ABSTRACT
The study evaluates the sub-acute toxicity and antioxidant potential of ethanolic leaf extract of Cymbopogon citratus against CCl4-induced toxicity in Sprague Dawley rats. The ethanolic leaf extract of C. citratus was prepared by solvent maceration method. The phytochemicals present in the extract were determined using standard methods. The potential sub-acute toxicities were evaluated using OECD procedure. The sub-acute toxicity of the extract at the doses of 125, 250 and 500 mg/kg, b.wt. was administered orally for 28 days. Another sets of rats were made hepatotoxic by orally administered with CCl4 (20% CCl4 in olive oil) twice per week for a period of five weeks. They were treated with C. citratus extract (300 and 600 mg/kg body weight) once a day for 35 days. Biochemical parameters were used to assess the hepatoprotective effects of the extract on liver tissues. Phytochemical screening of C citratus shows the presence of anthraquinones, alkaloids, flavonoids, etc. The administration of C. citratus is not hematotoxic and significantly reduced (P<0.05) elevated liver biomarker enzymes, urea, creatinine and the level of malondialdehyde. Treatment with the extract was found to significantly increase (P<0.05) TP level, the activities of superoxide dismutase and catalase. Liver histopathology shows that the extract reduced the incidence of liver lesions induced by CCl4. The administration of C. citratus did not produce any toxic effects in the sub-acute study. The plant exhibits potent protective effects in CCl4-induced liver damage due to decrease in liver biomarker enzymes activities, increase of antioxidant-defense system and inhibition of lipid peroxidation.
Key words: Sub-acute toxicity, protective effects, Cymbopogon citratus, carbon tetrachloride, hematological, oxidative stress parameters.
Cymbopogon citratus is prominent and commonly used in alternative medicine for the treatment of diverse ailments. C. citratus is a tropical monocotyledonous hypogeal perennial herb belonging to the family Poaceae and is commonly known as lemon grass. Several bioactive compounds have been reported to be isolated from the plant. The oil from C. citratus plant is used as culinary flavoring, scent, and medicine. Citronelle compound obtained from C. citratus, acts as an antihypertensive agent by inducing vasodilatation of vascular smooth muscles (Bastos et al., 2010; Chitra et al., 2012). Furthermore, citral obtained from the plant has been shown to possess activities like antiproliferative effect against Trypanosoma cruzi (Santoro et al., 2007), antiparasitic effects against leishmaniasis (Santin et al., 2009; Oliveira et al., 2009), anti-mutagenicity (Vinitketkumnuen et al., 1994) and antinociceptive (Viana et al., 2000). C. citratus effectively treats fever, infection, headaches, rheumatic pain, nervous and digestive disorders. The plant also acts as a sedative, antispasmodic, analgesic, and anti-inflammatory agent (Naik et al., 2010; Figueirinha et al., 2010). In Nigeria, lemon grass is used to treat fever, jaundice, hypertension, diabetes mellitus and obesity (Adeneye and Agbaje, 2007).
Hepato-toxicity is a method used in animal model, for liver damage investigation for screening the hepato-protective activity of natural medicinal plant. The use of natural products for liver diseases is growing because of their safety and efficacy as an alternative remedy compared with chemically synthesized drugs (Natanzi et al., 2009). Histo-pathological changes in liver tissue; activities of alkaline phosphatase (ALP), gamma glutamyltransferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH); levels of malondialdehyde (MDA), reduced glutathione (GSH) and other related parameters are used to assess liver toxicity and the hepato-protective activity of medicinal plants (Kumar et al., 2009; Uboh et al., 2012).
Liver helps in detoxification of drugs, exogenous toxins and therapeutic agents; it also helps in the bio-regulation of amino acids, proteins, carbohydrates, fats, blood coagulation and immunomodulation (Juza and Pauli, 2014). Impairment of the liver generally occurs from excessive exposure to toxicants, chemotherapeutic agents, alcohol, protozoan and viruses (Juza and Pauli, 2014). Experimental model used to induce liver damage in animals is by using carbon tetrachloride (CCl4). CCl4 is activated by cytochrome (CYP) 2E1, CYP2B1 or CYP2B2 and possibly CYP3A, to form the trichloromethyl radical (CCl3−) (Slater, 1984). This radical can bind to cellular molecules (protein, lipid, nucleic acid), impairing crucial cellular processes such as lipid metabolism, which results in fatty acid degeneration (steatosis) (Raucy et al., 1993). CCl3− forms adducts with DNA, which initiate the onset of hepatocellular carcinoma. This radical can also react with oxygen to form the trichloromethylperoxy radical CCl3OO−, which is a highly reactive species. The substance (CCl3OO−) reacts with polyunsaturated fatty acids and phospholipids to initiates the chain reaction of lipid peroxidation reaction.
This affects the permeabilities of mitochondrial, plasma membranes and endoplasmic reticulum resulting in the loss of cellular calcium sequestration and homeostasis, which may contribute heavily to subsequent cell damage (Weber et al., 2003; Mehendale et al., 1994). CCl4 intoxication is mediated by two types of nonparenchymal liver cells, viz., Kupffer and stellate cells. The activation of Kupffer cells by CCl4 mediate inflammatory processes via the nuclear factor kappa B (NF-kB) signal transduction pathway with production of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6(IL-6) and other inflammatory mediators; cyclooxygenase-2 (Cox-2) and inducible nitric oxide synthase (iNOS) (Gallucci et al., 2000; Gruebele et al., 1996), which in turn causes full activation of the mitogen activated protein kinase (MAPK)/extracellular signal-related kinase (ERK) and the Janus kinase (Jak)-signal transducer and activator of transcription protein (STAT) pathway. These pathways are involved in the regulation of cell proliferation and apoptosis (Bak et al., 2016). Stellate cells are normally quiescent and fat-storing cells, but after activation by agents like CCl4, they display a typical acute-phase response (Nieto et al., 2000), take on a fibroblast like appearance, release nitric oxide, begin to overproduce type-I collagen and thus promote hepatic fibrosis (Lee et al., 1995).
