis of Gmelina asiatica L. leaves. Sci. Res. Rep. 5(2):125-136.
|
|
|
|
|
Griffiths PR, Haseth JA (1986). Fourier Transform Infrared Spectroscopy. New York, Willey.
|
|
|
|
|
Guesnet P, Alessandri JM (2011). Docosahexanoic acid (DHA) and the developing central nervous system (CNS) – Implication for dietary recommendation. Biochimie 93(1):7-12.
Crossref
|
|
|
|
|
Hamadan DI, Abdulla RH, Mohamed ME, El-Shazly AM (2013). Chemical composition and biological activity of essential oils of Cleopatra mandarin (Citrus reshni) cultivated in Egypt. J. Pharmacogn. Phytother. 5(5):83-9.
|
|
|
|
|
Hites AR (1997). Gas Chromatography Mass Spectroscopy: Handbook of Instrumental Techniques for Analytical Chemistry. pp. 609-611.
|
|
|
|
|
Hutschenreuther A, Birkemeyer C, Grotzinger K, Straubinger RK, Rauwald HW (2010). Growth inhibiting activity of volatile oil from Cistuscreticus L. against Borrelia burgdorferi s.s in vitro. Pharmazie 65(4):290-295.
|
|
|
|
|
Ikeyi PA, Omeh NY (2014). A review of the Ethnotherapeutics of medicinal plants used in traditional/alternative medicinal practice in Eastern Nigeria. Int. J. Curr. Microbiol. Appl. Sci. 3(1):675-683.
|
|
|
|
|
Ju EM, SE Lee, HJ Hwang, JH Kim (2004). Antioxidant and anticancer activity of extract from Betulaplatyphylla var. Japonica. Life Sci. 74(8):1013-1026.
Crossref
|
|
|
|
|
Kalaivani CS, Sathish SS, Janakiraman N, Johnson M (2012). GC-MS medicinally important plant. Int. J. Med. Aromat. Plants 2(1):69-74.
|
|
|
|
|
Kiran SR, Devi PS (2007). Evaluation of mosquitocidal activity of essential oil and sesquiterpenes from leaves of Chloroxylon swietenia DC. Parasitol. Res. 101:413-418.
Crossref
|
|
|
|
|
Kumar KJ, Prasad DAG (2011). Identification and comparison of biomolecules in medicinal plants of Tephrosiatinctoria and Atylosia albicans by using FTIR. Rom. J. Biophys. 21(1):63-71.
|
|
|
|
|
Langenheim JH (1994). Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 20:1223-1280.
Crossref
|
|
|
|
|
Mishana NR, Abbiw DK, Addae-Mensah I, Adjanouhoun E, Ahyi MRA, Ekpere JA, Enow-Orock EG, Gbile ZO, Noamesi GK, Odei MA, Odunlami H, Oteng-Yeboah AA, Sarpong K, Sofowora A, Tackie AN (2000). Traditional Medicine and Pharmacopoeia, Contribution to the revision of ethnobotanical and Floristic Studies in Ghana. OAU/STRC Tech. Rep. P 67.
|
|
|
|
|
Mishra D, Joshi S, Sah SP, Dev A, Genga B (2011). Chemical composition and antimicrobial activity of the essential oils of Senecioru finervis DC. (Asteraceae). Indian J. Nat. Prod. Resour. 2(1):44-47.
|
|
|
|
|
Nnodim JK, Nwanjo HU, Okolie NJ, Opara AU, Nwosu DC, OKoroiwu I, Dike J, Okorie H, Nwadike CN, Uduji HI (2013). Effects of Xylopia Aethiopica fruits on reproductive hormonal level in rats. Glob. J. Med. Plant Res. 1(1):29-31.
|
|
|
|
|
Okwu DE, Jossiah C (2006). Evaluation of the chemical composition of two Nigerian medicinal plants. Afr. J. Biotechnol. 5(4):357-361.
|
|
|
|
|
Prakasia PP, Nair AS (2015). Chemical fingerprint of essential oil components from fresh leaves of Glycosmis pentaphylla (Retz.) Correa. Pharma Innov. J. 3(12):50-56.
|
|
|
|
|
Somova LI, FO Shode, K Moodley, Y Govender (2001).Cardiovascular and Diuretic activity of Kaurene Derivatives of Xylopia aethiopica and Alepidea amatymbica. J. Ethnopharmacol. 77:165-174.
Crossref
|
|
|
|
|
Srinivasan K, Sivasubramanian S, Kumaravel S (2014). Phytochemical profiling and GCMS study of Adhatodavasicaleaves. Int. J. Pharma Bio Sci. 5(4):714-720.
|
|
|
|
|
Telascrea M, de Araújo CC, Marques MOM, Facanali R, de Moraes PLR, Cavalheiro AJ (2007). Essential oil leaves of Cryptocaryam andioccana Meisner (Lauraceae): Composition and intraspecific chemical variability. Biochem. System. Ecol. 35:222-232.
Crossref
|
|
|
|
|
Uzer, A., Ercag, E. and Apak, R. (2005).Selective spectrophotometric determination of TNT in soil and water with dicyclohexylamine extraction. Anal. Chim. Acta, 534:307-317.
Crossref
|
|
|
|
|
Yun-Jie, X., Jui-Hsin, S., Bo-Wei, C., Yen-Ju, T., Yang-Chang, W. and Jyh-Horng, S. (2013).Oxygenated ylangene-derived sesquiterpenoids from the soft coral Lemnalia philippinensis. Marine Drugs 11: 3735-3741.
Crossref
|
|