ABSTRACT
The effect of varying NaCl concentrations on membrane lipids of Escherichia coli and Staphylococcus aureus cultivated at 37°C was studied. Lipid profiles of the membrane extracts were resolved by thin layer chromatography (TLC) using polar and neutral lipids solvent systems, with their fatty acid constituents determined via gas chromatography-mass spectrometry (GC-MS). Polar lipids identified were cardiolipin, phosphatidic acid, phosphatidylglycerol, phosphatidylethanolamine and lysophosphati-dylglycerol, while neutral lipids for both microorganisms were steryl-esters, triacylglycerols, diacylglycerols and monoacylglycerols at the different NaCl concentrations of growth however triacylglycerol was not detected in S. aureus at 3% NaCl. Fatty acids for E. coli were C15:0, C16:0 and C18:1 at 0% NaCl but C16:2 was also detected at 3% NaCl. S. aureus fatty acids were C13:0, C14:0, C16:0 and C18:1 for 0% NaCl while at 3% NaCl, additional fatty acids observed include C12:0, C15:0 and C16:1. The lipids expressed were relatively similar; however, their fatty acids differed in constituents and abundance.
Key words: NaCl concentration, lipid profiles, fatty acids, membranes, Escherichia coli, Staphylococcus aureus.
Every organism must find in its environment all of the substances required for energy generation and cellular biosynthesis. The chemicals and elements of this environ-ment that are utilized for bacterial growth are referred to as nutrients. Therefore, changes in composition of surrounding environment such as varying saline concen-trations, pH and other conditions may result in membranes with altered physical or thermal characteristics. It has been established that most microorganisms alter their membrane lipid composition when grown in the presence of high NaCl concentrations (Kogut and Russel, 1984; Komaratat and Kates, 1975). Also, it has been suggested that the phospholipid head group alterations of Gram-positive halotolerant bacteria differ from those of Gram-negative halophilic bacteria when grown in media containing high NaCl concentrations (Miller, 1985).
The plasma membrane of microorganisms enables the cell to sense changes in the surrounding osmotic conditions so that it can adjust its internal glycerol concentration to achieve osmotic equilibrium (Peeler et al., 1989). In fact, it has been suggested that the NaCl-induced alterations of membrane lipid composition may be important in controlling the ionic permeability of halotolerant or halophilic bacteria (Komaratat and Kates, 1975). For example, bacteria belonging to the Enterobacteriaceae family, such as Escherichia coli and Salmonella, do not tolerate high salt levels, but some species, such as Serratia rubidea, are very salt-tolerant (up to 10% NaCl) (Zambonelli et al., 1992). Also, halotolerant strains of E. coli are able to survive and grow even at very high salt concentrations; this high osmotic strength is due to the production of proline in the cells (Brewer, 2000; Tiecco, 2000). However, tolerance of Staphylococcus aureus to high concentrations of NaCl in liquid medium has been reported by Hurst et al. (1973). This study is aimed at identifying the phospholipids and fatty acids expressed by E. coli and S. aureus at different NaCl concentrations.
Bacterial culture conditions
Clinical isolates of E. coli and S. aureus were grown for 18 h in 150 ml nutrient broth containing additional NaCl concentrations to give a salt inclusion level of 0, 1 and 3% (w/v). Each culture medium was prepared in triplicate.
Culture harvesting
The cultures were harvested by centrifugation (1000 x g, 10 min). In addition, wet cell paste obtained after decanting the clear supernatant, was then re-suspended in 100 ml; 1.0% NaCl (w/v), and centrifuged. Second supernatant was discarded and cell pellet frozen overnight and stored in sealed McCartney bottles.
Lipid extractions
Lipids were extracted from bacterial cells following the procedure of Bligh and Dyer (1959). 3.75 ml (1:2 v/v) chloroform (CHCl3): methanol (MeOH) were added to each 1 ml of sample (wet cell paste), and then vortex well in a test-tube. 1.25 ml CHCl3 was later added to the mixture in test-tube and then vortex well. Finally, another 1.25 ml distilled H2O was added and vortex well before centrifuging at 6000 rpm in an automated cold centrifuge for 15 min at 4°C to give a two-phase system (aqueous top and organic bottom). The bottom phase which is the organic phase containing extracted lipids was then recovered through a Pasteur pipette, making sure to avoid the interface or upper face. Extracted lipids from the bottom phase were then placed in an evaporator and the residue obtained was later re-dissolved in 1 ml of CHCl3: MeOH (2:1 v/v) ready for further lipid analysis.