The efficient potency of C. citratus on free radical scavenging and other reactive oxygen species and antioxidation ability led us to evaluate the sub-acute toxicity and the protective effect of ethanolic leaf extracts of C. citratus on carbon tetrachloride-induced liver damage in male Sprague Dawley rats.
Collection and identification of plant material
The leaves of C. citratus were obtained from Ikorodu in Lagos State, Nigeria. The plant was authenticated by a botanist from the department of Botany, University of Lagos, Lagos, Nigeria.
Authentication number for C. citratus was given (6946).
Preparation of ethanolic leaf extract of C. citratus
The leaves of C. citratus were washed, air dried under shade in the Biochemistry Laboratory, pulverised to coarse power using blender. Extraction was carried out by dispersing 200 g of the ground C. citratus plant material in 1 L of 90% ethanol and shaking was done with GFL shaker for 72 h. This was followed by vacuum filtration and concentrated by rotary evaporator at a temperature not exceeding 40°C. The concentrated extract was dried to complete dryness in an aerated oven at 40°C for 48 h. The extract was later stored in a refrigerator at 4°C.
Phytochemical analysis of ethanolic leaf extract of C. citratus
Phytochemical tests for bioactive constituents were carried out on small portions of the plant extract using standard phytochemical procedures (Trease and Evans, 1986; Sofowora, 1993; Kokate, 1994).
Experimental animals
A total of 70 male Sprague Dawley albino rats with body weight ranging from 200 to 220 g were obtained from Ratzmattazz Nigeria enterprises, 21 insurance estate satellite town, Lagos, Nigeria. They were acclimatized for two week to laboratory condition of 23±2°C. They were kept in plastic cages and fed with commercial rat chow and supply with water ad libitum. The rats were used in accordance with NIH Guide for the care and use of laboratory animals; NIH Publication revised (2011).
Sub-acute toxicity test
The sub-acute toxicity test was conducted in accordance with the guidelines published by the Organization for Economic Cooperation and Development (OECD, 2007) No. 407 with slight modification. At the onset of dosing, the rats weighed 210 ± 10 g each. Twenty eight acclimatized rats were grouped into four groups. Each group contains seven animals. Group I served as the positive control group and received distilled water, for 28 consecutive days, while the other groups (II, III and IV) received a daily amount of 125, 250 and 500 mg/kg b.wt. of ethanolic leaf extract C. citratus orally, for 28 consecutive days, respectively. Food and water intake were given freely. After 28 days of the feeding trial, the rats were fasted overnight (for at least 20-24 h) before they were sacrificed.
Body weight determination
The individual body weights of all animals were recorded weekly (7 days interval) during the course of the sub-acute toxicity study. The body weights were also recorded prior to testing and terminally (after fasting) prior to when they were sacrificed.
Administration of CCl4
Male albino rats (Sprague Dawley) of about sixteen weeks old with weight range of 200 to 220 g were made hepatotoxic by orally administered with CCl4 (20% CCl4 in olive oil) dosage of 1 ml/kg body weight twice per week for a period of five weeks according to the method described by Momoh et al. (2018a). The animals were all treated once per day according to the grouping of the animals as shown in the following. Forty two acclimatized rats were grouped
into six groups. Each group contains seven animals as follows: Group A-Normal control; Group B-Negative control (CCl4 without treatment); Group C-Positive control (CCl4 + 100 mg/kg b.wt. silymarin); Group D-Olive oil only; Group E-CCl4 + 300 mg/kg b.wt. of C. citratus leaf extract; Group F-CCl4 + 600 mg/kg b.wt. of C. citratus leaf extract.
Collection of blood samples
All the albino rats were sacrificed by cervical decapitation after 20-24 h fasting. Blood was collected from the albino rats by ocular puncture into EDTA tubes for hematological analysis and the remaining blood was collected in heparinised tubes and centrifuge at 3000 rpm for 20 min and the plasma stored at -20°C to estimate biochemical parameters. The animals were dissected while their livers and kidneys were excised for biochemical and histological examinations.
Determination of hematological parameters
The hematological parameters were determined in the whole blood using BC-3200 Auto Hematology Analyzer in University of Lagos Teaching Hospitals (LUTH) in Idi-Araba, Lagos, Nigeria. The hematological parameters investigated were as follows: White blood cell count (WBC), Monocyte number (Mid#), Monocyte percent (Mid%), Granulocyte number (Gran#), Granulocyte percent (Gran%), Lymphocyte number (Lym#), Lymphocyte percent (Lym%), Hemoglobin (HGB), Red blood count (RBC), Hematocrit (HCT), Mean cell volume (MCV), Mean corpuscular hemoglobin (MCH), Mean corpuscular hemoglobin concentration (MCHC), Red Blood Cell Distribution Width Coefficient of Variation (RDW-CV), Red Blood Cell Distribution Width Standard Deviation (RDW-SD), Platelet count (PLT), Mean platelet volume (MPV), Platelet Distribution Width (PDW) and Plateletcrit (PCT).
Measurement of plasma liver biomarker enzymes and lipid profile
Liver damage was assessed by the estimation of plasma activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), total protein (TP), total cholesterol (TC), triglyceride (TG), high density lipoprotein-cholesterol (HDL-Chol), were measured using commercially available test kits from Randox Laboratories Ltd. (UK). LDL- Cholestrol was calculated according to Momoh et al. (2018b). LDL-C=TC - HDL-C - TG/5. Kidney damage was assessed using urea and creatinin Randox kits.
Hepatic antioxidant activities
Preparation of liver homogenate
The liver tissues of some of the sacrificed albino rats were excised and the liver samples were cut into small pieces and homogenized in phosphate buffer saline (PBS) to give a 10% (w/v) liver homogenate. The homogenates were then centrifuged at 12,000 rpm for 50 min. The supernatant obtained was later used for assay of thiobarbituric acid reactive substances (TBARS) content, superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and reduced glutathione (GSH).
Determination of lipid peroxidative (LPO) indices
Lipid peroxidation as evidenced by the formation of TBARS was
measured in the homogenate by the method of Jiang et al. (1992).
Determination of superoxide dismutase (SOD)
The SOD activity was estimated by its capacity of inhibiting pyrogallol autooxidation in alkaline medium. The liver homogenate was assayed for the presence of SOD by utilizing the technique described by Zou et al. (1986).
