ABSTRACT
Tamarindus indica L is a tropical plant that is used around the world mainly for medicinal purposes. Yet few scientific studies of its biological potential have been undertaken. The aim of this study was to assess the antioxidative potential of T. indica L leaves. The plant material was harvested and then dried in the absence of direct sunlight. The extraction was done by alcoholic decoction, and the fractions were obtained by liquid-liquid chromatography using solvents of increasing polarity. The reducing capacity of each sample was assessed using DPPH and FRAP methods. The yield of extract from the alcoholic decoction of the leaves was 8%. The hexane, dichloromethyl, acetyl acetate and aqueous fractions represent respectively 0.91, 1.17, 1.06 and 2.69% of the leaves. Evaluation’s results of the antioxidative capacity assessed by DPPH indicate that the best IC50 values were obtained from the extract (60.53 μg/mL) followed by the aqueous fraction (71.66 μg/mL), while the ethyl acetate fraction (453.33 μg/mL) was the least potent. As for the results obtained by the FRAP method, the best antioxidative activity was obtained with the extract (A = 0.066), followed by the hexane fraction (A = 0.016). This study shows that the extract and its chemical fractions reduce DPPH and the FRAP, thus indicating that T. indica L leaves have an antioxidative activity.
Key words: Tamarindus indica L leaves, antioxidative activity,
Research of plants that have antioxidative activity has become a topic of increasing interest in light of the important roles for antioxidative compounds in the treatment and prevention of pathologies linked to oxidative stress that is generated by free radicals (Cole et al., 2005; Bolignon et al., 2009).
Indeed, antioxidants help to neutralize free radicals which can damage cellular membranes and interact with the genetic material of cells.Antioxidants from fruits, vegetables and beverages play an important role in human health, for example preventing cancer and cardiovascular diseases, and reduce the incidence of different diseases (Mircea and Isabel, 2015). Tamarindus indica L is a tropical plant that is mainly used for medicinal purposes. With the exception of the forests of the Casamance, it is found throughout Senegal. At the chemical level, prior research has revealed the presence of phenolic constituents such as flavonoids, aljaloids, and tannins (Selvi et al., 2012), as well as the presence of amino acids, carbohydrates, and proteins (Kerharo and Adam, 1974). In terms of pharmacopoeia, the leaves are used against phagedenic ulcers (Kerharo and Adam, 1974). Leaf extracts have been shown to exhibit an antioxidative activity in hepatic disorders, and they are ingredients in the composition of medications used to prevent cardiovascular diseases (Diallo, 2001; Bowe, 2007). The leaves are also known to have laxative properties (Bhat et al., 1990). Further, the hydroalcoholic extract of the leaves has been shown to result in hypoglycemia in Wistar rats that have alloxane-mediated diabetes. The hypoglycemic effect was most pronounced at a dose of 200 mg/kg body weight (Ramchander et al., 2012). Since anti-diabetic effects often correlate with antioxidative effects, and its potential has not yet been demonstrated, in this study we decided to focus on the in vitro antioxidative potential of aqueous extract and subsequent fractions of T. indica L.
The plant material was harvested at the botanical garden of the Faculty of Sciences and Technologies at the Cheikh Anta Diop University of Dakar (Senegal) in May of 2013, and they were identified and dried away from direct sunlight at the Laboratory of Pharmacognosy of the Faculty of Medical Pharmacy and Odontology of Dakar. The following reagents were used to determine the various activities: DPPH: 2, 2, Diphényl-1-pycrylhydrazyl (Sigma Aldrich), L (+) Ascorbic acid (ACS ISO), absolute Ethanol (Scharlau Chemie S.A.), Trichloroacetic acid (Cl3CCO2H) (Alfa Aesar), Disodium phosphate Na2HPO4, Potassium hexacyanoferrate (K3Fe(CN)6) at 1% (EMSURE®).
Extraction
The extraction was performed by decoction of 75 g of leaves powder, brought to a boil under reflux in 750 mL of ethanol for 30 min. After filtration, the resulting ethanolic extract was evaporated using a Rotavapor (Stuart rotary evaporater, Stuart Equipment), yielding a dry residue (12 g) (Dieng et al., 2015).
Fractionation
The fractionation consisted of adding 250 mL of warm water to nine grams (9 g) of dry extract. Following complete dissolution, the aqueous extract was added to 100 mL of hexane and 100 mL of dichloromethane and 100 mL of ethyl acetate in a separating funnel for the fractionation. The organic hexane, dichloromethane, ethyl acetate phases and the aqueous phase obtained thereby were then evaporated with a Rotavapor to yield dry extracts. These extracts were then suspended in ethanol for the pharmacological testing (Sarr et al., 2015).
