Generation of reactive oxygen species (ROS) in biological systems has been reported to be a significant cause of inflammatory and metabolic diseases. More recently, ROS and in a particular ozone has also been implicated in the conversion of cholesterol to atherogenic compounds, secosterol A, and upon aldolization to secosterol-B. Secosterol-A is uniquely produced by cholesterol ozonolysis, while secosterol-B can also be generated through the reaction of cholesterol with singlet oxygen. On the other hand, lipid oxidation reactions generate hydroperoxides, which upon catalytic and/or enzymatic decomposition yields lipid peroxide products of significant importance to tissue health. The mechanism of formation of potent oxidants like ozone in biological systems has not been clearly demonstrated, with only a theory: That antibodies catalyze oxidation of water by singlet oxygen to yield a trioxidic species, like hydrogen trioxide, as an intermediate in hydrogen peroxide formation while a recent hypothesis indicates that ozone could also be an intermediate in the aforementioned pathway and could be generated from biological molecules in the presence of singlet oxygen. Similarly, there is new information being generated concerning the involvement of antioxidants and amino acids in either termination or propagation of oxidative processes in mammalian systems. This review explores mechanisms of ROS/ozone generation in tissues, lipid peroxidation, cholesterol oxidation and highlight dietary management of non-communicable diseases with a focus on the roles of antioxidants and amino acids.
Non-communicable diseases; the global and regional perspective
Non-communicable diseases (NCDs), also known as chronic diseases are diseases that are not transferrable from person to person directly, but occur as a result of a combination of genetic, physiological, environmental and behavior factors (WHO, 2017). The main types of NCDs include cardiovascular diseases (like heart attacks and stroke), cancers, chronic respiratory diseases such as chronic pulmonary disease, and asthma and diabetes. NCDs are driven by forces such as rapid urbanization, globalization of unhealthy lifestyles and population ageing. These are characterized by unhealthy diets, lack of physical activity and exposure to tobacco smoke and alcohol abuse. They result in metabolic risk factors including high blood pressure, high blood sugar, elevated blood lipids and obesity that eventually lead to cardiovascular disease, the principal cause of premature deaths in low and middle income economies (WHO, 2017).
The disease burden caused by NCDs continues to weigh down the global health budget. NCDs claim millions of lives prematurely in low and middle income countries (WHO, 2005). The “Global Action Plan for NCDs 2013 to 2020”, aims at reducing premature deaths from NCDs through banning tobacco and alcohol advertising, promoting healthy diets, disease prevention and increased physical activity (WHO, 2015; Lichtenstein et al., 2006; Hill et al., 2009). It is shown that atherosclerosis and clinical events are related to modifiable risk factors, and that lowering levels of these factors that results in reducing the incidence of metabolic disease (Board, 2014) points to a breakthrough.
Dietary approaches and lifestyle have been demonstrated to be effective in decreasing cardiovascular morbidity and mortality risk (Lichtenstein et al., 2006; Hill et al., 2009). However, the role of antioxidants and dietary supplements needs sufficient evidence for their efficacy before promotion for use in management of cardiovascular disease (Hill et al., 2009). This is due to pro-oxidant activities under certain conditions while unproven clinical claims on supplements could compromise disease management. The close link between healthy diet and physical activity could play a key role in managing NCDs. This would require an understanding of the etiology and progression of such disease conditions mainly attributable to reactive oxygen species and the development of oxidative stress.
Reactive oxygen species: Generation and roles in vivo
Reactive oxygen species (ROS) are a group of compounds which are either beneficial or harmful to the body that produced endogenously or exogenously. They are generated through irradiation with ultraviolet (UV) light, X-rays, γ-rays and metal catalyzed reactions. In addition, they are also generated during tissue inflammation and mitochondrial reactions (Kunwar and Priyadarsini, 2011). In vivo, free radicals are produced continuously, and are highly reactive with affinity for lipids, proteins and nucleic acids (Sivanandham, 2011). Primary ROS generated within tissues are superoxide, peroxide and hydroxyl radical which have attracted research focus (Kunwar and Priyadarsini, 2011). High amounts of ROS are generated in the liver by cytochrome P450 2E1 (CYP2E1) after heavy alcohol exposure (Yonge and Cederbaum, 2008).
The mitochondrial respiratory chain generates most of the ROS owing to its over 80% utilization of all oxygen in-take by the body. Low concentrations of ROS are essential for gene expression, cellular growth, biosynthesis of molecules such as thyroxin, prostaglandin and stimulate growth, and development processes (Droge, 2002). Recently, scientific evidence indicates that ROS and lipid peroxidation (LPO) products have been shown to be capable of acting as signaling mediator and induce adaptive response up-regulate defense capacity, mainly through nuclear factor erythroid 2-related factor 2 (Nrf2)-Kelch (Niki, 2009; Higdon et al., 2012; Ito et al., 2010). Immune cells macrophages and neutrophils generate ROS for destruction of invading pathogens (Rosen et al., 1995).