Lipid analysis
The first analysis was carried out using a preparative thin layer chromatography (TLC). The total lipids extract in chloroform: methanol, 2:1 (v/v) were run on the outside lanes of the same TLC plate to enable identification of the sample lipid classes; polar lipids were resolved on activated 0.25 mm layers of silica gel H (Merck)and, developed using chloroform: methanol: diisobutyl ketone: glacial acetic acid: water, 45:15:30:20:4 (v/v/v/v/v) (Hunter et al., 1981; Wuthier, 1966). Resolution of neutral lipids were carried out on activated 0.25 mm layers of silica gel G (Merck), with solvent system hexane: diethyl ether: acetic acid, 80:20:1 (v/v/v) (Hunter et al. 1981; Kupke and Zeugner, 1966). Lipids were detected by inserting the TLC plates in chamber containing few crystals of iodine which has been already saturated with iodine vapour, after which the TLC plates are then carefully removed and the spots are gently circled with a dull pencil. Lipid compositions were further analyzed by gas chromatography-mass spectrometry (GC-MS). The GC-MS analysis of lipid extracts was performed using a GC-MS QP2010 PLUS Shimadzu, Japan. Interpretation on mass spectrum of GC-MS was done using the database of National Institute Standard and Technology (NIST), having more than 62,000 patterns. The mass spectrum of the unknown component was compared with the spectrum of the known components stored in the NIST library. The name, molecular weight and structure of the components of the test materials were ascertained.
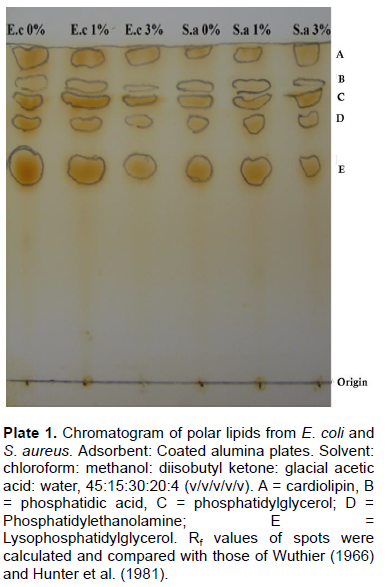
TLC of the resolved polar lipids of E. coli and S. aureus is presented in Plate 1. Five spots containing cardiolipin (CL), phosphatidic acid (PA), phosphatidylglycerol (PG), phosphatidylethanolamine (PE) and lysophosphatidyl-glycerol (LPG) were identified in both organisms at the different NaCl concentration investigated by comparing their Rf values with those of Hunter et al. (1981) who used the same solvent system. Also, Oku et al. (2004) reported the presence of phosphatidylethanolamine in S. aureus and E. coli at different salt stress condition. This further establishes the suggestion, that halophiles, including halococci exhibit highly similar lipid profiles (Kates, 1978).
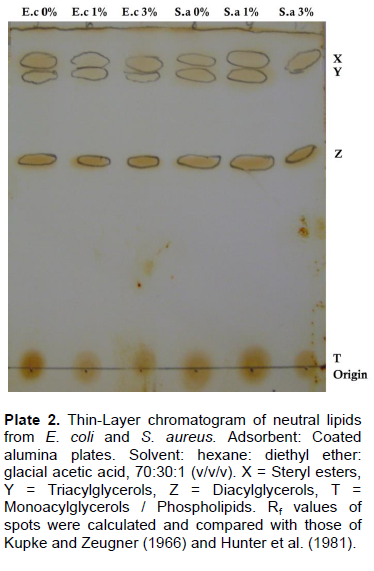
The resolved neutral lipids from these bacteria showed four components on TLC for both organisms at the different NaCl concentration as shown in Plate 2; however, the triacylglycerol spot was not detected for S. aureus at 3% NaCl inclusion. The lipids identified include: steryl esters (SE), triacylglycerols (TAG), diacylglycerols (DAG) and monoacylglycerols (MAG)/phospholipids (PL).
