ABSTRACT
Cadmium (Cd) toxicity is influenced by dietary components, such as fiber and minerals. Garden egg, carrot and oat are rich sources of fiber. Thus, this study examines the effect of Solanum melongena (garden egg), Daucus carota (carrot) and oat-supplements on selected biochemical parameters in the plasma and tissues of cadmium-exposed rats. Twenty-five healthy male Wistar rats (140±50 g) were distributed into five treatment groups in which rats in group one were not exposed to cadmium, and served as control while rats in group two were exposed to cadmium only in addition to their normal diet. Cadmium was administered by gastric intubation at a dose of 5 mg Cd/kg body weight as CdCl2. H2O was given three times a week for six weeks. Rats in Groups 3 to 5 were treated similarly with cadmium, but with their normal diet supplemented with 5% garden egg, carrot and oat, respectively. A significant (P<0.05) increase was observed in alanine aminotransferase (ALT)/aspartate aminotransferase (AST) activity in the plasma/kidney of rats exposed to Cd, while a significant (P<0.05) decrease was observed in liver ALT/AST activity. Likewise, the levels of liver, kidney and intestine alkaline phosphatase (ALP), superoxide dismutase (SOD) and lipid peroxidation were increased compared to the control. Conversely, feeding with garden egg, carrot and oat significantly (P<0.05) reversed these effects of cadmium, compared to rats maintained on cadmium only. The results suggest that garden egg, carrot and oat contain bioactive/antioxidant properties which help in ameliorating cadmium toxicity.
Key words: Cadmium, lipid peroxidation, dietary fiber, carrot.
Cadmium (Cd) is an abundant and ubiquitously distributed heavy toxic metal that is widely used in modern industries (Novelli et al., 2000). It is of great commercial importance due to its agricultural and industrial use (WHO, 2000; Jarup, 2003), but is a serious environmental and industrial pollutant because it easily contaminates soil, plants, air and water (Ognjanovic et al., 2010). Cd has a long biological half-life, contaminates water and food and accumulates in human tissues, especially the liver and kidney, causing damage (WHO, 2000). It is not biodegradable and thus the risk of human exposure is constantly increased as it enters the food chain (Agency for Toxic Substances and Disease Registry (ATSDR), 2008). Exposure to cadmium through food sources, especially leafy vegetables is also common and it is the main route of exposure for the non-smoking and non-occupationally exposed population (Kierstin, 2003). Cadmium toxicity results from its promotion of oxidative damage by increasing the cellular concentration of reactive oxygen species (ROS) and by reducing the cellular antioxidant capacity (Corticeiro et al., 2006). During exposure, cadmium accumulates predominantly in the liver, kidneys, reproductive organs and tissues (Godt et al., 2006; Takamure et al., 2006) and this is due to the liver and kidney being the most susceptible organs (Asagba, 2009). Increase in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities in the blood is a manifestation of Cadmium-induced damage of the liver (Kowalczyk et al., 2003). At the cellular level, cadmium induces oxidative stress, cell proliferation, and apoptosis (Turner and Lysiak, 2008; Tremellen, 2008).
Efforts to ameliorate cadmium (Cd) absorption and toxicity are thus of great importance since exposure to Cd due to environmental contamination is still inevitable in many populations (Callegaro et al., 2010). Ishihara et al. (2001) reported that nutritional deficiencies are believed to have aggravated the concurrent liver, kidney and bone disorders seen in “Itai-Itai” disease patients. Recent studies have shown a correlation between cadmium related kidney and bone diseases and nutritional deficiencies, such as proteins, trace elements and antioxidants (Asagba et al., 2004; Lawal and Ellis, 2011; Ferramola et al., 2012). This, according to Asagba et al. (2004), indicates that nutritional status greatly influences the metabolic fate and toxicity of cadmium. Liu et al. (2004) reported that diets containing high fiber content decrease absorption of cadmium and Asagba and Eriyamremu (2007) reported that the uptake, distribution and toxicity of cadmium are influenced by the type or composition of the diet of a population. Fruits and vegetables have long been recognized as very useful in controlling and modulating various functions in the body to maintain normal state of health and reduce the risk of diseases. This is because they contain phytochemicals that help in the prevention of diseases (Prior, 2003) and fiber, which pushes food through the digestive system, absorbing water and easing defecation. Fibers act by changing the nature of the contents of the gastrointestinal tract and influencing how other nutrients and chemicals are absorbed (Eastwood and Kritchevsky, 2005). Carrot, garden egg and oat are good sources of crude fiber (Ejoh et al., 1996), but few studies have evaluated their effects on Cd toxicity. The present study was aimed at investigating the effects of carrots, garden egg and oat- on some biochemical parameters in the plasma and tissues of cadmium exposed rats.