However, the mechanism of bacterial damage in the phagosome owing to ROS remains unclear. Relevant targets of the phagocytic oxidative burst have still not been clearly identified. In addition, the inability to produce singlet oxygen in the laboratory at concentrations that are comparable to amounts in generated phagosome is yet to be overcome. According to Slauch (2011), the application of molecular and genetic tools available in salmonella could significantly surmount this challenge. Macrophages and neutrophils contain Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase which generates superoxide radical and hydrogen peroxide. The hydrogen peroxide in turn reacts with chloride to generate hypochlorite which ultimately destroys the pathogens.
The NADPH oxidase and the resulting ROS are critical for defense against diseases. Neutrophils additionally express myeloperoxidase (MPO) (Virani et al., 2008) which in the presence of 'heme' produces hypochlorous acid (HOCl) from hydrogen peroxide and chloride anion (Klebanoff, 2005). MPO a recognized biomarker of atherosclerosis (Nambi, 2005) catalyzes key reactions in normal host cell defenses, and in inflammation defense. It is secreted from activated phagocytes and is present in human artherosclerotic lessions, and low density lipoprotein recovered from human atheroma. The MPO oxidizes tyrosine to tyrosine radical in the presence of hydrogen peroxide. Neutrophils kill pathogens through cytotoxicity using either by HOCl or tyrosine radical (Heinecke et al., 1993).
Hydrogen peroxide in the presence of free iron or copper ions can yield hydroxyl radical by removing an electron from the metal ion (McCord, 2004). However, superoxide radical regenerates the metal ions back to their original form making them available to react with hydrogen peroxide. These two reactions account for most of the hydroxyl radical generation in tissues owing to the role of metal ions. The contribution of iron in these reactions is linked to any increases of free iron in cells implying that it directly promotes ROS generation and oxidative stress (Tsukamoto and Lu, 2001).
Oxidative stress
The imbalance between ROS and the systems’ ability to readily detoxify or repair resulting tissue damage leads to oxidative stress that occurs due to excessive generation of ROS or diminishing levels of antioxidants. Oxidative stress results in damage of cellular components like proteins, lipids and deoxyribonucleic acid (DNA), and is believed to have a role in pathogenesis of cancers, cardiovascular diseases, diabetes, atherosclerosis among others (Loscalzo, 2004; Lien et al., 2008; Sivanandham, 2011; Board, 2014; Mollazadeh et al., 2016). Oxidative stress may cause DNA fragmentation through activated endonucleases as a result of increased levels of calcium ions in cells leading to apoptosis (Zhivotovsky and Orrenius, 2011). Despite oxidative stress being a major cause of complications like in diabetics, Mollazadeh et al. (2017) recently demonstrated that pomegranate seed oil significantly decreased oxidative stress in tissues and mitochondrial fractions of diabetic rats and remarkably decreased glucose-induced toxicity, ROS levels and lipid peroxidation in H9c2 cell lines. In another study, Sadeghnia et al. (2017) demonstrated that alcoholic extracts of Terminalia chebula exhibited neuroprotection and oligoprotection aganist quinolinic acid induced oxidative stress via ROS.
Lipid peroxidation
Unsaturated fatty acids in foods and biological systems may react with oxygen or ROS, and become oxidized. LPO involves the autoxidation of unsaturated fatty acid esters and sterols. In foods, LPO may lead to development of rancidity, loss of essential fatty acids and formation of toxic compounds (Frankel, 1985). While, in pharmaceuticals emulsions, the presence of LPO in may initiate oxidative stress conditions in invalids leading to serious health challenges (Khanum and Thevanayagam, 2017). Increasing evidence indicates that lipid oxidation products have two faces just like ROS and reactive nitrogen species (RNS) (Niki, 2009; Higdon et al., 2012). Lipid peroxidation, which is the free radical-mediated lipid oxidation, has been implicated in pathogenesis of various diseases.
It induces disturbance of fine structure, alteration of integrity, fluidity, and permeability, and functional loss of bio-membranes, modifies low density lipoprotein (LDL) and high density lipoprotein (HDL) to pro-atherogenic and pro-inflammatory forms, and generates potentially toxic products (Niki, 2012). They also exhibit carcinogenesis and mutagenesis. Secondary products of LPO (reactive carbonyl compounds), modify proteins and DNA bases (Poli et al., 2008). Therefore, LPO has been implicated as the underlying mechanisms in numerous disorders and diseases such as cardiovascular diseases, cancer, neurological disorders and aging (Leonarduzzi et al., 2012; Negre-Salvayre et al., 2010). Model studies have suggested possible beneficial effects of LPO products including antitumor and physiological signaling messenger (Niki, 2012). Experimentally, increased LPO products have been detected in biological fluids and tissues from patients with these disease conditions as compared with healthy subjects (Niki, 2009; Sayre et al., 1997).