Gram-negative bacteria are surrounded by two membranes, and the major lipids of such enteric bacteria cells are phosphatidylethanolamine, phosphatidylglycerol and cardiolipin (diphosphatidylglycerol) in varying ratios (Goldfine, 1982), while in the Gram-positive cocci and the lactobacilli the major polar lipids are phosphatidylglycerol and cardiolipin (Goldfine, 1982). The presence of phospha-tidylethanolamine and phosphatidylglycerol in the bacteria considered is not unusual as most groups of bacteria, with the exception of the Lactobacillaceae and Micrococcaceae, contain phosphatidylethanolamine (Shaw, 1970). Phosphatidylglycerol, its O-amino acid esters, and cardiolipin are even more widely distributed among bacterial lipids (Shaw, 1970). To survive in this salt stressed environment, microorganisms considered would have tried to modify their membrane structures to modulate the chemical composition of lipids (Morrow et al., 1995).
Nagamachi et al. (1992) in their study reported that cardiolipin can stabilize liposomes during osmotic stress. It is also required for the growth of Escherichia coli and Bacillus subtilis under high-salt conditions (Lopez et al., 2006; Romantsov et al., 2007) hence, some strains of E. coli and coliforms are able to tolerate quite high NaCl levels (Colavita et al., 2003). Therefore, it can be inferred that membrane lipid like cardiolipin plays a major role in the adaptive mechanism of the bacteria subjected to the condition of high salt stress. Also, other mechanisms, including species-specific systems such as variations in cell wall proteins (Kuroda et al., 2008), enhanced Staphylococci ability to cope with high-salt stress (Wilkinson, 1996). In addition, the free glycerol, a well-known osmoprotectant, could contribute to the resistance of the cell to osmotic stress. Miller (1985) however observes a significant change in the amount of Phosphatidylethanolamine upon increase in the NaCl concentration of the medium where organism grows.
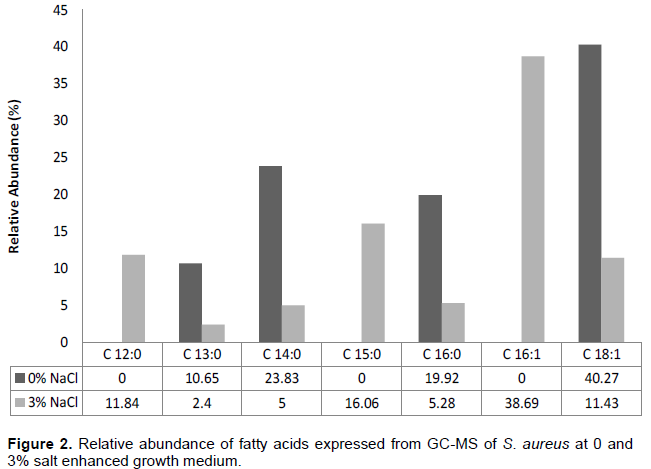
From the GC-MS analysis of E. coli at 0 and 3% salt inclusion as seen in Figure 1, four compounds; three of which are fatty acids namely: pentadecylic acid, oleic acid and palmitic acid where resolved for control condition, while for salt stressed condition, five compounds where resolved with four of them being fatty acids: pentadecylic acid, palmitinoleic acid, oleic acid and palmitic acid. Also, GC-MS analysis of S. aureus at 0 and 3% salt inclusion (Figure 2), reveals six compounds with four of them being fatty acids, namely: malonic acid, myristic acid, oleic acid and palmitic acid, while for salt stressed conditions, eight compounds were resolved with seven, being fatty acids: lauric acid, malonic acid, myristic acid, pentadecylic acid, palmitoleic acid, oleic acid and palmitic acid, respectively.
The level of unsaturation of fatty acids was generally observed to increase in the salt stressed S. aureus condition (3% salt inclusion) as compared to the control, just as the crucial role of unsaturated fatty acids in the microbial stress response mechanisms has been previously reported by Russel et al. (1995). There was a decrease in relative abundance of C 18:1 (oleic acid) observed in both Gram negative E. coli and Gram positive S. aureus at salt stressed condition than in control conditions. Gale and Llewellin (1971) suggested that oleate of the membrane may be linked with K+ accumulation; therefore, they inferred that oleate is also necessary for the stability of S. aureus membrane. The inner concentration of NaCl depends on the medium in which S. aureus is grown (Heller et al., 1998) and appears to be strictly related to internal K+ concentration, indicating membrane activity (Hurst et al., 1973).