Determination of catalase (CAT)
The liver homogenate was assayed for catalase colorimetrically at 620 mm and expressed as μmoles of H2O2 consumed/min/mg protein by the method of Rukkumani et al. (2004).
Determination of reduced glutathione (GSH)
Reduced glutathione (GSH) was determined in the liver homogenate using the method of Rukkumani et al. (2004).
Determination of glutathione peroxidase (GPx)
Reduced glutathione (GSH) was determined in the liver homogenate using the method of Rukkumani et al. (2004).
Histopathological studies
The histopathological analyses were assayed in the Department of Anatomy, College of Medicine, University of Lagos, Idi-Araba, Lagos, Nigeria. The albino rats were sacrificed and their abdomens were cut open to remove their liver and kidney. Some of the organs were fixed in Boucin’s solution (mixture of 75 ml of saturated picric acid, 25 ml of 40% formaldehyde and 5 ml of glacial acetic acid) for 12 h, and then embedded in paraffin using conventional methods (Galighor and Kozloff, 1976). They were cut into 5 μm thick sections and stained using haematoxylin-eosin dye and finally mounted in di-phenyl xylene. The sections were then observed under microscope for histopathological changes in the liver and kidney architecture and their photomicrographs were taken.
Data analysis
The results were calculated and expressed as Mean ± Standard deviation. Data analyses were done using the GraphPad prism computer software version 5.01. One-way analysis of variance (ANOVA) was used for comparison for determining the significant difference. The inter group significant was analysed using Posthoc Turkey’s and Bonferroni’s multiple comparison test. A P-value < 0.05 was considered significant.
Phytochemical screening of ethanolic leaf extract of C. citratus
Phytochemical screening of ethanolic leaf extract of C. citratus shows the presence of secondary metabolite like
tannins, steroid, anthraquinones, triterpenoids and saponin (Table 1).
Sub-acute toxicity study
Clinical observations and survival of animals administered with C. citratus
The study shows no mortalities were recorded in the rats over the period of 28 days of treatment with C. citratus leaf extract at the doses of 125, 250 and 500 mg/kg, b.wt., through oral gavage. None of the animals after administration of C. citratus at the doses of 125, 250 and 500 mg/kg, b.wt., showed any obvious morbidity or clinical symptoms of toxicity such as changes in the eyes, skin and fur, autonomic (salivation, perspiration and piloerection), stereotype activities and respiratory rate problem throughout the experimental period of 28 days.
Body weight determination of experimental animals
The body weight of the animals administered with the plant extract were recorded at an interval of 7 days over the treatment period of 28 days and there were significant increase (P<0.05) in the body weight of the animals administered with the plant extract at different concentrations when compared with the healthy control group (Figure 1). The increase in the body weight for all groups was mostly dose dependent as a greater increase in body weight was observed in high dose group.
The effect of C. citratus ethanolic leaf extract on liver biomarker enzymes and lipid profile in male albino rats
There were significant reduction (P<0.05) in AST activity, LDL-Chol and creatinine levels in animals administered C. citratus extract (groups III and IV) compared to group I animals. ALT and GGT activities, TC and TG levels did not show any significant different (P>0.05) in all the rats administered C. citratus extract for the sub-acute toxicity test when compared with the non-treated animals (group I). The plasma total protein (TP) concentration and HDL-Chol was significant increased (P<0.05) in the treated group (groups II-IV) animals compared to group I animals (Table 2).
Sub-acute toxicity test
The oral administration of C. citratus leaf extract (125, 250 and 500 mg/kg b.wt.) in sub-acute toxicity study showed no toxic sign or death of rats after 28 days. Animals administered C. citratus extract showed significant increase (P<0.05) in catalase (group III), GPx (group IV) SOD% (group III and IV), SOD units (groups II - IV) and TP (groups III and IV) in their liver homogenate while MDA values reduces significantly (P<0.05) in groups II to IV animals when compared with group I rats (Table 3).
Sub-acute histological study
The histological study for the kidney and liver are as shown in Figure 2.
Hematological analysis
Table 4 shows that there were significant increase (P<0.05) in WBC, Mid#, Mid%, Gran%, Gran#. MCH, HGB, HCT, MCHC, RBC and their Lymph# and Lymph% were significantly lowered (P<0.05) in the animals treated with C. citratus leaf extract compared to the animals administered with CCl4 without treatment. The animals in group A showed significant increase (P<0.05) in Lymph#, MCV and decrease (P<0.05) in Mid%, Gran%, HGB, MCH, and PLT when compared with animals administered with C. citratus extract (Table 4).
Analysis of liver biomarker enzymes and lipid profile
There were significant (P<0.05) increase in liver biomarker enzymes (AST, ALT, ALP and GGT), urea and creatinine in group B untreated animals compared to all other animals in other groups. Group B animals also have lower level of TP value compared to healthy animals (group A) and animals administered with C. citratus extract (Table 5).
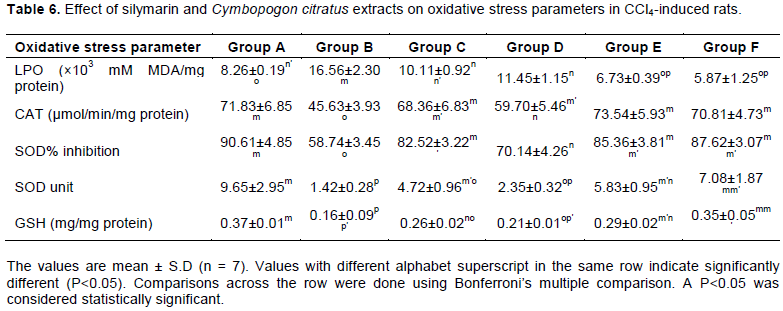
Determination of oxidative stress parameters
Oxidative stress parameters (SOD% inhibition, SOD unit, CAT and GSH) were significantly (P<0.05) reduced in animals administered with CCl4 without treatment compared to the control group animals (group A) and animals treated with C. citratus extract. The MDA values of group B rats were significantly (P<0.05) increased compared to other groups (Table 6).