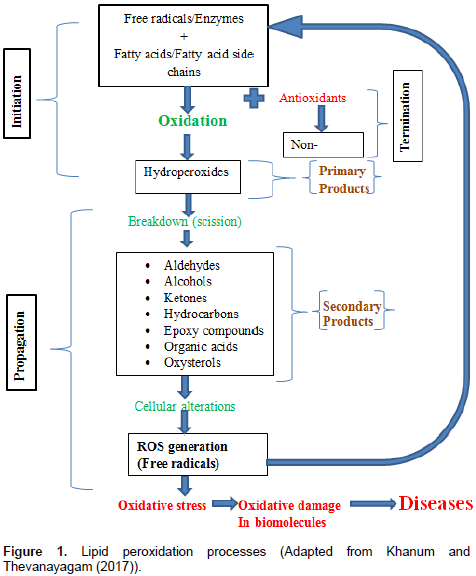
Recently, LPO products have been shown to be capable of acting as signaling mediator and induce adaptive response up-regulate defense capacity, mainly through nuclear factor erythroid 2-related factor 2 (Nrf2)-Kelch (Niki, 2009; Higdon et al., 2012; Ito et al., 2010). Radical scavenging antioxidants such as vitamin C and Vitamin E do not scavenge physiologically important signaling ROS such as hydrogen peroxide and super oxidase, nor will they inhibit enzymatic lipid oxidation. Therefore, these antioxidants may not be potent inhibitors of myeloperoxidase-mediated reactions (Davies, 2011). Even at high concentrations, these antioxidants may likely not impair physiological signaling by ROS/RNS and LPO products. LPO in foods and biological systems may occur by a free radical mechanism starting with initiation, propagation and ending with termination as shown in Figure 1 (Khanum and Thevanayagam, 2017).
Oxidative stress and chronic diseases
Increasing evidence indicates the role of oxidative damage in chronic diseases. Chen et al. (2007) observed that long-term exposure to ozone as an atmospheric pollutant led to significant correlation with increases in lipid peroxidation. According to the findings from this study, 8-isoprostane 98-iso-PGF was found to be a good biomarker of oxidative damage related to air pollution. Long et al. (2001) in another study, indicated that ozone induced inflammation and biomolecule oxidation in the lungs, whereas extracellular antioxidant levels were relatively unchanged. Plasma antioxidants like urate, ascorbate, glutathione (GSH) and vitamin E, defend the lungs by reacting with oxidizing agents, hence it was expected that they would decrease upon exposure to ozone, and an increase in F2-isoprostanes (lipid peroxidation products). Exposure to 3ppm of ozone for 6 h resulted in increase in Broncho alveolar lavage fluid (BALF) neutrophil which indicated inflammation and elevation of BLAF F2-isoprostanes (Long et al., 2001). Only higher doses of ozone were observed to cause elevation of urate but a decrease in ascorbate. However, there was no effect on other plasma antioxidants upon exposure to ozone (Long et al., 2001).
Ozone is a powerful oxidant which has wide application in medicine and dentistry (Kumar et al., 2014), food processing (O’Donnel et al., 2012) and industrial processes (Cook, 1982). In the atmosphere, the ozone layer prevents dangerous UV rays from reaching the earth’s surface (Loscalzo, 2004). However, it has been shown to cause damage to mucosa and respiratory tissues in animals and plant tissues even at low concentrations from 100ppb (Loscalzo, 2004). The exposure to ozone alone or as combination with other atmospheric pollutants like diesel exhausts has been observed to induce decrements in lung function (Madden et al., 2014). The exposure is elevated by physical activities like exercising which increases frequency and depth of breathing, hence exposing sensitive lung tissue to the pollutants.
Previously, Hackey et al. (1975) demonstrated that individuals with hyper reactive air waves developed decrements to their pulmonary functions when exposed to ozone than healthy volunteers. The discovery of endogenous generation of ozone in biological systems has raised a lot of interest to the possibilities of pathways involved and mechanisms of body protection from any adverse effects (Wentworth et al., 2003). This type of oxidant in biological systems could contribute towards the pathogenesis of inflammatory diseases. Currently, inflammation is considered to have a role in the increasing health conditions including autoimmunity, atherosclerosis and ageing related complications. This occurs when ozone cleaves to any compound that contains an alkene or olefin (an unsaturated hydrocarbon) like unsaturated lipids, or oxidized proteins. In addition, ozone reacts with other chemicals to generate more toxic and harmful materials such as hydrotrioxy and hydroxyl radicals. The resultant modified proteins from these reactions may be noted as foreign leading to an autoimmune response in addition to signal amplification of inflammation in tissues.