Chemicals
The reagents used in this study were of analytical grade from the British Drug House.
Plant materials
Garden egg, carrot and oat were purchased from Effurun market, Effurun, Delta State, Nigeria. Garden egg and carrot were grated and sun-dried to constant weight, while the oat was mashed with hands. Experimental diets were then prepared by supplementing grower’s mash with 5% of the processed garden egg, carrot and oat.
Experimental animals
Twenty five healthy male Wistar rats with an average weight of 140±5 g were used for this study. They were obtained from the animal house, Faculty of Basic Medical Sciences, Delta State University, Abraka, and housed in standard cages. They were allowed for two weeks to acclimatize to laboratory condition before the commencement of the experiment. The animals were treated in accordance with internationally accepted standards and protocols for animal care.
Experimental design
The rats were distributed into five treatment groups with five rats in each group. Rats in group one were not exposed to cadmium and served as control. Rats in group two were exposed to cadmium only in addition to their normal diet. Cadmium was administered by gastric intubation at a dose of 5 mg Cd/kg body weight as CdCl2. H20 three times a week for six weeks. Rats in Groups 3 to 5 were treated similarly with cadmium, but with their normal diet supplemented with 5% garden egg, carrot and oat, respectively. At the end of the treatment period, the rats were weighed and sacrificed by cervical dislocation.
Collection and treatment of samples
The abdominal and thoracic region of each rat was opened with the aid of dissecting kit to expose the kidney, heart, intestines, etc. Blood was collected from the heart by means of 5 ml hypodermic syringe and needle into a heparinised tube; it was centrifuged at 3,000 g for 10 min, and the plasma was obtained for biochemical assay. The liver, kidney and intestine of each rat were excised and weighed, and 10% homogenates were prepared under cold conditions with phosphate buffer (pH 7.4). The homogenates were centrifuged at 5000 g for 10 min and the supernatants were carefully separated from the residue and used for biochemical assays.
Biochemical analysis
The activities of ALT and AST in the plasma and tissues were assayed by the method of Reitman and Frankel (1957). The activity of the aminotransferases is expressed in units/ml. Assay for superoxide dismutase (SOD) was done by the method of Misra and Fridovich (1972) based on the inhibitory effect of SOD on the initial rate of epinephrine auto-oxidation. The activity of SOD is expressed in unit/g tissue. One unit is the amount of the enzyme necessary to cause 50% inhibition of the oxidation of epinephrine to adenochrome for 1 min. The activity of alkaline phosphate (ALP) in the tissues was determined by the method of Annino and Giese (1976). The activity of the enzyme is expressed in units/g tissue. One unit is the amount of the enzyme that forms one micromole of p-nitrophenol per minute. The level of thiobarbituric acid reactive substances (TBARS) which is an index of lipid peroxidation was determined by the method of Gutteridge and Wilkins (1982). Values of TBAS are reported in terms of malondialdehyde (MDA) and expressed as µmole MDA/g tissue. The amount of MDA in the samples was quantified using a molar extinction coefficient of 1.56ËŸ105 m/cm.
Data analysis
The values are reported as Mean ± SEM. The mean values between the groups were compared by using analysis of variance (ANOVA) and least significance test (LSD) procedure using the statistical package for the social sciences software (SPSS). The results were considered significant at P<0.05 level.