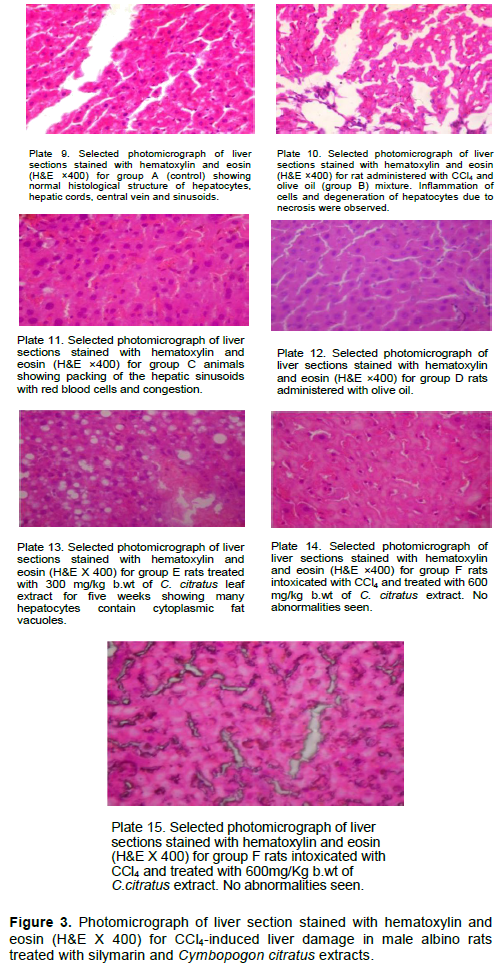
Histopathological studies
The liver architecture of the healthy animal, animal infected with CCl4 without treatment and animals treated with C. citratus extract are as shown in Figure 3.
Phytochemical screening of ethanolic leaf extract of C. citratus shows the presence of secondary metabolite like flavonoids, alkaloids, tannins, steroid, anthraquinones, triterpenoids, saponin, etc. (Table 1). The presence of these secondary metabolites in C. citratus may be responsible for the antioxidant and protective properties of the plant. Studies have shown that reactive oxygen species (ROS) are not only responsible for oxidative stress at low levels, they are also considered to play an important role in normal cell physiological functions, acting as modulators of redox regulated processes (Droge, 2002; Schreck and Baeuerle, 1991). These ROS are continuously produced during normal physiologic events and normally removed by antioxidant defence mechanisms (Zorov et al., 2006; Chen et al., 2006). Plants are potential sources of antioxidants, since synthetic antioxidants have side effects when consumed in vivo (Ghasemzadeh and Ghasemzadeh, 2011). Polyphenols (total phenolic, flavonoids and proanthocyanidin contents) are the major plant compounds with antioxidant activity. This antioxidant activity is believed to be mainly due to their redox properties (Zheng and Wang, 2001), which play an important role in adsorbing and neutralizing free radicals, decomposing peroxides, quenching singlet and triplet oxygen. The results from this study strongly suggest that phenolics are important components of these plants, and some of their pharmacological effects could be attributed to the presence of these important secondary metabolites.
General behavioral changes in body weight are preliminary indicators of early signs of toxicity caused by various drugs and chemicals (Ezeja et al., 2014). The body weight of the animals administered with C. citratus extract increases significantly (P<0.05) when compared with the control group and was considered normal. Thus, it can be concluded that C. citratus oral administration did not produce any major clinical toxicological signs and did not affect the normal growth pattern of the animals throughout the treatment period of 28 days.
In toxicity rating by joint FAO/WHO Expert Committee on Food Additives (WHO,1966), if at 2 g/kg oral dose of a substance causes no death, it is sufficient to assume that the substance is relatively non-toxic. The sub-acute toxicity study shows that the plant extract of C. citratus is non-toxic and no mortality was observed in all the groups. The calculated LD50 value was greater than 500 mg/kg b.wt. The kidney is susceptible to damage caused by various toxic substances as large volume of blood flows through it and the toxins filtered usually gets concentrated in the kidney tubules (Al-Attar et al., 2017).
Clinical biochemistry analysis was conducted to investigate any possible influence of the extract on hepatic and renal functions of the rats. Biochemical parameters are considered as an important marker for toxicity evaluation, as both liver and kidney are necessary for the survival of an organism (Suganthy et al., 2018). The extract did not damage the liver as evidenced by significant decreased (P<0.05) in the level of plasma activity of AST (group III). ALT, GGT, TC, and TG did not show any significant difference while plasma concentration of TP and HDL-C (groups III and IV) significantly increases (P<0.05) in the animals administered with the extract (Table 2). Increase of these transaminases (AST, ALT and GGT) in the plasma is an indication of necrotic lesions within the liver. AST and ALT are mainly used to detect injury to liver cells (hepatocytes). Under normal circumstances, these enzymes (AST and ALT) reside in the hepatocytes. However, these enzymes will leak into the blood stream if the liver is injured, thus raising their levels in the blood (Oriakhi et al., 2018). In a research work carried out by Eraj et al. (2016) aqueous extract of C. citratus was administered at a dose of 200 mg/kg body weight orally for 15 days to healthy rabbit. The extract exhibited significant reduction in biochemical parameters (ALP, SGOT, SGPT, GT and TB) as observed in their study (Eraj et al., 2016). GGT acts as an indicator for cholestasis (e.g. biliary duct obstruction). Obstructed bile duct will induce the synthesis of GGT, thus elevating the levels in the blood (Bulle et al., 1990). The animals administered C. citratus extract (groups III and IV) had significant (P<0.05) reduced creatinine level compared to group I animals. Serum creatinine level is a good indicator of renal function since elevation of serum creatinine level is associated to a marked failure of nephron functions (Lameire et al., 2005). The study shows that the plant does not have toxic effect on the kidney.