Ozone generation in the human body
The identification of the cholesterol ozonolysis product 3β-hydroxy-5-oxo-5, 6-secocholestan-6-al (secosterol A), whose aldolization produces 3β-hydroxy-5β- hydroxyl-B-norcholestane-6β-carboxaldehyde (secosterol-B) in human atherosclerotic plague and brain tumor tissue was cited as evidence for the endogenous ozone production in human tissues (Wentworth et al., 2003). The signature products that are unique to cholesterol ozonolysis within atherosclerotic tissues during carotid endarterectomy confirm ozone production during lesion development (Wentworth et al., 2003). In vitro activation of these atherosclerotic plagues generated steroids that possessed cytotoxicity, lipid-loading in microphages, and deformation of alipoprotein B, hence participated fully in pathogenesis of atherosclerosis (Wentworth et al., 2003). Further work by Brinkhorst et al. (2008) found that cholesterol-5-hydroperoxide (obtained by oxidation of cholesterol with singlet oxygen) underwent a Hock cleavage to form mainly secosterol B, and concluded that ozone may not be necessary for formation of the secosterols in vivo (Brinkhorst et al., 2008). However, it has been confirmed that secosterol A, the major secosterol in atherosclerotic plaques, is only a major product of ozone or ‘an oxidant with the chemical signature of ozone’ (Wentworth et al., 2009).
Mechanisms of ozone generation in biological systems
The generation of ozone or an oxidant with the chemical signature of ozone is still debatable with various research outputs differing on it. What has not been disputed is the generation of this potent oxidant. Despite the ongoing challenges on differing research hypotheses and outputs, the pathway through which this oxidant is generated has not been clearly established. The pathway through which ozone or ozone like oxidant is formed in the body can answer some of the unresolved research questions about this oxidant. In addition, this pathway can bring closer the utilization of this knowledge in disease prevention or management. The specificity of information could augment the photodynamic therapy for cancer treatment, and also frontiers in treatment of drug resistant parasitic infections like malaria. In biological systems, ozone formation has been proposed to occur through the water oxidation pathway where antibodies and /or amino acids act as the catalysts.
Water oxidation pathway
Hydrogen peroxide and ozone were hypothesized to be formed through the antibody-catalyzed water oxidation pathway. Antibodies catalyze the reaction between singlet oxygen and water to give hydrogen peroxide as a frontline in immune defense (Nieva and Wentworth, 2004). The antibody-catalyzed ozone formation results in high amounts of hydrogen peroxide (H2O2) (in excess of 500 H2O2 molecules) per antibody molecule (Wentworth et al., 2001; Wentworth et al., 2002). Peng et al. (2006) observed production of ozone and hydrogen peroxide from human leukemia THP-1 monocytes when incubated with human immunoglobulin G and phorbol myristate acetate. The ozone generated significantly inhibited accumulation of intracellular lipids through vinylbenzoic acid than by catalase. Following this path, it can be established that ozone is involved in the pathogenesis of atherosclerosis through the antibody-catalyzed water oxidation pathway more than hydrogen peroxide.
The reaction of water with singlet oxygen generates hydrogen trioxide as an intermediate (Wentworth et al., 2002), which theoretically either reacts further with singlet oxygen or with another hydrogen trioxide (Cerkovnic and Plesnicar, 2013). In addition, it has been demonstrated experimentally that under aqueous conditions, hydrogen trioxide is highly unstable and decomposes to singlet oxygen and water (Cerkovnic and Plesnicar, 2013). The formation of hydrogen peroxide and ozone through water oxidation pathway was proposed to occur at the hydrophobic site of the poly peptide, where hydrogen trioxide is shielded from water (Wentworth et al., 2001) however, up to date it has not been possible to detect the hydrogen peroxide and ozone from the decomposition of hydrogen trioxide (Cerkovnic and Plesnicar, 2013).
Alternative to the water oxidation pathway; the role of amino acid oxidation in the antibody catalyzed formation of hydrogen peroxide is not feasible owing to the high quantity of hydrogen peroxide formed per antibody molecule (Wentworth et al., 2001). However, four amino acids; methionine, cysteine, tryptophan and histidine were found to catalyze ozone formation in the presence of singlet oxygen (Yamashita et al., 2008). The common feature of these amino acids is that they are all photo-oxidizable (Wentworth et al., 2001) as evidenced through numerous studies both in free form and as components of proteins (Zhu et al., 2004; Pattison et al., 2012; Lundeen and McNeill, 2013; Sreethara et al., 2013; Amano et al., 2014; Liu et al., 2014).
The reactivity of these amino acids with singlet oxygen largely depends on their position in the protein as exposed residues are readily oxidized while the inaccessible residues remain un-oxidized (Lundeen and McNeill, 2013). Despite only a few amino acids being oxidized in the presence of singlet oxygen, they are able to generate adequate ozone and hydrogen peroxide (Sreethara et al., 2013). Therefore, it is possible to mention that protein molecules lacking these amino acids are not able to generate ozone. On the other hand, formaldehyde, cinnamic acid and resveratrol have been reported as precursors of ozone at molecular level (Tyihak et al., 2013). Tyihak hypothesized that formaldehyde reacts with hydrogen peroxide to generate activated formaldehyde and singlet oxygen. The singlet oxygen generated from this reaction participates in the water oxidation pathway to yield ozone (Tyihak et al., 2013).