The effect of garden egg, carrot and oat on body weight gain and organ/body weight ratio of rats exposed to cadmium is presented in Table 1. The body weight gain of rats exposed to cadmium (Cd) significantly (p< 0.05) decreased in comparison to the control. However, this parameter significantly (p< 0.05) increased on feeding the Cd exposed rats with diet supplemented with garden eggs, carrots and oats in relation to rats fed on Cd+ Normal Diet only (control, not included). The above observation was the same trend for organ/ body weight ratio. Consequently, the study indicates that feeding of garden egg, carrots and oats to rats can reduce the effect of cadmium on body weight gain and organ/ body weight ratio of the rats. The effect of garden eggs, carrot and oat on levels of SOD and lipid peroxidation in the liver, kidney and intestine of rats exposed to cadmium are presented in Table 2. The liver SOD level of rats administered Cd significantly (p <0.05) decreased compared to the control. However, feeding cadmium exposed rats with garden egg, carrot and oat increased the SOD level significantly (p<0.05) in relation to rats maintained on Cd and normal diet only. The garden egg, carrot, and oat caused 105.1, 57.9 and 82.4%, increase respectively. Like in the liver, the kidney SOD level of rats exposed to Cd decreased significantly (p<0.05) in relation to the control.
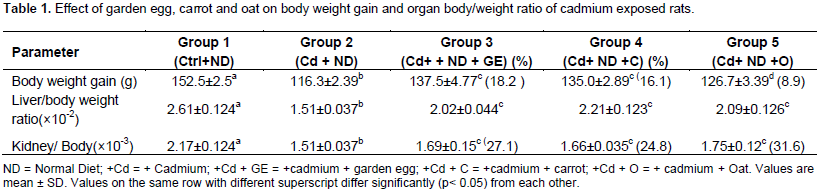
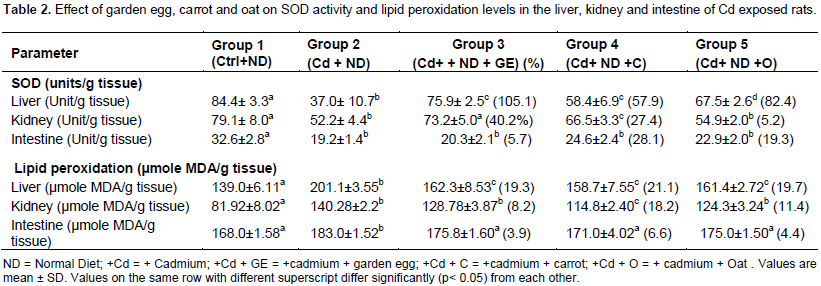
Conversely, this enzyme increased significantly (p<0.05) on feeding cadmium exposed rats with garden egg, carrots, and oat in relation to rats maintained on Cd and normal diet only. Garden egg, carrots and oat caused 40.2, 27.4 and 5.2%, increase respectively. Also, like in the liver and kidney, the intestine SOD level of cadmium exposed rats decreased significantly (p<0.05) in relation to the control. However, unlike the liver and the kidney, the SOD level of intestine of cadmium exposed rats fed with garden egg, carrots and oat was not significantly different in relation to rats maintained on Cd and normal diet only. Thus, the study indicates that feeding of rats with garden egg, carrot and oat ameliorated effect of cadmium on SOD activities in both liver and kidney, but not in intestine. The effect of garden egg, carrot and oat on the level of lipid peroxidation in the liver, kidney and intestine of rats administered cadmium is presented in Table 2. The liver lipid peroxidation of rats exposed to Cd increased significantly (p<0.05) compared to the control. This however decreased significantly (p<0.05) on feeding the Cd exposed rats with garden egg, carrots and oat by percentage of 19.3, 21.1 and 19.7, respectively, in relation to the rats exposed to Cd only. Similarly, the kidney lipid peroxidation level of rats administered Cd increased significantly (p<0.05) in comparison to the control.
However, when the Cd exposed rats were fed garden egg, carrot and oat, there was an insignificant decrease (for garden egg and oat) and a significant (P<0.05) decrease (for carrot) in the level of lipid peroxidation compared to rats maintained on Cd only. The percentage decrease in kidney lipid peroxidation was 8.2, 18.2 and 11.4% of garden egg, carrot and oats, respectively. Like the liver and kidney, the intestine LPO level of rats administered Cd increased significantly in relation to the control, but insignificantly decreased on feeding with garden egg, carrot and oat in relation to the rats maintained on Cd only. The decrease caused by garden egg, carrot and oat was 3.9, 6.6 and 4.4%, respectively. The study thus indicates that garden egg, carrot and oat have the ability to reduce the effect of cadmium on tissue membrane lipid peroxidation. Table 3 represents the effects of garden egg, carrot and oat on ALP activity in the liver, kidney and intestine of cadmium exposed rats. In the liver, the alkaline phosphates activity of Cd exposed rats was significantly (p<0.05) reduced compared to the control. However, feeding Cd exposed rats with garden egg, carrot and oat had no significant effect (except with carrot which was observed to have a significant increase) on ALP activity in relation to rats maintained on Cd only.