Calculation of yields
The yields were calculated using the following formula:
Yield= (residue weight/ original dry weight) × 100
The residue represents the extract or the fraction following fractionation, while the dry weight becomes the residue after evaporation. Determination of the free radical scavenging capacities was performed by the DPPH and the FRAP methods.
DPPH
The determinations of DPPH activities were performed according to the method of Molyneux (Molyneux, 2003). The extract was tested at various concentrations (that is, at 6.25-12.5-25-50-100-200 μg/mL) with the DPPH at a ratio ¼ in the extract/DPPH mixture. Ascorbic acid was used as a reference antioxidant, and tested at the same concentrations. Absorbance was measured at 517 nm using a spectrophotometer after an incubation time of 30 min (T30) using ethanol as a blank. Three assays were performed for each concentration of entity tested (n=3). The results were first expressed as percentage inhibition (that is, PI, which is equal to the absorbance of DPPH on its own minus the absorbance after adding the extract at a given concentration, divided by the absorbance of DPPH on its own), the antiradical activity, and as an IC50 (that is, the antiradical concentration that allows for scavenging of 50% of the free radicals). The values of EC50 were then calculated based on the IC50 divided by the molar mass of DPPH, and the AP (the antiradical potential) was equal to the inverse of the effective concentration (Bassène, 2012).
FRA
The reducing capacity of the extract of T. indica L leaves was assessed according to the method of Oyaizu (Oyaizu, 1986). Briefly, various concentrations of each extract (that is, 200; 100; 50; 25; 12.5 and 6.25 μg/mL) were diluted in half with distilled water and then mixed with 2.5 mL of the phosphate buffer solution (0.2M; pH 6.6) and 2.5 mL of 1% potassium ferricyanide [K3Fe(CN)6]. The mixtures obtained thereby were incubated at 50°C for 30 min, after which 2.5 mL of trichloroacetic acid (10%) was added. After centrifugation at 3,000 rpm for 10 min, 2.5 mL of the supernatant fraction of each concentration was mixed with distilled water and 0.5 mL FeCl3 (0.1%). The absorbance was measured at 700 nm using a spectrophotometer of Biosystems (BTS 350, Biosystems, Barcelona, Spain).
Statistical analysis
Statview software was used for the statistical analyses. An analysis of the variance from the norm, followed by a Fisher’s test were performed. The difference was deemed to be significant when P < 0.05 relative to the negative control (i.e. the DPPH solution). Statgraphics 5.0 software was used to determine the inhibitory concentrations. Analysis of the variances was performed using the Fisher’s test with a threshold for significance of 0.05 using Statview software.
DPPH
The results for the determination of the antioxidizing activity of the extracts and ascorbic acid by the DDPH method, as expressed in percentage inhibition, are presented in Figure 1. The ethanolic extract and its fractions hexane, dichloromethane, ethyl acetate and aqueous inhibited the DPPH• free radical dose-dependent manner. At concentrations from 6.25 to 25 µg/mL, the ethanolic extract and its fractions showed moderate antioxidant activity; while the best activities were observed at concentrations of 100 and 200 µg/mL. The ethanolic extract at concentrations of 100 and 200 µg/mL inhibited more significant absorbance of radical DPPH• compared to the aqueous fraction and other fractions.
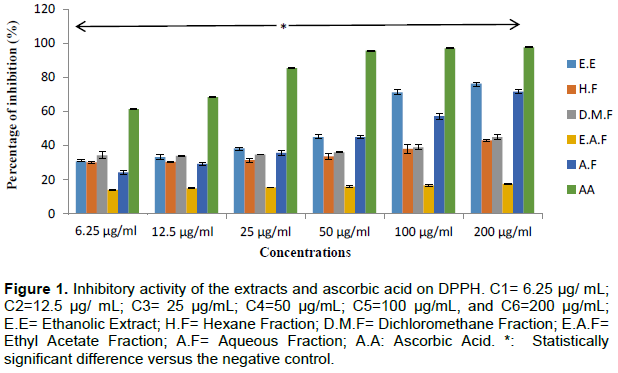
The 50% inhibitory and effective concentrations, and the antiradical activity were calculated and the values obtained are presented in Table 1. As shown in Table 1, the ethanolic extract had the best IC50, EC50, AP, followed by the aqueous fraction results while the ethyl acetate had the lowest values.