Oxidative stress is caused by the presence of ROS in excess of the available of antioxidant buffering capacity. Many studies have showed that ROS can damage proteins, lipids and DNA, thus altering the structure and function of the biological cell, tissue, organ and system, respectively (Momoh et al., 2018a). Catalase catalyzes the conversion of hydrogen peroxides into oxygen and water and protects the tissue from oxidative damage by highly reactive oxygen free radicals and hydroxyl radicals (Momoh et al., 2018a). Glutathione (GSH) is a dipeptide compound containing glutamate, cysteine and glycine amino acids whose antioxidant function is facilitated by the sulphydryl group of cysteine. In the oxidation reaction of glutathione, the sulphur forms a thiyl radical that reacts with a second oxidized glutathione forming a disulphide bond (GSSG). GSH is found in most plant and animal tissues, cells and subcellular compartments of higher plants. GSH can reacts chemically with superoxide, singlet oxygen and hydroxyl radicals and therefore function directly as a free radical scavenger. Glutathione may stabilize membrane structure by removing acyl peroxides formed by lipid peroxidation reactions (Price et al., 1990). Glutathione peroxidase is a selenium-dependent enzyme, which decomposes H2O2 and various hydro- and lipid peroxides (Kinnula et al., 1995). SOD is an effective defence enzyme that catalyzes the dismutation of superoxide anions into hydrogen peroxide (Momoh et al., 2018a). We observed significant increase (P<0.05) in catalase, SOD%, SOD unit and total protein in the animals’ administered C. citratus compared to the control healthy animals in the sub-acute toxicity test. The level of GSH and GPX (except for group IV) did not show any significant difference while MDA values were lower in the rats administered with C. citratus. This is an indication that the plant can reduce oxidative stress caused by the presence of ROS.
Histological study shows that the tissue shows normocellular glomerular tufts disposed on a background containing normal renal tubules and no abnormalities are seen in Plates 1 to 4. Plates 5 to 7 histopathology of the liver shows normal radially arranged hepatocytes extending from portal tracts to central veins and no fatty change or sinusoidal congestion are seen but Plate 8 shows small cytoplasmic fat microvesicles (Figure 2). Sub-acute administration of C. citratus did not cause any major toxic effects on the biochemical parameters, liver and kidney architectures. The hepato-protective effects of C. citratus extract in rats with oxidative stress induced by CCl4 was investigated. It is generally believed that the hepatotoxicity induced by CCl4 is due to the formation of the active metabolite, trichloromethyl free radical (CCl3•). This then readily interacts with molecular oxygen to form the trichloromethyl peroxy radical (CCl3OO•). Both radicals are capable of binding to lipids, proteins and other macromolecules with simultaneous attack on poly-unsaturated fatty acids to produce lipid peroxidation leading to hepatotoxicity (Momoh et al., 2018a). H2O2 have also been used as an animal model for the induction of liver damage (Mello et al., 1984; Ganie et al., 2011).
Hematological and biochemical indices are reliable parameter for the assessment of the health status of animals (Momoh et al., 2018a). Evaluation of hematological parameters would be helpful in determining the toxic effects of C. citratus extract on animal blood. WBC helps the body to fight against infection, defend the body by phagocytosis against invasion by foreign organisms and to produce or at least to transport and distribute antibodies in immune response. RBC helps to check the level of anemia and to evaluate normal erythropoiesis. HGB level shows the amount of intracellular iron present while HCT indicates the volume of RBC in 100 ml of blood and it helps to determine the degree of anemia or polycythemia (Momoh et al., 2018a). The study shows that there are significant decrease (p<0.005) in the level of blood WBC, Mid#, Mid%, Gran%, Gran#, HGB, RBC, HCT, MCH and MCHC of the CCl4 intoxicated rats (Group B) compared to the animal treated with C. citratus extract (Table 4). The significant reduction (P < 0.05) in these hematological parameters in Group B animals may be attributed to the cytotoxic effects and suppression of the erythropoiesis caused by the administration of CCl4. There were significant increase (P<0.05) in the Lymph#, Lymp% in the animals administered CCl4 without treatment compared with animals treated with C. citratus extract (groups E and F). C. citratus extract causes significant increase (P<0.05) in Mid%, Gran%, HGB, MCH, PLT, and a decrease in Lymph# and MCV values in groups E and F rats compared to healthy animals (group A). This is an indication that the plant may aid in the increase of the immune system against infections and stimulate the production of hemoglobin. Other haematological parameters like RDW-CV, RDW-SD, MPV, and PDW showed no significant differences in the entire groups. The results obtained from this study showed clearly that ethanolic leaf extracts of C. citratus is not hematotoxic.
The present study demonstrates that C. citratus extract attenuates liver damage due to CCl4 administration as indicated by the significant reduction in the elevated levels of AST, ALT, ALP, GGT and increase in TP levels of groups E and F animals. The administration of C. citratus extract displayed similar results as that of the control (group A), with slight amelioration in most of the studied parameters. The result obtained from this study showed that there were significant increase (P<0.05) in the levels of AST, ALT, ALP and GGT values of group B animals compared to other animals in other groups. This may imply that severe damage occurs in the liver cells of the animals administered with CCl4 since the activities of these enzymes are reported to be increased in liver damage. Treatment with C. citratus extract and silymarin markedly reduced the effect of CCl4 induced liver damage as evidenced by decreased in the level of these plasma liver biomarker enzymes activities (AST, ALT, ALP and GGT). The significant increase in these liver biomarker enzymes in the plasma of these animals is an indication of hepatotoxicity of the liver in the animals administered with CCl4 (Mahesh et al., 2009) and this causes cellular leakage and loss of functional integrity of the hepatic cell membrane (Gupta and Singh, 2007; Kalegari et al., 2014). The study shows that there were significant increase (P<0.05) in the urea and creatinine levels of group B animals compared to other groups. This is an indication of severe kidney damage in group B animals. Group B rats have lower level of TP value compared to healthy animals (group A) and animals administered with C. citratus extract. The significant decrease (P<0.05) in the total protein values of animals administered with CCl4 without treatment compared to other animals in groups A, C, E and F, respectively, may be due to considerable liver damage through induction of peroxidation of lipids and inhibiting protein synthesis due to trichloromethyl free radical covalent bindings (Momoh et al., 2018a; Lee et al., 2004). In this study, there was significant increase (P<0.005) in the catalase, SOD% inhibition, SOD unit and decrease in MDA values in the liver tissue homogenate of the rats treated with C. citratus extract and group A animals compared with group B animals. MDA increased after oral administration with CCl4, treatment with C. citratus leaf extracts and silymarin reduce the level of MDA (P<0.05). Inhibition of elevated MDA levels observed in C. citratus extract and silymarin treated groups may be due to their antioxidant and free radical scavenging activities through re-establishment of biomembranes of hepatic parenchymal cells. Nwosu’s study shows that aqueous leaf extract of C. citratus exhibits protective role in animals exposed to toxic dose of paracetamol by its ability to enhance free radical scavenging activity which lead to increase in the levels of antioxidants measured (Nwosu et al., 2015). Furthermore, it was observed that aqueous leaf extracts of C. citratus has an antihepatotoxic action against dimethylnitrosamine (DMN) induced hepatic oxidative damage in rats which might be ascribed to its antioxidant and free radical scavenging property (Naglaa et al., 2015). The observed protective effect of silymarin against lipid peroxidation could be related to its antioxidant effects which assist in the preservation of membrane integrity. Silymarin can chelate transition metal ions such as copper and iron rendering them effective antioxidants (Momoh et al., 2018a).