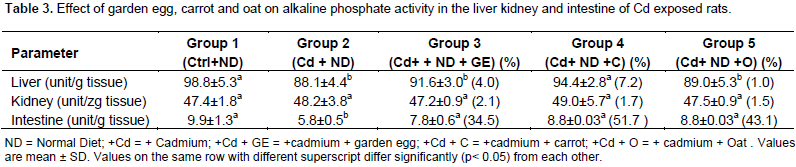
The Kidney ALP activity of rats treated with Cd was not significantly different from control. The activity of the enzyme remained comparable to the control even after the feeding of carrot, garden egg and oat to cadmium treated rats. The intestine ALP activity of Cd exposed rats was observed to be significantly reduced in relation to control. On the other hand, the activity of the enzyme increased significantly (p<0.05) on feeding with garden egg, carrot and oat by 34. 5, 5.7 and 43.1%, respectively in relation to rats maintained on Cd only. Table 4 shows the effect of garden egg, carrot and oat on ALT and AST activities in the liver, plasma and kidney of rats administered cadmium. The kidney ALT activity of Cd exposed rats was significantly (p<0.05) increased in relation to control. This was reversed when garden eggs, carrot and oat were added to the diet of the exposed rats as the ALT activity was reduced significantly (p<0.05) in comparison with those maintained on Cd only. The garden egg, carrot and oat caused 48.9, 13.8 and 8.8%, decrease respectively. The ALT activity of the liver of Cd exposed rats decreased significantly (p<0.05) in relation to control. However, with garden egg, carrots and oat supplements in the diet of the Cd exposed rats, there was significant (p<0.05) increase in the ALT activity in relation to rats maintained on Cd only. The increase caused by garden egg, carrot and oat was to the tune of 166.7, 162.5 and 170.8%, respectively.
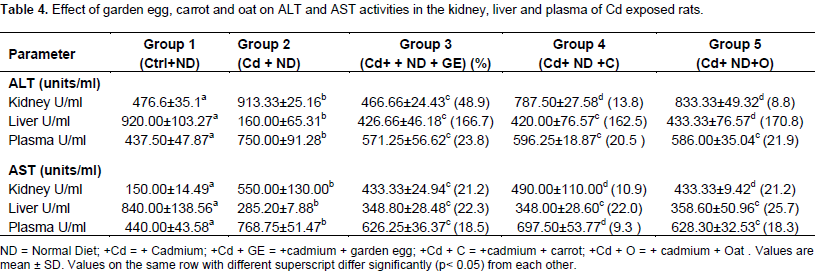
The plasma of rats administered cadmium had a significantly (p<0.05) increased ALT activity in comparison to the control. When fed with garden egg, carrot and oat, the activity of plasma ALT of Cd exposed rats was grossly reversed significantly (p<0.05) in relation to those maintained on Cd only. Garden egg, carrot and oat reduced the effect of Cd on plasma ALT activity by 23.8%, 20.5% and 21.9%, respectively. The study therefore shows that supplementing diet of rats with garden egg, carrot and oat reversed the cadmium decrease of liver ALT activity and Cd induced increase of both kidney and plasma ALT activity.The kidney AST activity of Cd exposed rats significantly (p<0.05) increased in relation to control. Conversely, the activity of the enzyme was significantly reduced by 21.2, 10.9 and 21.2% relative to those maintained on Cd only when Cd exposed rats were fed with garden egg, carrots and oat supplemented diets, respectively. The liver AST activity was significantly (p<0.05) reduced in rats administered Cd compared to the control. However, on feeding with garden egg, carrot and oat, there was a significant (p<0.05) increase in the liver AST activity in relation to rats maintained on Cd only. The increase caused by garden egg, carrot and oat was 22.3, 22.0 and 25.7%, respectively. The plasma AST activity of Cd treated rats was significantly (p<0.05) increased in relation to the control. However, when fed with garden egg, carrot and oat, there was a significant reduction of AST activity compared to rats maintained on Cd only by 18.5, 9.3 and 18.3%, respectively. Thus, the study indicates that feeding of garden egg, carrot and oat ameliorates the effects of cadmium on plasma and tissue AST activity.