Tomono et al. (2011) demonstrated that activated neutrophil-like differentiated human leukemia HL60 (nHL-60) cells cultured in a medium containing cholesterol significantly increased the levels of secosterol A production by myeloperoxidase-dependent generation of singlet oxygen. In a cell-free study, when singlet oxygen was produced in aqueous solutions of immunoglobulins, albumin and 19 amino acids (excluding tyrosine) by ultraviolet A (UVA) irradiation of 6-Formylpterin, formation of ozone occurred in the presence of the proteins and the four amino acids; methionine, histidine, tryptophan and cysteine. Ozone formation was evidenced by conversion of indigo carmine and vinylbenzoic acid to isatin sulfonic acid and 4-carboxybenzaldehyde, respectively (Yamashita et al., 2008).
In related studies, ozone generation by neutrophils was evidenced by the conversion of indigo carmine to isatin sulfonic acid (Wentworth et al., 2002; Babior et al., 2003). These indicators for ozone generation have been challenged when Kettle et al. (2004) demonstrated that superoxide generated an equivalent amount of isatin sulfonic acid from indigo carmine just as neutrophils. According to their findings, the bleaching of indigo carmine by neutrophils to isatin sulfonic acid cannot be used as an exclusive indicator to support ozone production in cells. However, Wentworth et al. (2003) showed that both ozone and neutrophils converted vinyl benzoate to 4-carboxybenzaldehyde as evidence for ozone formation. The oxidative burst of phagocytosing neutrophils due to reduced NADPH oxidase leads to formation of hypochlorous acid, singlet oxygen and hydroxyl radical. However, the antimicrobial activity of ROS is not well elucidated (Wentworth et al., 2002; Williams, 2006).
Wentworth proposed that neutrophils produce ozone which contributed to bacterial killing, where antibodies catalyze the production of ozone from singlet oxygen and water. The mechanisms still remains unclear. Kettle and Winterbourn (2005) challenged the validity of detection of ozone generated basing on their findings that superoxide converted indigo carmine to isatin sulfonic acid and vinyl benzoate to 4-carboxybenzaldehyde as evidence for ozone formation. There is growing evidence to support ozone generation in presence of singlet oxygen in vivo. A rare variant of chronic granulomatous disease (CGD); an inherited disorder where phagocytes are unable to kill certain bacteria and fungi produced significant amounts of singlet oxygen but very little superoxide and neutrophils provided a useful model to check oxidative burst (Aussel et al., 2011; Slauch, 2011).
It was found out that superoxide (SOD) mutant mice as compared with wild mice, singlet oxygen was consumed by some reaction that did not result in the production of hydrogen peroxide (Aussel et al., 2011). The results from this study clearly points to the validity of the existence of a potent oxidant responsible for the reactions. Findings from this study have since not been challenged implying that they could have provided compelling evidence to support ozone generation in vivo. On the other hand, MPO deficient mice failed to produce hypochlorous acid and singlet oxygen, and showed increased susceptibility to infections especially pneumonia and death when exposed to high doses of bacteria and fungi (Aratani et al., 2006).
The exposure of human neutrophils to large quantities of invading pathogens like E-coli in ratios of greater than 5:1, initiates amino acid catalyzed oxidant defense system with high bactericidal activity. This implies that ozone produced by neutrophils only swings into action when the host is challenged by high doses of infectious agents (Yamashita et al., 2008). It is worthwhile to consider pathways of singlet oxygen mediated amino acid and formaldehyde oxidation with the aim of identifying potential steps that could be involved in ozone formation.
Antibody catalyzed ozone generation by amino acids
Ozone generation was found to occur in a dose-dependent and at comparable levels to the immunoglobulins in the presence of four amino acids (Yamashita et al., 2008). Therefore, the residues of these amino acids could be responsible for the production of ozone by antibodies and other proteins. The side chains of all amino acid residues of tryptophan, cysteine and methionine are susceptible to ROS oxidation to result in carbonyls such as aldehydes and ketones. It had earlier been reported that formation of ozone (O3) and hydrogen peroxide (H2O2) by proteins occurred through a pathway that antibodies catalyze oxidation of water by singlet oxygen (1O2) to form trioxidic species like hydrogen trioxide , which then reacts with 1O2 to form O3 and H2O2 (Wentworth et al., 2002; Nyffeler et al., 2004). This hypothesis has been challenged owing to the fact that the high quantity of ozone and hydrogen peroxide formed cannot be fully accounted for through the water oxidation pathway, implying that other biological materials are involved.