The results of the histological study are as shown in Figure 3. Histological examination results are consistent with that of the biochemical analysis. The liver of the control animals (group A) showed a normal arrangement of hepatocytes and sinusoids. The cytoplasm was not vacuolated. Areas of infiltration by inflammatory cells, changes in fats and necrosis were not observed (Plate 9). Group B rats, which were exposed to CCl4 for 35 days, exhibited severe histo-pathological alterations which include cytoplasmic vacuolization, inflammation of cells, congestion, infiltration, and degeneration of hepatocytes due to necrosis (Plate 10). The rats (group C) treated with silymarin showed sinusoidal congestion (Plate 11). The group D rats showed normal arrangement of hepatocytes and sinusoids, the olive oil did not affect the liver architecture of the animals. Histologic section of tissue shows parallel plates of hepatocytes with oval nuclei and moderate eosinophilic cytoplasm. All the vessels appear normal; no abnormalities are seen (Plate 12). The group E rat showed many hepatocytes containing cytoplasmic fat vacuoles (Plate 13), while animals administered with higher concentration of C. citratus (group E) showed significant improvement evident through a well arranged of hepatocytes with cytoplasm not vacuolated (Plates 14 and 15). Sinusoids well preserved, no fat inclusions or atypia is seen and no abnormalities seen when compared with CCl4 intoxicated rats without treatment (Figure 3). In another research work carried out by Naglaa et al. (2015), it was observed that animals administered with C. citratus significantly reversed the effect of dimethylnitrosamine on the liver structure in the histopathological study (Naglaa et al., 2015). This study shows that C. citratus ethanolic leaf extracts significantly (P<0.05) reduces the damage effect of CCl4 on liver architecture of male Sprague Dawley rats.
The current results demonstrate that C. citratus has a potent hepatoprotective effect against CCl4-induced liver injury in Sprague Dawley rats. C. citratus treatment significantly reduced increase in liver biomarker enzyme activities and attenuates oxidative stress-induced pathological changes.
This research work was financially supported by Tertiary Education Trust Fund (TETFUND) from Nigeria. The authors are grateful to the Rector (MR. SAMUEL O. SOGUNRO) and Management Staff of Lagos State Polytechnic Ikorodu, Lagos, Nigeria for their support.
The authors have not declared any conflict of interests.
REFERENCES
|
Adeneye AA, Agbaje EO (2007). Hypoglycemic and hypolipidemic effects of fresh leaf aqueous extract of Cymbopogon citratus Stapf. in rats. Journal of Ethnopharmacology 112:440-444
Crossref
|
|
|
|
Al-Attar AM, Alrobai AA, Almalki DA (2017). Protective effect of olive and juniper leaves extracts on nephrotoxicity induced by thioacetamide in male mice. Saudi Journal of Biological Sciences 24:15-22.
Crossref
|
|
|
|
|
Bak J, Je NK, Chung HY, Yokozawa T, Yoon S, Moon JO ((2016). Oligonol ameliorates CCl4-induced liver injury in rats via the NF-Kappa B and MAPK signaling pathways. Oxidative Medicine and Cellular Longevity 1:1-12.
Crossref
|
|
|
|
|
Bastos JF, Moreira IJ, Ribeiro TP, Medeiros IA, Antoniolli AR, De Sousa DP (2010). Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic and Clinical Pharmacology and Toxicology 106:331-337.
Crossref
|
|
|
|
|
Bulle F, Mavier P, Zafrani ES (1990). Mechanism of gammaglutamyl transpeptidase release in serum during intrahepatic and extrahepatic cholestasis in the rat: a histochemical, biochemical and molecular approach. Hepatology 11:545-550.
Crossref
|
|
|
|
|
Chitra R, Sim SM, Ismil R (2012). Effect of Cymbopogon citratus and citral on vascular smooth muscle of the isolated thoracic rat aorta. Evidence-Based Complementary and Alternative Medicine 53:94-75.
Crossref
|
|
|
|
|
Chen TJ, Jeng JY, Lin CW, Wu CY, Chen YC (2006). Quercetin inhibition of ROS-dependent and - independent apoptosis in rat glioma C6 cells. Toxicology 223:113-126.
Crossref
|
|
|
|
|
Droge W (2002). Free radicals in the physiological control of cell function. Physiological Reviews 82:47-95.
Crossref
|
|
|
|
|
Eraj A, Sarfaraz S, Usmanghani K (2016). Hepato-Protective Potential and Phytochemical Screening of Cymbopogon citratus. Journal of Analytical and Pharmaceutical Research 3(6):00074.
Crossref
|
|
|
|
|
Ezeja MI, Anaga AO, Asuzu IU (2014). Acute and sub-chronic toxicity profile of methanol leaf extract of Gouania longipetala in rats. Journal of Ethnopharmacology 151:1155-1164.
Crossref
|
|
|
|
|
Figueirinha A, Cruz MT, Francisco V, Lopes MC, Batista MT (2010). Anti-inflammatory activity of Cymbopogon citratus leaf infusion in lipopolysaccharide-stimulated dendritic cells: Contribution of the polyphenols. Journal of Medicinal Food 13:681-690.