An attempt was made in this study to examine the effect of garden egg, carrot and oat on some biochemical parameters in the organs and plasma of Cd-exposed rats. The significant decrease (P<0.05) of body weight gain of Cd exposed rats when compared to control is consistent with previous studies (Eriyamremu et al., 2005; Asagba and Eriyamremu, 2007). The significant difference (P<0.05) noticed in the weight gain and organ/body weight ratio in the Cd exposed rats (compared to the control) is an indication of Cd toxicity which is also consistent with previous reports (Asagba et al., 2004; Asagba and Eriyamremu, 2007; Eriyamremu et al., 2005). It has been established that weight loss caused by cadmium can be linked to its influence on nutrient digestion and availability (Eriyamremu et al., 2005). The reversal of the effect of Cd on body weight gain and organ/body weight ratio by garden egg, carrot and oat suggests strongly that these plants have bioactive components that may be protective in Cd toxicity. The result of the present study indicates that the administration of Cd induces peroxidative injury in the liver, kidney and intestine of rats, which is mediated by the inhibition of SOD activity and increase in the level of lipid peroxidation (Table 2). This is consistent with findings of previous studies (Asagba et al., 2004; 2006; Asagba and Eriyamremu, 2007; Ding et al., 2013, Nazima et al., 2015; Ayala et al., 2014; Akomolafe et al., 2016, Oyinloye et al., 2016).
One of the mechanisms attributed for the inhibition of SOD by Cd is the displacement of essential metals such as copper and zinc from the enzyme (SOD) and the inhibition of SOD activity might lead to oxidative stress (Timbrell, 1995). Under the condition of oxidative stress, lipid peroxidation occurs as evidenced by the production of malondialdehyde (MDA), which can be used as an index of per oxidative injury in vivo and susceptibility of tissues to oxidative stress. In addition, Arroyo et al. (2012) showed that Cd competes with essential metals such as zinc, selenium, copper and calcium and thus interfere with various cellular processes such as metal membrane transport and energy metabolism. The plasma, liver and kidney were assayed for amino transferees (ALT/AST) activities, which are the indices of tissues (particularly liver) damage (WHO, 2000). According to reports, exposure to Cd may lead to kidney and liver damage (Alfvén et al., 2002; Asagba et al., 2002, 2006). Thus, the activity of plasma ALT/AST is expected to increase in Cd exposed rats and decrease correspondingly in the liver as damage to this organ may lead to leakage of these enzymes into the plasma (Lee et al., 2009; Kowalczyk et al., 2003; Shati, 2011). The result of the study confirmed this for plasma and liver ALT/ AST activities (Table 4). However, it is noteworthy that the kidney ALT/AST activity of Cd exposed rats was higher compared to control (Table 4).
Reports show that amino transferees are involved in amino acid metabolism (Kowalezyk et al., 2003). Thus, the observed increase in the activity of kidney amino transferees may be an indication of increased metabolism of amino acid occasioned by Cd stress in this organ. Cd contamination has severally been shown to induce oxidative stress, which leads to the progression of severe pathological conditions after prolonged retention in tissues (Baba et al., 2013; Matović et al., 2011). Many authors have shown that Cd metabolism in the body promotes the generation of reactive oxygen species (ROS) such as superoxide ion, hydroxyl radicals and hydrogen peroxide (Baba et al., 2013; El-Refaiy and Eissa, 2013). The toxicity of Cd can also be accounted for by the reduction in the activity of alkaline phosphatase (ALP) in the liver, kidney and intestine of Cd exposed rats as recorded in this study. Cadmium, being a non-redox metal, is capable of indirectly eliciting oxidative damage to the liver by depleting cellular antioxidant levels especially glutathione as well as depleting protein-bound sulfhydryl groups (Wang et al., 2015). According to Timbrell (1995), the inhibition of enzymes such as ALP by Cd may be by the displacement of essential co-factors in the active site of the enzyme by Cd and also by binding of Cd to SH groups which are essential for the activity of enzyme.
