ABSTRACT
The effect of some traditionally extracted edible seed oils (sesame, peanut and melon oils) on some sex hormones - prolactin, progesterone, testosterone, estradiol, luteinizing hormone (LH) and follicle stimulating hormone (FSH) of albino Wistar rats was studied. Sixty (30 - 50 g) weanling rats (20 males and 40 females) were purchased and housed separately until they weighed 120 to 150 g. Afterwards, the rats were cohabited in a mating ratio of 1 male : 2 females respectively, to give rise to 10 groups (n = 6), and fed rat chow (control), rat chow supplemented with 5, 10 and 20% sesame oil, 5, 10 and 20% peanut oil or 5, 10 and 20% melon oils, respectively. The animals were observed and pregnant females were separated into individual cages, allowed to litter and after weaning; the parent male and female rats were sacrificed and blood samples collected for hormonal assays. The results indicate that 5 and 10% supplemented seed oils caused significant increase (p < 0.05) in prolactin level (with a corresponding decrease in progesterone), LH, estradiol and testosterone relative to the controls. This favourable impact on endocrine environment suggests that the consumption of these seed oils, especially sesame oil, may enhance fertility.
Key words: Sesame oil, peanut oil, melon oil, sex hormones.
The term seed oils refer generally to vegetable oils obtained from the seeds (endosperm) of food plants, rather than the fruit (pericarp). The oil-seed-bearing plants are usually grown for the economic and nutritional importance of the oils extracted therefrom; although a few serve for food or textile fibers production, in which case oil is obtained only as a by-product. Sesame, groundnut and melon oils were studied in the present investigation.
Sesame oil is an edible vegetable oil extracted from sesame seeds (also known as beniseed in Nigeria), produced by the sesame plant (Sesamum indicum). Sesamum indicum (family Pedaliacea) originated from tropical Africa (RMRDC, 2004) and is cultivated in the middle belt and some northern states of Nigeria (Olanyanju et al., 2006); hence the seeds constitute a staple food among many ethnic groups in Nigeria. The seeds are either eaten fresh, dried, fried or as a blend with sugar; as well as a paste in some traditional soups.
Several studies have demonstrated the nutritional and health benefits of sesame seeds. The seeds are known to contain reasonable amounts of dietary proteins and fibre and minerals such as iron, magnesium, manganese, copper, phosphorous, zinc and calcium (Aremu et al., 2006). The chemical constituents of the Nigerian variety are akin to those grown in other parts of the world (Bamigboye et al., 2010). Lignans obtained from sesame seeds have been shown to possess antihypertensive, hypolipidemic, anti-inflammatory, anti-cancer and anti-oxidant properties (Ide et al., 2003; Kang et al., 1998; Matumura et al., 1995; Yamashita et al., 1992 and Hirose et al., 1992).
The seed oil is said to combat health conditions like cold, chronic cough and in turn prevents bronchial lung disease (Kanu, 2011). Besides its broad use in cooking, the oil is also used in the manufacture of margarine and some pharmaceuticals (Sangha et al., 2004). Sesamin, the most abundant lignan in sesame oil (Fukuda et al., 1986), is known to contain unique amounts of phyto-estrogens (Jacklin et al., 2003), and hence may exert some profound effects on sex physiology. It is thought also that sesame oil can boosts sex drive and improve sperm counts in males because of its reported high zinc content (Bamigboye et al., 2010). Zinc-rich diet and supplementary zinc are shown to be effective in raising testosterone level, hence improved body composition, sex drive and increase semen volume in men (Shafiei et al., 2011). This claim has however not been established firmly by scientific research, hence form part of the major objectives of the present investigation.
Peanuts oil is a vegetable oil obtained from the grains/seeds of the Arachis hypogaea plant (family Fabaceae). The oil is available in refined, unrefined, cold pressed and roasted varieties; the latter with a strong peanut flavor and aroma, analogous to toasted sesame seeds (Liu et al., 2011). It is used as general cooking oil, with a high smoke point relative to many other cooking oils, which makes it suitable for frying of foods. The major fatty acid components are oleic acid (46.8%), linoleic acid (33.4%) and palmitic acid (10.0%) (USDA, 2011). The oil also contains some stearic, arachidic, arachidonic, behenic and lignoceric acids and other fatty acids. The oil is a significant source of resveratrol, a compound associated with reduction in risk of cardiovascular disease and cancer (Sanders et al., 2000), lowering blood cholesterol level (Kritchevsky, 1988), weight loss (by decreasing appetite), and constipation relieve when topically applied to the rectum. Topical application to the skin is also known to provide relieve from arthritic and joint pains, scalp crusting and scaling, dry skin and other skin problems (Stampfer et al., 1998). However, to our knowledge, there is no available information in respect to its effect on reproductive function.