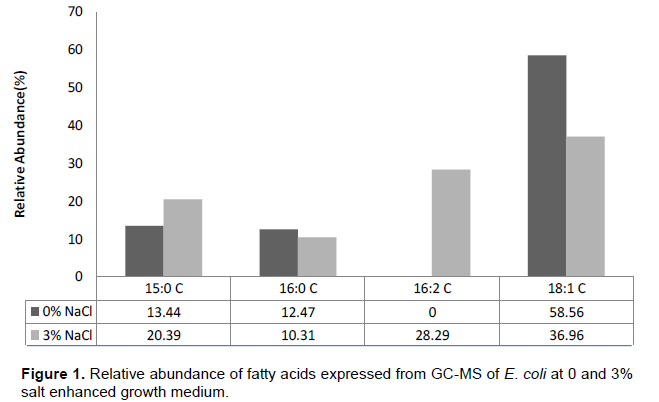
The presence of C16:2 in the cell extracts of E. coli (3% NaCl conc.) contradict the generally accepted observa-tion that polyunsaturated fatty acids do occur among members of the order Eubacteriales (Shaw and Stead, 1971). However, many authors have reported polyun-saturated fatty acids in bacteria. Katalinic and Fischer (1998), reported a C18:2 among fatty acids of Bacillus megaterium about 5% of the total acids. Yano et al. (1971), studied the fatty acids of Arthrobacter simplex grown in a variety of hydrocarbon-based media and reported the presence of C18:2 to be about 20% of the total fatty-acid composition, and confirmed their results by using mass spectroscopy. Therefore, that bacteria are capable of de novo synthesis of polyunsaturated fatty acids is evidenced by the work of Yano et al. (1971), who showed conversion of hydrocarbons, used as sole carbon sources, to polyunsaturated fatty acids.
Medium chain fatty acids with chain length between C8 to C14 were observed to be expressed in S. aureus at both control and salt -stressed condition which was not seen in E. coli at any of its salt conditions. This may further suggest one of the mechanisms S. aureus uses naturally to survive in salt stressed environment confirming it been halotolerant.
The lipid and fatty acid compositions of both bacteria considered were altered when the NaCl concentration of the growth medium were varied and in response to changes in growth salt conditions, the alteration of lipids obtained by on-probe GC-MS sample pre-treatment showed increased expression of unsaturated fatty acids at 3% NaCl concentration than 0% NaCl condition. Findings of this work also contribute to the compre-hension of the membrane fatty acids modulation as it occur in bacteria in relation to their exposure to salt environment as previously suggested.
The author(s) did not declare any conflict of interest.
We wish to appreciate Mrs. Kolawole of the department of Biochemistry, FUTA for her kind assistance.
REFERENCES
|
Bligh EG, Dyer WJ (1959). A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911-917.
Crossref
|
|
|
|
Brewer MS (2000). In: R.K. Robinson, C.A. Batt and P. Patel (Eds). Encyclopedia of Food Microbiology, (Academic Press, New York).
|
|
|
|
|
Colavita G, Sessa M, Giaccone V, Vergara A (2003). Effect of NaCl concentration on the survival and growth of coliforms in raw seasoned sausages. Vet. Res. Commun. 1:293-295
Crossref
|
|
|
|
|
Gale EF, Llewellin JM (1971). Effect of unsaturated fatty acids on aspartate transport in Staphylococcus aureus and on staphylococcal lipid monolayers. Biochim. Biophys. Acta 233:237-242.
Crossref
|
|
|
|
|
Goldfine H (1982). Lipids of prokaryotes: structure and distribution. Curr. Top. Membr. Transp. 17:1-43.
Crossref
|
|
|
|
|
Heller R, Holler C, Sussmuth R, Gundermann KO (1998). Effect of salt concentration and temperature on survival of Legionella pneumophila. Lett. App. Microbiol. 26(1):64-8.
Crossref
|
|
|
|
|
Hunter MIS, Olawoye TL, Saynor DA (1981). The effect of temperature on the growth and lipid composition of the extremely halophilic coccus, Sarcina marina. Antonie Van Leeuwenhoek 47:25-40.