On the other hand, methionine, histidine, tryptophan and cysteine easily react with singlet oxygen to form peroxides and other oxidation products (Min and Boff, 2002). In addition, 1O2 inactivates enzymes whose catalytic site contains cysteine or histidine residues, indicated a modification of these amino acid residues (Suto et al., 2007). The accelerated riboflavin-sensitized destruction of ascorbic acid in the presence of histidine and tyrosine suggesting that the intermediate reaction products of amino acid and 1O2 were responsible (Jung et al., 1995). Therefore, it is possible to note that ozone could be formed from intermediate products of the reaction of 1O2 with amino acids. Moreover, the fact that the sulfur-containing amino acids react with O3 to produce 1O2 (Kanofsky and Sima, 1991) points to the potential reversibility of O3 and 1O2 from common intermediaries.
A potential mechanism can be speculated for cysteine and methionine that alkyl sulfides react with 1O2 to form a nucleophilic peroxysulfoxide (RSOO-) (Jensen et al., 1998). Since 1O2 is electrophilic, (Min and Boff, 2002), it might easily react with the peroxysulfoxide to form a tetroxysulfoxide RSOOOO-, which could decompose to form ozone and a sulfoxide RSO. Notably, sulfoxides are major products of sulfide oxidation (Jensen et al., 1998; Min and Boff, 2002). The sulfoxide can behave as a nucleophile and thus react with 1O2 to form RSOOO-, which could decompose to form ozone and regenerate the sulfide. In this way, many molecules of ozone could be generated from a single methionine molecule, which is consistent with the results of Yamashika et al. (2008).
Interestingly, it has been reported that the products of O3 reacting with methionine are methionine sulfoxide and 1O2 (Mudd, 1998), and this is likely to form RSOOO- as an intermediate. This intermediate product may therefore be involved in the conversion of 1O2 to O3 and vice versa, depending on the concentrations of either oxidant. Ozone readily absorbs UV radiation at 254 nm producing H2O2 as an intermediate (Munter, 2001) in studies where singlet oxygen is generated by irradiation of amino acids or antibodies. The loss of cyclooxygenase activity by endothelial cells due to formation of H2O2 in presence of O3 (Madden et al., 1987) implies that O3 may also be converted to H2O2 by a mechanism not involving irradiation.
Mechanism of action
Ozone and hydrogen peroxide combine to form peroxone, a potent bacterial and viral inactivator (Merenyi et al., 2010). The mechanisms of ozone in neutralizing microorganisms have focused on the oxidation of bacterial lipids and proteins found in bacterial cell membranes and viral envelope, phospholipids, cholesterols and glycoproteins. At molecular level, it has been shown that ozone still performs the same fundamental function.
Endogenous and exogenous management of ROS related health conditions
The body can protect itself against oxidative damage through endogenous or exogenous antioxidants, which even at low concentrations can significantly delay or prevent oxidation in tissues (Kohen and Nyska, 2002). The exogenous use of antioxidants through food or supplements to counter oxidative stress is worth exploring. Antioxidants protect the cells against adverse effects of ROS by terminating the chain reaction before vital molecules are damaged through scavenging free radicals or repair of damaged molecules (Loscalzo, 2004; Zhivotovsky and Orrenius, 2011). Diets rich in fruits and vegetables have been associated with lower cancer rates leading to various theories that their antioxidant content has protective effect against cancer development. Clinically, non-steroidal anti-inflammatory drugs have been demonstrated to inhibit the generation of hypochlorous acid (a ROS) thereby, suppress the oxidative functions of neutrophils (Paino et al., 2005).
On the other hand, among obese individuals, the contribution of overweight on the incidence of metabolic and inflammatory disease cannot be over emphasized. Obesity being an independent factor for cardiovascular disease (CVD) and insulin resistance in diabetics is critical in health management. Primarily, the target of managing CVD is lowering of low density lipoprotein cholesterol (LDL-C) through adoption of a therapeutic lifestyle change diet characterized by weight loss and increased physical activity (Hill et al., 2008). Both obesity and metabolic syndrome (MetS) are associated with higher levels of C-reactive proteins (CRP) and insulin resistance as key biomarkers and independent predictors of CVD events.
In, obesity elevated CRP and insulin resistance may impede the lipid lowering effects of dietary interventions. Weight loss has been shown to be a successful strategy to reduce CRP and increase insulin sensitivity, but the effects of different macronutrients on inflammation are largely unknown (Hill et al., 2008). Bo et al. (2006) demonstrated that type 2 diabetes mellitus (DM), Mets, and inflammation were linked to reduced magnesium and fiber intakes, and these associations were reduced by adjustments for each of these nutrients. The prevalence of DM, Mets and highly sensitive C-reactive protein (hs-CRP) > 3mg/L significantly reduced with increases in magnesium and fiber intake. Low magnesium and fiber intakes were linked to hs-CRP > 3mg/L in the entire population under study (Bo et al., 2006). Therefore, high fiber diets that are rich in magnesium could be ideal for reduction of these risk factors across populations.