The Citrullus lanatus plant seeds commonly called melon (or “egusi” in Nigeria), are a source of another traditional cooking oil - melon oil. The seeds have notable nutritional and cosmetic importance. The seeds are famous for their high oil (50%) and protein (35%); vitamins C and B2, minerals, fat and carbohydrate compositions (Rûgheimer, 1997). Its industrial importance includes use in production of moisturizers and skin regenerating/restruc-turing products (Jacks et al., 1976, Lazos, 1986). The oil predominantly contains unsaturated fatty acids hence exhibits high antioxidant activity (Lazos, 1986).
In spite of the extensive literature reports on the nutritional and medicinal properties of these oils, as well as the several claims to their role on reproduction, no detailed scientific study has been carried out to validate or invalidate this claim on the reproductive functions, particularly the oils extracted from the seeds grown in Nigeria. The present study was therefore undertaken to evaluate the possible impact of these vital and commonly consumed oils on reproductive functions in male and female rats using the sex hormones as the biochemical markers of reproduction. The study ensures that the oils were extracted using procedures akin to the traditional methods.
Collection of seeds and extraction of the oils
Sesame, peanut and melon seeds were purchased from Watt market, Calabar in Cross River State, Nigeria. The seeds were cleaned, washed and sun-dried and afterwards ground thoroughly into a paste using a manual grinder (Corona). The extraction method employed was the traditional approach used in Nigeria, described by Tunde-Akintunde et al. (2012). In the method, water at 50 - 60°C was added to the blended seed paste in small quantities, periodically and massaged with the hands. The hot water permeates and percolates flour particles and causes the less dense oil to float as the uppermost layer, from where it is carefully removed by skimming. The extract was heated on a cooking mantle to evaporate the water (moisture); allowed to cool, then stored in air-tight coloured bottles with screw cap to prevent peroxidation. From 1 kg each of the sesame, peanut and melon seeds, 263.9 ml (26.4%), 333.3 ml (33.3%) and 319.4 ml (31.9%) of oil was extracted, respectively.
Preparation of experimental diets
Rat chow purchased from Vital Feed Depot in Calabar, Cross River State, Nigeria, was used to compound the experimental diets thus: oil : feed (w/w) - 1: 19 (5% oil); 1: 9 (10% oil) and 1 : 4 (20% oil). These ratios were similarly used for the three different oils - sesame, peanuts and melon oils.
Experimental animals
Sixty (60) weanling albino Wistar rats (20 males and 40 females) obtained from the animal house, Faculty of Science, University of Calabar, were used for this study. The animals were housed and fed in the animal house of the Department of Biochemistry until they weighed between 120 to 150 g. During this period, the males were separated from the females to avoid mating. The animal housing facility was maintained under standard conditions. Thereafter, the rats were cohabited in a mating ratio of 1 male: 2 females on the basis of their within and between groups average weight to give 10 mating groups of six animals each (two males and four females). From commencement of cohabiting, the animals were fed the oils-supplemented diets and water ad libitum according to the schedule shown in Table 1.
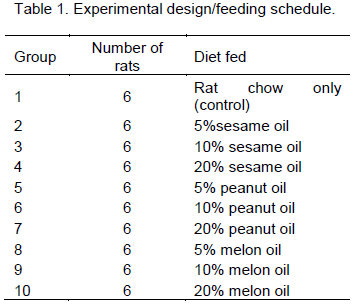
During the mating period, the rats were closely observed for signs of pregnancy and the pregnant females were separated into individual cages while maintaining the initial groupings until they produce litters. The litter size was recorded for each animal and allowed to cohabit with mother until the litters were weaned; the parent animals were removed and sacrificed. This procedure lasted for about seven months - January to July 2013. The animal procedures were carried out in line with the University of Calabar; College of Medical Sciences approved protocol.
Collection of samples and hormonal assays
At the end of study period, each of the adult rats was anaesthetized in chloroform vapour and immediately dissected. Blood samples were collected by cardiac puncture into plain sample tubes and allowed to stand for about an hour at room temperature; after which they were centrifuged at 3000 rpm for 10 min using a bench top centrifuge (MSE, England). Sera obtained from the respective sample tubes were stored frozen until ready for hormonal assays. Heart, kidney and liver of each animal were surgically removed and weighed, and the relative organ weight was calculated thus:
The sex hormones including prolactin, progesterone, estradiol, luteinizing and follicle stimulating hormones, were evaluated by enzyme linked immunosorbent assay (ELISA) using ELISA assay kits (Accu-Bind Elisa microwell, monobind, USA, 100 North Pointe Drive, Lake Forest California, 92630, USA). The procedure followed was as contained in the kit inserts.
Statistical analysis
All values were expressed as the mean ± SEM. The data obtained were analyzed by one way ANOVA using the SPSS statistical package, version 17 and the post hoc comparison with LSD. Differences test were considered significant at p < 0.05.