FRAP
The results for the determination of the antioxidizing activity of the extracts and ascorbic acid by the FRAP method, as expressed in absorbance, are presented in Figure 2. The ethanolic extract and its fractions reduce in a dose-dependent manneriron. At concentrations from 6.25 to 25 µg/mL, the ethanolic extract and its fractions showed moderate antioxidant activity; while the best activities were observed at concentrations of 100 and 200 µg/mL. The ethanolic extract at concentrations of 200 µg/mL reduced more significantly the iron compared to the hexane fraction and other fractions. The results of the ethanolic extract wasn’t similar to the references of antioxidants.
The aim of our study was to investigate the antioxidizing potential of T. indica L. leaves. The results obtained regarding the antioxidizing activity of the extract and the fractions of T. indica L leaves by the DPPH method have shown that the extract and the fractions in question significantly inhibited DPPH at all of the concentrations tested (P<0.05). This effect occurred in a dose dependent manner. The ethanolic extract and the aqueous fraction of the leaves had the best antioxidative activities, followed by the dichloromethane and hexane fractions. This may be explained by the extraction of polyphenols by the polar solvents (Bassène, 2012).
Thus, the best antioxidative capacity can be assigned to the ethanolic extract and the aqueous fraction with IC50’s of 60.53 and 71.66 μg/mL, respectively.
Further, the lowest antioxidative activity was seen with the dichloromethane, hexane and ethyl acetate fractions of the leaves, with IC50 inhibitory concentrations of 205.33, 232.66 and 453.33 μg/mL, respectively. The statistical test reveals a highly significant difference with p<0.05. The apolar extracts (that is, hexane, dichloromethane, and ethyl acetate) exhibited a very weak antiradical activity, for which the percentage of antiradical activity did not even reach 50%. This shows that the apolar antioxidants are inactive when it comes to DPPH.
It is clear that the potent activity of the extracts is due to the abundance of phenolic components, of which the ethanol extract has the highest content of dosed molecules (e.g. polyphenols, flavonoids and tannins), followed by the aqueous fraction. A study done by Kang et al. (2003) suggests that the polar molecules in plant extracts contribute to the high antiradical activity.
The ascorbic acid that we used as an antioxidant reference shows a higher activity than T. indica L’s extract at all three concentrations that were tested (P < 0.05). The relative IC50 indicates that the extract of T. indica L leaves corresponds to 183.42 equivalents of ascorbic acid (P< 0.05). This same trend is seen for the EC50 (the EC50 of the extract was equal to 182 Equivalents of ascorbic acid), although in regard to the apolar fractions, such as dichloromethane for example, 205.33 μg/mL is required for an efficacy of 50%. Lastly, the antiradical potential shows that ascorbic acid is 187 times more active than the extract, since ascorbic acid is a purified compound. The FRAP method is based on the capacity of polyphenols to reduce ferric iron Fe3+ to ferrous iron Fe2+. The reducing power is one of the antioxidative mechanisms. Furthermore, the reducing capacity of a constituent can serve as an important indicator of its antioxidative potential (Yang et al., 2008). The reducing activity may be due to polyphenols, such as flavonoids and anthocyanins (Kanoun, 2011).
Some authors have reported that there is a direct correlation between antioxidizing activities and the ability of plant constituents to reduce ferric iron (Fe3+) to ferrous iron (Fe2+) (Yildirim et al., 2001). Indeed, the presence of reducing agents in plant extracts results in the reduction of Fe3+ to the ferrous form by the ferricyanide complex. Consequently, Fe2+ can be assessed by measuring and monitoring the increase in the intensity of the blue color of the reaction mixture at 700 nm (Chung et al., 2002). In other words, the FeCl3/KFe3(CN)6 system provides the method with sufficient sensitivity to “semi-quantitatively” determine the concentration of polyphenols which participate in the redox reaction (Amarowicz et al., 2004).
In our extract, as well as its fractions, we have demonstrated activities that reduce ferric ions to ferrous ions with values of 0.066 to 0.016 at concentrations of200 μg/mL. The highest optical densities were observed in the ethanol extract (0.066 ± 0.003) followed by hexane fractions (0.058 ± 0.002) and lastly the dichloromethane fraction (0.051 ± 0.0024). The lowest optical densities were detected in the ethyl acetate fractions (0.039 ± 0.002) and the aqueous fraction (0.016 ± 0.008). The statistical test reveals a highly significant difference with p<0.05. These results may be due to the presence of polyphenols in the leaves, such as tannins, flavonoids, and alkaloids (Selvi, 2011). Polyphenolic constituents are known to be good antioxidants. These polyphenols could therefore be partially responsible of the antioxidative activity of the leaves. The antioxidative activity of several plant-derived entities is proportional to their total polyphenol content, which indicates that there is a link between the level of polyphenols and the antioxidative activity (Djeridane et al., 2006; Lamien-Meda et al., 2008).