Crossref
|
|
|
|
|
Galighor AE, Kozloff EN (1976). Essentials of practical microtechnique 2nd edn, Lea and Febiger, NewYork.
|
|
|
|
|
Gallucci RM, Simeonova PP, Toriumi W, Luster MI (2000). TNF-α regulates transforming growth factor-α expression in regenerating murine liver and isolated hepatocytes. Journal of Immunology 164:872-878.
Crossref
|
|
|
|
|
Ganie SA, Haq E, Hamid A, Masood A, Zargar MA (2011). Long dose exposure of hydrogen peroxide (H2O2) in albino rats and effect of Podophyllum hexandrum on oxidative stress. European Review for Medical and Pharmacological Sciences 15:906-915.
|
|
|
|
|
Ghasemzadeh A, Ghasemzadeh N (2011). Flavonoids and phenolic acids: Role and biochemical activity in plants and human. Journal of Medicinal Plants Research 3:6697-6703.
Crossref
|
|
|
|
|
Gruebele A, Zawaski K, Kaplan D, Novak RF (1996). Cytochrome P450 2E1- and cytochrome P450 2B1/2B2-catalyzed carbon tetrachloride metabolism: effects on signal transduction as demonstrated by
|
|
|
|
|
altered immediate-early (c-Fos and c-Jun) gene expression and nuclear AP-1 and NF-κB transcription factor levels. Drug Metabolism and Disposition 24:15-22.
|
|
|
|
|
Gupta RS, Singh D (2007). Hepatomodulatory role of Enicostemma littorale Blume against oxidative stress induced liver injury in rats. African Journal of Agricultural Research 2:131-138.
|
|
|
|
|
Jiang ZY, Hunt JY, Wolff SP (1992). Detection of lipid hydroperoxides using the 'Fox method'. Analytical Biochemistry 202:384-389.
Crossref
|
|
|
|
|
Juza RM, Pauli EM (2014). Clinical and surgical anatomy of the liver: a review for clinicians. Clinical Anatomy 27:764-769.
Crossref
|
|
|
|
|
Kalegari M, Gemin CA, Araújo-Silva G, Brito NJ, López JA, Oliveira Tozetto SD, das Graças AM, Miguel M.D, Stien D, Miguel OG (2014). Chemical composition, antioxidant activity and hepatoprotective potential of Rourea induta Planch. (Connaraceae) against CCl4-induced liver injury in female rats. Nutrition 6:713-718.
Crossref
|
|
|
|
|
Kinnula VL, Crapo JD, Raivio KO (1995). Generation and dis¬posal of reactive oxygen metabolites in the lung. Laboratory Investigation 73:3-19.
|
|
|
|
|
Kokate CK (1994). Practical Pharmacognosy. Fourth ed. Delhi: Vallabh Prakashan.
|
|
|
|
|
Guide for the Care and Use of Laboratory Animals (2011). Washington NIH Publication:2011.ISBN-13: 978-0-309-15400-0ISBN-10: 0-309-15400-6.
|
|
|
|
|
Kumar SS. Kumar BR, Mohan GK (2009). Hepatoprotective effect of Trichosanthes cucumerina var. cucumerina L. on carbon tetrachloride induced liver damage in rats. Journal of Ethnopharmacology 123:347-350.
Crossref
|
|
|
|
|
Lameire N, Van Biesen W, Vanholder R (2005). Acute renal failure. Lancet 365(9457):417-430.
Crossref
|
|
|
|
|
Lee KS, Buck M, Houglum K, Chojkier M (1995). Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through cmyb expression. Journal of Clinical Investigation 96:2461-2468.
Crossref
|
|
|
|
|
Lee KJ, Woo E, Choi CY, Shin DW, Lee DG, You HJ, Jeong HG (2004). Protective effect of acteoside on carbon tetrachlorideinduced hepatotoxicity. Life Science 74:1051-1064.
Crossref
|
|
|
|
|
Mahesh A, Shaheetha J, Thangadurai D, Rao DM (2009). Protective effect of Indian honey on acetaminophen induced oxidative stress and liver toxicity in rat. Biologia 64:1225-1231.
Crossref
|
|
|
|
|
Mehendale HM, Roth RA, Gandolfi RA, Klaunig JE, Lemasters JJ, Curtis LR (1994). Novel mechanisms in chemically induced hepatotoxicity. FASEB Journal 8:1285-1295.
Crossref
|
|
|
|
|
Mello FAC, Hoffmann ME, Meneghini R (1984). Cell killing and DNA damage by hydrogen peroxide are mediated by intracellular iron. Biochemical Journal 218:273-275.
Crossref
|
|
|
|
|
Momoh JO, Adeniyi MO, Aderele OR (2018a). Experimental and Mathematical Model for the Hepatoprotective Effect of Methanolic Extract of Moringa oleifera Leaf against CCl4-induced Hepatotoxicity in Sprague Dawley Male Albino Rats JAMMR. 26(5):1-14. Article no.JAMMR.32062 ISSN: 2456-8899.
Crossref
|
|
|
|
|
Momoh JO, Osuntoki AA, Ebuehi OAT (2018b). Hepatic Lipase Influences Plasma Lipid Profiles and Lipoprotein Ratios in Regional Hospital Patients with Ischemic Stroke. International Journal of Biochemistry Research and Review 21(3):1-13, Article no.IJBCRR.35257 ISSN: 2231-086X, NLM ID: 101654445
Crossref
|
|
|
|
|
Naglaa ASS, Usama FA, Ali MA, Saleh SI (2015). The Role of Cymbopogon Citratus Extract in Protecting the Liver Against Injurious Effect of Dimethylnitrosamine in Rats. International Journal of Clinical and Developmental Anatomy 1(4):89-95.
Crossref
|
|
|
|
|
Naik MI, Fomda, BA, Jaykumar E, Bhat JA (2010). Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacteria. Asian Pacific Journal of Tropical Medicine pp. 535-538.