Crossref
|
|
|
|
|
Hurst A, Hughes A, Beare-Rogers JL, Collins-Thompson DL (1973). Physiological Studies on the Recovery of Salt Tolerance by S. aureus after Sublethal Heating. J. Bacteriol. 116(2):901-907.
|
|
|
|
|
Katalinic JP, Fischer W (1998). α-D-Glucopyranosyl-, D-alanyl- and L-lysylcardiolipin from Gram-positive bacteria: analysis by fast atom bombardment mass spectrometry. J. Lipid Res. 39:2286-2292.
|
|
|
|
|
Kogut M, Russel NJ (1984). The growth and phospholipid composition of a moderately halophilic bacterium during adaptation to changes in salinity. Curr. Microbiol. 10:95-98.
Crossref
|
|
|
|
|
Komaratat P, Kates M (1975). The lipid composition of a halotolerant species of Staphylococcus epidermidis. Biochim. Biophys. Acta 398:464-484.
Crossref
|
|
|
|
|
Kuroda M, Tanaka Y, Aoki R, Shu D, Tsumoto K, Ohta T (2008). Staphylococcus aureus giant protein Ebh is involved in tolerance to transient hyperosmotic pressure. Biochem. Biophys. Res. Commun. 374(2):237-241.
Crossref
|
|
|
|
|
Lopez CS, Alice AF, Heras H, Rivas EA, Sanchez-Rivas C (2006). Role of anionic phospholipids in the adaptation of Bacillus subtilis to high salinity. Microbiology 152(3): 605-616.
Crossref
|
|
|
|
|
Miller KJ (1985). Effects of temperature and Sodium Chloride concentration on the phospholipid and fatty acid compositions of a halotolerant Planococcus sp. J. Bacteriol. 162:263-270.
|
|
|
|
|
Morrow MR, Singh DM, Grant CW (1995). Glycosphingolipid headgroup orientation in fluid phospholipid/ cholesterol membranes: similarity for a range of glycolipid fatty acids. Biophys. J. 69(3): 955-964.
Crossref
|
|
|
|
|
Nagamachi E, Hirai Y, Tomochika K, Kanemasa Y (1992). Studies on osmotic stability of liposomes prepared with bacterial membrane lipids by carboxyfluorescein release. Microbiol. Immunol. 36(3):231-234.
Crossref
|
|
|
|
|
Oku Y, Kurokawa K, Ichihashi N, Sekimizu K (2004). Characterization of the Staphylococcus aureus mprF gene, involved in lysinylation of phosphatidylglycerol. Microbiology 150:45-51.
Crossref
|
|
|
|
|
Peeler TC, Stephenson MB, Einspahr KJ, Thompson GA (1989). Lipid Characterisation of an enriched Plasma membrane fraction of Dunaliella salina Grown in Media of Varying Salinity. Plant Physiol. 89: 970-976.
Crossref
|
|
|
|
|
Romantsov T, Helbig S, Culham DE, Gill C, Stalker L, Wood JM (2007). Cardiolipin promotes polar localization of osmosensory transporter ProP in Escherichia coli. Mol. Microbiology 64(6):1455-1465.
Crossref
|
|
|
|
|
Russel NJ, Evans RI, Ter Steeg PF, Hellemons J, Verheul A, Abee T (1995). Membranes as a target for stress adaptation. Int. J. Food Microbiol. 28: 255–261.
Crossref
|
|
|
|
|
Shaw N (1970). Bacterial Glycolipids. Bacteriol. Rev. 34(4): 365-377.
|
|
|
|
|
Shaw N, Stead D (1971). Lipid composition of some species of Arthrobacter. J. Bacteriol. 107:103-133.
|
|
|
|
|
Tiecco G (2000). Microbiologia degli Alimenti di Origine Animale, Calderini Edagricole, Bologna
|
|
|
|
|
Wilkinson BJ (1996). The staphylococci in human disease. In: Crossley KB, Archer GL, editors. Biology. Churchill Livingstone.
|
|
|
|
|
Wuthier RE (1966). Two-dimensional chromatography on silica gel-loaded paper for the micro-analysis of polar lipids. J. Lipid Res. 7: 544-550.
|
|
|
|
|
Yano I, Furukawa Y, Kusunose M (1971). Fatty-acid composition of Arthrobacter simplex grown on hydrocarbons. Occurrence of ahydroxy- fatty acids. Eur. J. Biochem. 23:220-228.
Crossref
|
|
|
|
|
Zambonelli C, Papa F, Romano P, Suzzi G, Grazia L (1992). Microbiologia dei Salumi. Calderini Edagricole, Bologna.
|
|