In this study, a good correlation was obtained with the results of the phytochemical characterization, the antioxidative and antimicrobial activities of the methanol extract of T. indica L leaves, with 50% tannins, 60-70% flavonoids, and 80% of the content as phenolics (Selvi, 2011). The DPPH scavenging activity was similar, with a PI of 72%. This is close to our values, such as 76.28 ± 0.9% at the highest concentration (200 μg/mL). This minor difference may be due to the solvents used for extraction. This shows that it is the polyphenolic constituents that contribute to the anti-oxidative activity of leaves that are used for various pathologies.
Flavonoids and tannins have been described to be the main constituents of T. indica leaf extract (Selvi, 2011; De Caluwe et al., 2010). The structure/activity relationship of flavonoids and phenolic constituents has shown that the antioxidative activity is determined by the position and extent of hydroxylation (Igor, 2002), and their ability to scavenge free radicals reflects (Nijidveltd et al., 2001; Pyo et al., 2004). The antioxidative activity of plant constituents is increasingly becoming relevant to research of medicinal plants. Indeed, the cellular and oxidative damage derived from free radicals, or by ROS, currently appears to be one of the principal causes of human pathologies relating to hypertension, cancer, atherosclerosis, arthritis, Alzheimer’s disease, cardiovascular diseases, auto-immune diseases, digestive disorders such as gastro duodenal and intestinal ulcers as well as inflammation of the gut, neurodegenerative disorders, diabetes, and viral infections ( Bonnefoy et al., 2002; Huang et al., 2005; Ziyatdiniva et al., 2005; Li et al., 2009). The free radicals that occur naturally as a result of normal metabolism in living organisms can be scavenged by chemo-prevention using antioxidative constituents present in the food consumed by humans and by medicinal plants (Kubola and Siriamornpun, 2008; Verma et al., 2009).
This study has shown that T. indica L leaves contain a proven antioxidative activity. This explains their use to treat liver pathologies. The results obtained support the use of the whole extract, as well as chemical fractions, or as food additives complements. The use of the leaves, which are more readily accessible, could be an alternative to achieve equilibrium in the pro-oxidant/antioxidant balance among disadvantaged sectors of the population. Lastly, follow-up studies could lead to ways to isolate and identify the antioxidative molecules by bioactivity-guided methods, as well as permitting determination of acute and sub-acute toxicity of the leaves.
The authors declare that there is no conflict of interests regarding the publication of this paper.
REFERENCES
|
Amarowicz R, Pegg RB, Rahimi-Moghaddamc P, Barl B, Weil JA. (2004). Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 84:551-562.
Crossref
|
|
|
|
Bassene E (2012). Initiation à la recherche sur les substances naturelles Extraction-Analyses – Essais biologiques. Presses universitaires de Dakar. P 150.
|
|
|
|
Bhat RB, Eterjere EO, Oladipo VT (1990). Ethnobotanical studies from Central Nigeria. Econ. Bot.44:382-390.
Crossref
|
|
|
|
Boligon AA, Pereira RP, Feltrin AC, Machado MM, Janovik V, Rocha JBT, Athayde ML (2009). Antioxidant activities of flavonols derivatives from the leaves and stem bark, of Scutia buxifolia Reiss. Biores. Technol. 100:6592-6598.
Crossref
|
|
|
|
Bonnefoy M, Drai J, Kostka T (2002). Les antioxydants pour retarder les effets du vieillissement, faits et perspectives. Peut-on prévenir le vieillissement ? Presse Méd. 31(24):1174-1184.
|
|
|
|
Bowe C (2007). Predicting suitable areas for the production of tamarind (Tamarindus indica L.) and underutilised fruit tree species. PhD, thesis, University of Southampton, Southampton, UK. P 230.
|
|
|
|
Chung YC, Chang CT, Chao WW, Lin CF, Chou ST (2002). Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 50:2454-2458.
Crossref
|
|
|
|
Cole GM, Lim GP, Yang F, Teter B, Begum A, MA Q, Harris-White ME, Frautschy A (2005). Prevention of Alzheimer's disease: Omega-3 fatty acid and phenolic antioxidant interventions. Neurobiol. Aging 26:133-136.