Crossref
|
|
|
|
|
Natanzi AE, Ghahremani, Monsef-Esphani MH, Minaei HR, Nazarian B, Sabzevari HO (2009). An experimental model for study of the hepatoprotective activity of Nasturtium officinale (Watercress) against acetaminophen toxicity using in situ rat liver system. European Journal of Scientific Research 38:556-564.
|
|
|
|
|
Nieto N, Dominguez-Rosales JA, Fontana L, Salazar A, Armendariz-Borunda J, Greenwel P, Rojkind M (2000). Rat hepatic stellate cells contribute to the acutephase response with increased expression of alpha1(I) and alpha1(IV) collagens, tissue inhibitor of metallo-proteinase-1, and matrixmetalloproteinase- 2 messenger RNAs. Hepatology 33:597-607.
|
|
|
|
|
Nwosu DC, Obeagu EI, Nwanna CA,. Nkwocha BC, IKE KO, Nwankpa P, Uloneme GC, Elendu HN, Ofodeme CN, Ezenwuba C, Oluh CC, Ozims J, Nwanjo HU (2015). Antioxidative role of aqueous leaf extract of Cymbopogon citratus (Lemon grass) on paracetamolinduced hepatoticity in albino rats. International Journal of Current Research in Chemistry and Pharmaceutical Sciences 2(12):64-70.
|
|
|
|
|
Oliveira VC, Moura DM, Lopes JA, de Andrade PP, da Silva NH, Figueiredo RCBQ (2009). Effects of essential oils from Cymbopogon citratus (DC) Stapf., Lippia sidoides Cham., and Ocimum gratissimum L. on growth and ultrastructure of Leishmania chagasi promastigotes. Parasitology Research 104:(5):1053-1059.
Crossref
|
|
|
|
|
Organization for Economic Cooperation and Development (OECD) (2007). Draft updated test guidelines 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents.
|
|
|
|
|
Oriakhi K, Patrick OU, Ikechi GE. (2018). Hepatoprotective potentials of methanol extract of T. conophorum seeds of carbon tetrachloride induced liver damage in Wistar rats. Clinical Phytoscience 4:25.
Crossref
|
|
|
|
|
Price A, Lucas PW, Lea PJ (1990). Age dependent damage and glutathione metabolism in ozone fumigated barley: a leaf section approach. Journal of Experimental Botany 41:1309-1317.
Crossref
|
|
|
|
|
Rukkumani R, Aruna K, Varma PS, Rajasekaran KN, Menon VP (2004). Comparative effects of curcumin and an analog of curcumin on alcohol and PUFA induced oxidative stress. Journal of Pharmaceutical Sciences 20:7(2):274-283.
|
|
|
|
|
Raucy JL, Kramer JC, Lasker JM (1993). Bioactivation of halogenated hydrocarbons by cytochrome P450 2E1. CRC Critical Reviews in Toxicology 23:1-20.
Crossref
|
|
|
|
|
Santoro G, Cardoso M, Guimar˜aes L, Freire J, Soares M (2007). Anti-proliferative effect of the essential oil of Cymbopogon citratus (DC) Stapf (lemongrass) on intracellular amastigotes, bloodstream trypomastigotes and culture epimastigotes of Trypanosoma cruzi (Protozoa: Kinetoplastida). Parasitology 134(11):1649-1656.
Crossref
|
|
|
|
|
Santin MR, dos Santos AO, Nakamura CV, Dias Filho BP, Ferreira ICP, Ueda-Nakamura T (2009). In vitro activity of the essential oil of Cymbopogon citratus and its major component (citral) on Leishmania amazonensis," Parasitology Research 105(6):1489-1496.
Crossref
|
|
|
|
|
Schreck R, Baeuerle DA (1991). A role for oxygen radicals as second messengers, Trends Cell Biology 1:39-42.
Crossref
|
|
|
|
|
Slater TF (1984). Free radical mechanisms in tissue injury. Biochemical Journal 222:1-15.
Crossref
|
|
|
|
|
Sofowora A (1993). Medicinal plants and traditional medicines in Africa Spectrum Book Ltd. Ibadan, Nigeria P 289.
|
|
|
|
|
Suganthy N, Muniasamy S, Archunan G (2018). Safety assessment of methanolic extract of Terminalia chebula fruit, Terminalia arjuna bark and its bioactive constituent 7-methyl gallic acid: In vitro and in vivo studies. Regulatory Toxicology and Pharmacology 92:347-357.
Crossref
|
|
|
|
|
Trease GE, Evans WC (1986). Pharmacognsy. 11th edition. London: Brailliar Tiridel Can Macmillian Publishers pp. 60-75.
|
|
|
|
|
Uboh FE, Ebongi PE, Akpan HD, Usoh IF (2012). Hepatoprotective effect of vitamins C and E against gasoline vaporinduced liver injury in male rats. Turkish Journal of Biology 36:217-223.
|
|
|
|
|
Viana G, Vale T, Pinho R, Matos F (2000). Antinociceptive effect of the essential oil from Cymbopogon citratus in mice. Journal of Ethnopharmacology 70(3):323-327.
Crossref
|
|
|
|
|
Vinitketkumnuen U, Puatanachokchai R, Kongtawelert P, Lertprasertsuke N, Matsushima T (1994). Antimutagenicity of lemon grass (Cymbopogon citratus Stapf) to various known mutagens in salmonella mutation assay. Mutation Research/ Genetic Toxicology 341(1):71-75.
Crossref
|
|
|
|
|
Weber LW, Boll M, Stamp FA (2003). Hepatotoxicity and mechan- ism of action of haloalkanes: carbon tetrachloride as a toxicological model. Critical Reviews in Toxicology 33(3):105-136.
Crossref
|
|
|
|
|
World Health Organization (WHO) (1966). Specifications for identity and purity and toxicological evaluation of food colours. WHO/Food Add/66.25 Geneva WHO; 1966.
|
|
|
|
|
Zheng W, Wang SY (2001). Antioxidant activity and phenolic compounds in selected herbs. Journal of Agriculture and Food Chemistry 49:5165-5170.
Crossref
|
|
|
|
|
Zorov DB, Juhaszova M, Sollott SJ (2006). Mitochondrial ROS induced ROS release: an update and review. Biochimica et Biophysica Acta1757:509-517.
Crossref
|
|
|
|
|
Zou GL, Gui XF, Zhong XL, Zhu YF (1986). Improvements in pyrogallol autoxidation method for the determination of SOD activity. Progress in Biochemistry and Biophysics 4:71-73.
|
|