Furthermore, the inclusion of garden egg, carrot and oat to the diet of Cd exposed rats ameliorated the effect of Cd on the above parameters in the indicated organs. This shows that these plants may be effective against Cd induced stress. This may be because they contain antioxidants and high fiber content, which has been shown to terminate formation of free radicals (Sarkar et al., 1995; Anderson et al., 2009; Noda et al., 2001) and Chelates metals (Asagba et al., 2004) in biological systems, respectively. Reports show that these plants contain antioxidants such as Vitamin A, C, and E, Caffeic acid, chlorogenic acid, amongst others and are rich in crude fiber, which have been shown to reduce Cd uptake (Jung et al., 2011; Bliss and Elstein, 2004; Berglund et al., 1994; Asagba et al., 2004). Comparatively, no consistent trend is noticeable for the effect of these plants on Cd induced toxicity in rats. It can therefore be assumed that any of these plants is equally effective in ameliorating Cd toxicity in rats.
The results of the presents study indicate that cadmium caused an increase in the level of lipid peroxidation and the activity of plasma and kidney ALT/AST and a decrease in the activity of liver ALT/AST. Likewise, the activity of ALP and SOD in the liver, kidney and intestine of rats was also decreased. But feeding with garden egg, carrot and oat reversed these effects of cadmium, except for the effect on intestine SOD activity. These results indicate that these plants are protected from Cd toxicity.
The authors have not declared any conflict of interests.
We sincerely appreciate the laboratory staff of the Department of Biochemistry, Delta State University, Abraka for their technical assistance and the Department for making some equipment and reagents available.
REFERENCES
|
Agency for Toxic Substances and Disease Registry (ATSDR) (2008). Toxicological Profile for Cadmium. Draft for public comment. US Department of Health and Human Services. Atlanta, US.
|
|
|
|
Akomolafe RO, Imafidon CE, Olukiran OS, Oladele AA, Ajayi AO (2016) livolin forte® ameliorates cadmium-induced kidney injury in wistar rats. Ser. J. Exp. Clin. Res. 17(2):107-116.
Crossref
|
|
|
|
|
Alfvén T, Järup L, Elinder CG (2002). Cadmium and lead in blood in relation to low bone mineral density and tubular proteinnuria. Environ. Health Perspect. 110:699-702.
Crossref
|
|
|
|
|
Anderson JW, Baird P, Davis RH (2009). Health Benefits of dietary fiber. Nutr. Rev. 67(4):188-205.
Crossref
|
|
|
|
|
Annino JS, Giese RW (1976) Clinical Chemistry. Principles and procedure, 4th edn. Boston: Little Brown.
|
|
|
|
|
Arroyo VS, Flores KM, Ortiz LB, Gómez-Quiroz LE, Gutiérrez-Ruiz MC (2012). Liver and cadmium toxicity. J. Drug Metab. Toxicol. S5:001
|
|
|
|
|
Asagba SO (2009) Role of diet in absorption and toxicity of oral cadmium-A review of literature. Afr. J. Biotechnol. 8(25):7428-436.
|
|
|
|
|
Asagba SO, Adiakpoh MA, Kadiri H, Obi FO (2006). Influence of aqueous extract of Hibiscus sabdariffa L. Petals on cadmium toxicity in rats. Biol. Trace Elem. Res. 115:37-47
|
|
|
|
|
Asagba SO, Eriyamremu GE (2007) Oral cadmium exposure alters haematological and liver function parameters of rats fed a Nigerian-like diet. J. Nutr. Environ. Med. 16(3-4):267-274
Crossref
|
|
|
|
|
Asagba SO, Eriyamremu GE, Adaikpoh MA Ezeoma A (2004) Levels of lipid peroxidation, superoxide dismutase and Na+/K+ ATPase in some tissues of rats exposed to a Nigerian-like diet and cadmium. Biol. Trace Elem. Res. 100(1):75-86.
Crossref
|
|
|
|
|
Asagba SO, Isamah GK, Ossai EK, Ekakitie AO (2002). Effect of oral exposure to cadmium on the levels of vitamin A and lipid per oxidation in the eye. Bull. Environ. Contam. Toxicol. 68:18-21.
Crossref
|
|
|
|
|
Ayala A, Munoz MF, Arguelles S (2014). Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 31:2014
Crossref
|
|
|
|
|
Baba H, Tsuneyama K, Yazaki M, Nagata K, Minamisaka T, Tsuda T, Nomoto K, Imura J (2013). The liver in itai–itai disease (chronic cadmium poisoning): pathological features and metallothionein expression. Mod. Pathol. 26(9):1228-1234.