Despite the mixed evidence of the relationship between fiber intake and control of diabetes, Post et al. (2012), evaluated this relationship and demonstrated that fiber supplementation for type 2 DM can reduce fasting blood glucose and glycosylated hemoglobin in patients with type 2 DM. Increasing dietary fiber intake for diets for diabetic mellitus patients could be beneficial for disease management. On the other hand, weight loss and changes in macronutrient content of diets constitute two main approaches in managing insulin resistance according to Reaven (2005). Weight loss enhances insulin sensitivity among the obese and overweight individuals with insulin resistance, while changes in macronutrient content of diets manages adverse effects of compensatory hyperinsulinemia.
The slow and continuous release in the gut of the dietary fiber bound antioxidants influences the health benefits to the host in disease prevention and management (Vitaglione et al., 2008). Dietary patters containing fiber rich foods may offer a protective role in managing diabetes mellitus (Maghsoudi and Azadbakht, 2012). The ‘healthy’, ‘Mediterranean’, ‘prudent’ and dietary approach to stop hypertension diets were associated with lower risk of hyperglycemia. Separately, Du et al. (2010) observed that higher intake of cereal fiber helped the prevention of body weight and waist circumference gain. Similar results have been recorded in dietary fiber being inversely associated with insulin levels, weight gain and other risk factors for cardiovascular disease CVD in young adults (Ludwig et al., 1999; Rimm et al., 1996; Ascherio, et al., 1996).
This was also collaborated with follow up studies, like 10 years after initial studies. Interestingly, the fiber type (for example, soluble or insoluble), source (for example, whole grain, refined grain, vegetable or fruit), or form (for example, intact, or processed) was not examined. These variables in addition to other biologically active constituents like magnesium and vitamin E may affect insulin response to ingested carbohydrates as well as CVD risk in significant ways. Shai et al. (2010) observed that low weight induced by low fat, Mediterranean and low carbohydrate diets over a period of time resulted in significant reduction in coratid atherosclerosis. Atherosclerosis develops over several decades beginning in youthful years of individuals. It is believed that lipid retention, oxidation and modification provoke chronic inflammation at susceptible sites on the arterial walls (Insull, 2008).
Hypertension, diabetes mellitus, obesity, genetic disposition and smoking risk factors accelerate development of atherosclerosis. Although inevitably being a progressive disease, clinically, atherosclerosis can be treated by 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) (Insull, 2008). Clinical use of non-steroid anti-inflammatory drugs has been shown to inhibit hypochlorous acid generation hence suppress oxidative functions of neutrophils (Paino et al., 2005). Clinically, ozone therapy has been successfully applied in the treatment of spinal disk herniation as compared to surgical procedures (Bocci, 2005; Bocci et al., 2015). Despite nano-medicine being known for anti-cancer therapy, it’s potential in clinical diagnosis, and treatment of atherosclerosis has been demonstrated (Lobatto et al., 2011).
The role of some tested antioxidants in quenching reactive oxygen species
The body protects itself against oxidative damage through endogenous or exogenous antioxidants. Antioxidants are classified as either enzymatic (protective) and non-enzymatic. Enzymatic antioxidants act as the first line of body defense against ROS by converting them to less reactive species. They include superoxide dismutase (SOD), catalase and glutathione peroxidase. Secondary defense against generation of ROS is through non-enzymatic antioxidants like alpha-tocopherol, glutathione and ascorbate which scavenge free radicals or chelate metal ions like iron and copper (Seifried et al., 2007). Ozone has been shown to react with biomolecules apart from cysteine and methionine, and the cysteine-containing glutathione, other biomolecules not containing amino acids, such as uric acid, ascorbic acid, NADH and NADPH to form singlet oxygen (Kanofsky and Sima, 1991).
Ascorbic acid
Ascorbic acid has been reported to promote the decomposition of linoleic acid hydrogen peroxides to genotoxic aldehydes such as 4-oxo-2-nonenal and 4-hydroxy-2-nonenal (Lee et al., 2001), and has also been shown to produce hydroxyl and alkoxyl radicals in the presence of active metals (iron and copper) ions thereby increasing oxidative damage (Jansson et al., 2003). In the stomach, ascorbic acid was found to exhibit pro-oxidative properties in the presence of ferrous ions (Kanner, 2007). At high concentrations, ascorbic acid exhibit pro-oxidative effects in blood cell from healthy donor, as evidenced by ROS and interleukin-6 (IL-6) production (Oliviera et al., 2012). Ascorbic acid reacts with O3 to produce 1O2 (Kanofsky and Sima, 1991), and reacts with 1O2 to produce H2O2 as one of its products (Mudd, 1998). In plant cells, ozone challenges the antioxidant protection in the extracellular matrix (Baier et al., 2005). Conklin and Barth (2004) found sensitivity to ozone correlated with ascorbate status of the leaf.