Crossref
|
|
|
|
De Caluwe E, Halamoud K, Van Damme P (2010). Tamarindus indica L.: a review of traditional uses, phytochemistry and pharmacology. Afrika Focus 23(1):53-83.
|
|
|
|
Diallo BO (2001). Biologie de la reproduction et évaluation de la diversité génétique chez une légumineuse : Tamarindus indica L. (Caesalpinioideae). Thèse Université Montpellier II. Science et technique du Languedoc. P 119.
|
|
|
|
Dieng M, Fall AD, Diatta K, Diatta W, Bassène E (2015). Dosage des polyphenols et activité antioxydante de feuilles et d'inflorescences mâles de Borassus aetiopicum Mart. (Arecaceae). Int. J. Biol. Chem. Sci. 9(1):1067-1071.
Crossref
|
|
|
|
Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stoker P, Vidal N. (2006). Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 97:654-660.
Crossref
|
|
|
|
Huang D, Ou B, Prior RL (2005). The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 53:184-1856.
Crossref
|
|
|
|
Igor Passi LB (2002). Etude des activités biologiques de Fagara zanthoxyloides Lam. (Rutaceae). Thèse Pharmacie, Bamako. P 133.
|
|
|
|
Kang DG, Yun CK, Lee HC (2003). Screening and comparison of antioxidant activity of extracts of herbal medicines used in Korea. J. Ethnopharmacol. 87:231-236.
Crossref
|
|
|
|
Kanoun K (2011). Substances naturelles, activités biologiques et synthèse. Mémoire en vue de l'obtention du Diplôme de Master en Biologie, Tlemcen. P 76.
|
|
|
|
Kerharo J, Adam JG (1974). La Pharmacopée Sénégalaise Traditionnelle, Plantes médicinales et toxiques,Ed. Vigot Frères, Paris. P 1012.
|
|
|
|
Kubola J, Siriamornpun S (2008). Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 110:881-890.
Crossref
|
|
|
|
Lamien-Meda A, Lamien LE, Compaore MM, Meda RN, Kiendrebeogo M, Zeba B, Millogo JF, Nacoulma OG (2008). Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules 13(3):581-94.
Crossref
|
|
|
|
Li HY, Hao ZB, Wanga XL, lei Huang L, Li JP (2009). Antioxidant activities of extracts and fractions from Lysimachia foenum-graecum Hance. Bioresour. Technol. 100:970-974.
Crossref
|
|
|
|
Mircea O, Isabel E (2015). Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 74:10-36.
Crossref
|
|
|
|
Molyneux P (2003). The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 26: 211-219.
|
|
|
|
Nijidveltd RJ, Van Nood E, Van Hoorn REC, Boelens PG, Van Norren K, & Van Leeurwen PAM (2001). Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 74:418-425.
|
|
|
|
Oyaizu M (1986). Studies on products of browning reaction-Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J. Nutr. 44:307-315.
Crossref
|
|
|
|
Pyo YH, Lee TC, Logendra L, Rosen RT (2004). Antioxydant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts. Food Chem. 85:19-26.
Crossref
|
|
|
|
Ramchander T, Rajkuma D, Sravanprasad M, Venkateshwarlu G, Dhanalakshm CH, Arjun I (2012). Antidiabetic Activity of Aqueous Methanolic Extracts of leaf of Tamarindus indica L. Int. J. Pharmacogn. Phytochem. Res. 4(1):5-7.
|
|
|
|
Sarr SO, Fall AD, Gueye R, Diop A, Sene B, Diatta K, Ndiaye B, Diop YM (2015). Evaluation de l'activité antioxydante des extraits des feuilles de Aphania senegalensis (Sapindaceae) et de Saba senegalensis (Apocynaceae). Int. J. Biol. Chem. Sci. 9(6):2676-2684.
Crossref
|
|
|
|
Selvi A,Tamil, Shipra S, Krithiga G, Chandrasekaran B, Rose C (2011). Antioxidant and Antimicrobial activity of leaves extract (Tamarindus indica) and it's phytochemical characterization. J. Pharm. Res. 4(12)4435.
|
|
|
|
Yang J, Guo J, Yuan J (2008). In vitro antioxidant properties of rutin. LWT 41:1060-1066.
Crossref
|
|
|
|
Yildirim A, Mavi A, Kara AA (2001). Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 49:4083-4089.
Crossref
|
|
|
|
Ziyatdinova G, Gilametdinova D, Budnikov G (2005). Reactions of superoxide anion radical with antioxidants and their use in voltammetry. J. Anal. Chem. 60(1):49-52.
Crossref
|