Crossref
|
|
|
|
|
Berglund M, Akesson A, Nermell B, Vahter M (1994). Intestinal absorption of dietary cadmium. Toxicol. Lett. 113:219-225.
|
|
|
|
|
Bliss RM, Elstein D (2004). Scientists get under eggplant's skin. ARS magazine.
|
|
|
|
|
Callegaro MG, Milbradt BG, Diettrich T, Alves E, Duarte FA, Flores EM, Dressler VL, Silva LP, Emanuelli T (2010) Influence of cereal bran supplement on cadmium effects in growing rats. Hum. Exp. Toxicol. 6:467-76.
Crossref
|
|
|
|
|
Corticeiro SC, Lima AIG, Figueira EMP (2006). The importance of glutathione in oxidative status of Rhizobium leguminosarum biovar viciae under Cd exposure. Enzyme Microb. Technol. 40:132-137.
Crossref
|
|
|
|
|
Ding Y, Zhang T, Tao JS, Zhang LY, Shi JR, Ji G (2013) Potential hepatotoxicity of geniposide, the major iridoid glycoside in dried ripe fruits of Gardenia jasminoides (Zhi-zi). Nat. Prod. Res. 27(10):929-933
Crossref
|
|
|
|
|
Eastwood M, Kritchevsky D (2005). Dietary Fiber: How did we get where we are? Annu. Rev. Nutr. 25:1-8.
Crossref
|
|
|
|
|
Ejoh A, Mbiapo F, Fakou E (1996). Nutritional composition of the leaves and flowers of Colocasia esculentum and Solanum melongena. Plant Foods Hum. Nutr. 49:107-112.
Crossref
|
|
|
|
|
El-Refaiy AI, Eissa FI (2013). Histopathology and cytotoxicity as biomarkers in treated rats with cadmium and some therapeutic agents. Saudi J. Biol. Sci. 20(3):265-280.
Crossref
|
|
|
|
|
Eriyamremu GE, Asagba SO, Onyeneke EC, Adaikpo MA (2005). Changes in carboxypeptidase A, dipeptidase and Na+/K+ ATPase activities in the intestine of rats orally exposed to different doses of cadmium. BioMetals 18:1-6.
Crossref
|
|
|
|
|
Ferramola ML, Pérez Díaz MF, Honoré SM, Sánchez SS, Antón RI, Anzulovich AC, Giménez MS (2012). Cadmium-induced oxidative stress and histological damage in the myocardium: Effects of a soy-based diet. Toxicol. Appl. Pharmacol. 265:380-389.
Crossref
|
|
|
|
|
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P (2006). The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 10(1):22.
Crossref
|
|
|
|
|
Gutteridge JMC, Wilkins S (1982). Copper-dependent hydroxyl radical damage to ascorbic acid: formation of a thiobarhuric acid reactive product. FEBS Lett. 137(2):327-330.
Crossref
|
|
|
|
|
Jarup L (2003) Hazards of heavy metal contamination. Br. Med. Bull. 68:167-182.
Crossref
|
|
|
|
|
Jung EJ, Bae MS, Jo EK, Jo YH, Lee SC (2011). Antioxidant activity of different parts of eggplant. J. Med. Plants Res. 5(18):4610-4615.
|
|
|
|
|
Kierstin PG (2003). Lactational transfer of cadmium in rodents – CNS: Effects in the offspring. Acta Universitalis Agriculturae Sueciae. Veterinaria Vol 150. Doctoral Thesis. pp. 10-11.
|
|
|
|
|
Kowalczyk E, Kopff A, Fijalkowski P, Kopff M, Niewarok J, Blaszezyk J, Kedziora J, Tyslerowicz P (2003) Effect of anthocyanins on selected biochemical parameters in rats exposed to cadmium. Acta Biochim. Pol. 50(2):543-548.
|
|
|
|
|
Lawal AO, Ellis EM (2011). The chemopreventive effects of aged garlic extract against cadmium-induced toxicity. Environ. Toxicol. Pharmacol. 32:266-274.
Crossref
|
|
|
|
|
Lee JR, Park SJ, Lee HS, Jee SY, Seo J, Kwon YK (2009). Hepatoprotective Activity of Licorice Water Extract against Cadmium-induced Toxicity in Rats. Evid. Based Complement. Alternat. Med. 6(2):195-201.