Glutathione
Glutathione (GSH) being a three amino acid peptide effectively neutralizes ROS in cytosol and cell organelles. In the presence of ROS, GSH is oxidized to glutathione disulphide (GSSG), which reverts back to GSH by glutathione reductase. The hydrogen molecule donor is the sulfhydryl residue of cysteine amino acid in the peptide (Aw, 1997). In addition, the neutralization of H2O2 to water in the glutathione-ascorbate cycle by glutathione and ascorbic acid (Noctor and Foyer, 1998) points to added value in reducing ROS.
Uric acid
Uric acid is the most abundant antioxidant in body fluids (Inoue et al., 2003), and estimated to possess 60% of antioxidant capacity of plasma antioxidants (Benzie and Strain, 1996). It has been detected at high concentrations in liver and lungs under oxidative stress (Glantzounis et al., 2005), and has been found to inhibit formation of toxic nitric oxide in the stomach (Pietraforte et al., 2006). Additionally, uric acid, ascorbic acid and glutathione have been confirmed to react with O3 (Kermani et al., 2006). Uric acid reacts with O3 to produce high amounts of singlet oxygen (Kanofsky and Sima, 1991) and intermediate products. In the presence of metal ions, and depending on the extent of oxidative reactions, uric acid has been reported to exert both antioxidant and pro-oxidant activities (Bagnati et al., 1999).
Curcumin
Curcumin, a powerful antioxidant found in turmeric, has been shown to have antimicrobial and anti-inflammatory activities especially when it is irradiated with UV light (Aggarwal and Sung, 2009). Irradiation of curcumin produces singlet oxygen however it is equally a powerful quencher of singlet oxygen (Das and Das, 2002). It remains to be demonstrated whether the enhanced antimicrobial activity of irradiated curcumin is merely due to singlet oxygen generation or the formation of ozone by reaction of singlet oxygen with curcumin.
Alpha tocopherol
Alpha-tocopherol found in vegetables and fish oil has been found to inhibit lipid oxidation by affecting the pathway of lipid hydro peroxides. However, α-tocopherol at high concentrations has pro-oxidant effect such as increased low-density lipoprotein (LDL) oxidation due to tocopheryl radicals (Upston et al., 1999). Being a powerful quencher of 1O2 (Kim et al., 2009) oxidized α-tocopherol has pro-oxidant properties due to tocopheryl radicals, hydroxyl radical and 1O2 (Kim et al., 2007). There is growing interest in non-enzymatic cholesterol oxidation due to the fact that the resulting oxysterols could be used as non-invasive markers of oxidative stress in vivo (Miyoshi et al., 2014). Singlet oxygen and ozone are the non-radical molecules involved in non-enzymatic oxidation of cholesterol.
The reaction of ozone with cholesterol is very fast at the 5, 6 –double bond to yield 1, 2, 3-trixolane, which decomposes to 3b-hydroxy-5-oxo-5,6-secocholestan-6-al (secosterol A or 5,6-secosterol) resulting from cleavage of the B-ring and the aldolation product secosterol B. These two have been proposed as specific marker of ozone-associated tissue damage and ozone production in vivo (Miyoshi et al., 2014). However, secosterol A and B can also be generated from singlet oxygen through the Hock cleavage of 5α-hydroperoxy cholesterol or via dioxietane intermediate. Since seco A and B are generated via non-enzymatic routes in vivo, they are ideal biomarkers to indicate oxidative stress pathways and assist in development of pharmacological agents (Miyoshi et al., 2014). In addition, cholesterol oxidation LPO products such as hexanal have been detected in breath of lung cancer patients, which indicates a role as non-invasive determination of inflammation in vivo (Fuchs et al., 2010).
Mechanisms of formation of atherogenic aldehydes
Ozone reacts with cholesterol to form secosterol A, while singlet oxygen reacts with cholesterol to form cholesterol 5-hydroperoxide, which easily undergoes Hock cleavage under acidic conditions to form secosterol B. Both secosterol A and B are active contributing to atherogenesis by different mechanisms. Atherogenic lesions are characterized by accumulation of LDL through ozone oxidation. The high lipid hydroperoxide concentration, thiobarbituric acid reactive substances, relative electrophoretic mobility (REM) and oxidation-specific immune isotopes characterized LDL oxidation by ozone. The lipid portion of LDL was oxidized first followed by the protein portion rendering it atherogenic (Horl et al., 2014).
The discovery of the formation of stable complexes of glycyrrhizic acid with products of cholesterol oxidation points to a new frontline in struggle against atherosclerosis (Glushchenko et al., 2011). There is therefore drive into increasing knowledge in understanding pathogenesis, management and possible treatment of arterial disease conditions including dietary interventions. Given the fact that the role of antioxidants in slowing down the ageing process and prevention of CVD is not comprehensively conclusive, it is therefore important to study them in relation to oxidative stress and any resultant atherogenic compounds. The effects of antioxidants on the formation of secosterol A and B depend on the quenching of singlet oxygen and ozone, respectively. Since the quenching of singlet oxygen may lead to production of ozone and vice versa, it is important to determine the effects of antioxidants on the formation of both secosterols simultaneously.