Crossref
|
|
|
|
|
Liu L, Zubik L, Collins FW, Mark M, Meydani M (2004). The anthiatherogenic potential of oat phenolic compounds. Atherosclerosis 175(2):39-49.
Crossref
|
|
|
|
|
Matović V, Buha A, Bulat Z, Äukić-Ćosić D (2011). Cadmium toxicity revisited: focus on oxidative stress induction and interactions with zinc and magnesium. Arh Hig Rada Toksikol 62(1):65-75.
Crossref
|
|
|
|
|
Misra HP, Fridovich I (1972). The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. Biol. Chem. 247:3170-3176.
|
|
|
|
|
Nazima B, Manoharan V, Miltonprabu S (2015). Grape seed proanthocyanidins ameliorates cadmium-induced renal injury and oxidative stress in experimental rats through the up-regulation of nuclear related factor 2 and antioxidant responsive elements. Biochem. Cell Biol. 93(3):210-226.
Crossref
|
|
|
|
|
Noda Y, Kneyuki T, Igarashi K (2001). Antioxidant activity of Nasunin, an anthocyanin in egg plant peels. Toxicology 748 (2-30):119-123.
|
|
|
|
|
Novelli F, Novelli E, Manzano MA, Lopes AM, Cataneo AC, Barbosa LL, Ribas BO (2000). Effects of tocopherol on superoxide radical and toxicity of cadmium exposure. Int. J. Environ. Health Res. 10:125-134.
Crossref
|
|
|
|
|
Ognjanovic BI, Marković SD, Ethordević NZ, Trbojević IS, Stajn AS, and Saicić ZS (2010). Cadmium-induced lipid peroxidation and changes in antioxidant defense system in the rat testes: Protective role of coenzyme Q(10) and vitamin E. Reprod. Toxicol. 29:191-197.
Crossref
|
|
|
|
|
Oyinloye BE, Adenowo AF, Osunsanmi FO, Ogunyinka BI, Nwozo SO, Kappo AP (2016). Aqueous extract of Monodora myristica ameliorates cadmium‑induced hepatotoxicity in male rats. SpringerPlus 5(641):1-7.
Crossref
|
|
|
|
|
Prior RL (2003). Fruits and vegetables in the prevention of cellular oxidative damage. Am. J. Clin Nutr. 78(3):570S-578S.
Crossref
|
|
|
|
|
Reitman S, Frankel S (1957). Glutamic – pyruvate transaminase assay by colorimetric method. Am. J. Clin. Pathol. 28:56.
Crossref
|
|
|
|
|
Sarkar S, Yadav P, Trivedi R, Bansal AK, Bhatnagar D (1995) Cadmium-induced lipid peroxidation and status of the antioxidant system in rat tissues. J. Trace Elem. Biol. 9(3):144-147.
Crossref
|
|
|
|
|
Shati AA (2011). Effects of Origanum majorana L. on cadmium induced hepatotoxicity and nephrotoxicity in albino rats. Saudi Med. J. 32(8):797-805.
|
|
|
|
|
Takamure Y, Shimada H, Kiyozumi M, Yasutake A, Imamura Y (2006). A possible mechanism of resistance to cadmium toxicity in male long-Evans rats. Environ. Toxicol. Pharmacol. 21:231-234.
Crossref
|
|
|
|
|
Timbrell JA (1995). Cadmium. In. Introduction to Toxicology. 2nd Ed. Taylor and Francis, London. pp. 76-77.
|
|
|
|
|
Tremellen K (2008). Oxidative stress and male infertility: A clinical perspective. Hum. Reprod. Update 14(3):243-258.
Crossref
|
|
|
|
|
Turner TT, Lysiak JJ (2008). Oxidative stress: a common factor in testicular dysfunction. J. Androl. 29(5):488-498.
Crossref
|
|
|
|
|
Wang J, Hao M, Liu C, Liu R (2015). Cadmium induced apoptosis in mouse primary hepatocytes: the role of oxidative stress-mediated ERK pathway activation and the involvement of histone H3 phosphorylation. RSC Adv. 5(40):31798-31806.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2000). Environmental Health Criteria. Cadmium International Programme on Chemical Safety (IPCS) Monograph. Geneva (Switzerland): WHO.
|
|