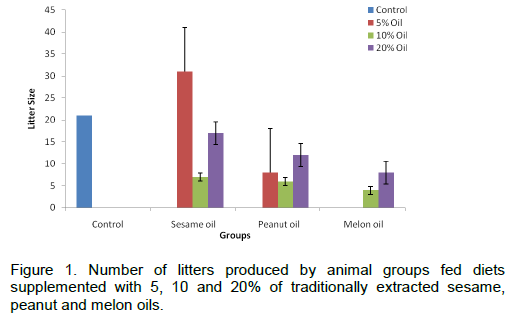
Effect of seed oils-supplemented diets on litter size
The result of the litter sizes of the various groups of rats treated with graded percentages of the three traditionally extracted oils is shown in Figure 1. The total number of litters produced was highest in sesame oil supplemented group (55), followed by peanut oil group (26) and melon oil fed group produced the lowest number of litters (12). Considering the individual treatment groups shows clearly that the oils exerted a non-significant effect on number of litters produced, except in the 5% sesame oil - fed group 31 l were produced as against 21 l in the control group (p>0.05). Moreover, there was no dose dependent effect of the oils on litter size.
Effect of seed oils-supplemented diets on body weight
The weight gain of the experimental female and male rats fed the seed oils-supplemented diets is shown in Table 2. The 5% peanut oil supplemented group showed the greatest gain in weight (41.2 ± 0.001 g) followed by 10% sesame oil fed group (39.75 ± 11.85 g) and lastly the 5% melon oil-supplemented group (39.20 ± 6.70 g). Although not statistically significant, the weight gain decreased with increasing percentage of peanut oil supplementation was evident in both female and male rats. A similar trend was also observed in the melon oil supplemented male rats. The relative organ weights (hearts, kidney and liver) of the test groups were not significantly different from that of the control group (data not shown).
Effect of seed oils-supplemented diets on sex hormones of female rats
The effect of seed oils-supplemented diets on sex hormones of female rats is shown in Table 3. As indicated in the result, the serum prolactin concentration was significantly increased in animal groups fed the 5 and 10% seed oils-supplemented diets (p < 0.05). However the 20% seed oils-supplemented diet exerted a null effect
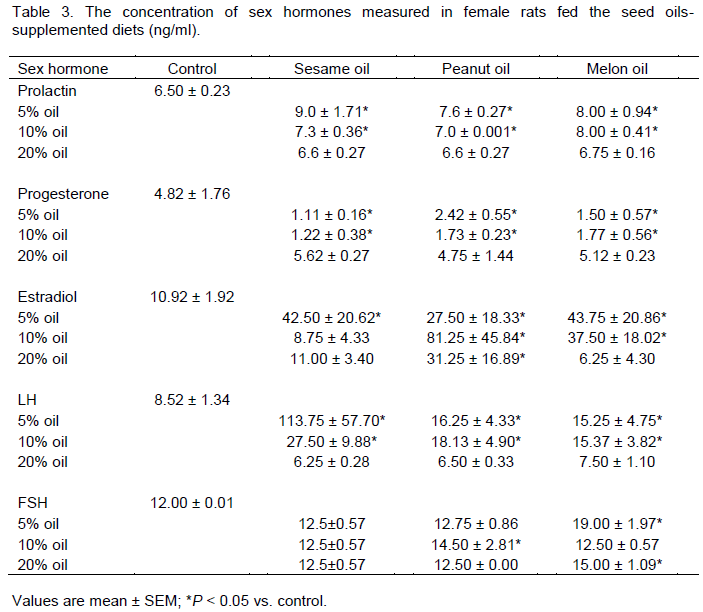
on prolactin level. Conversely, the progesterone concen-tration was decreased significantly in those groups with increased concentrations of prolactin (p < 0.05). Also, 20% seed oils-supplemented showed no observable effect on serum progesterone concentration. The estradiol concentration in the entire peanut oil supplemented group showed a significantly higher level than that of the control (p < 0.05). A similar effect was observed in the animals fed the low % supplemented sesame and melon oils, but not at 20% supplementation. Serum LH concentration was increased in the three test groups at 5 and 10% seed oils replacements only (p < 0.05); whereas the effect of the seed oils on FSH concentration in the female rats was rather irregular.
Effect of seed oils-supplemented diets on sex hormones of the male rats
The result of male sex hormones assayed in rats fed traditional oils-supplemented diets is shown in Table 4. From the results, the LH and testosterone concentrations were significantly increased in animal groups fed 5 and 10% supplemented with the sesame, peanut and melon oils (p < 0.05). The 20% oils-supplemented diets-fed groups showed no significant effects on the levels of these hormones. The FSH was not significantly impacted by any of the three traditional oils fed as supplemented diets.
The present investigation was carried out to assess the impact of traditionally extracted sesame, peanut and melon oils on sex hormones and hence fertility in Wistar rats. The result obtained, showed statistically significant improvement in biochemical markers of fertility in both males and females along with a comparative increase in the litter number (though not reaching statistical significance) in the animals fed with sesame oil-supplemented diet compared to peanut and melon oils, indicating an overall positive effect of the oil on fertility. It is likely that the phytoestrogens contained in the oil as earlier reported by Jacklin et al. (2003) may be responsible for this enhanced fertility action of the sesame oils in the experimental rats in a manner akin to animal estrogens. Yildiz (2005) had shown that sesamin, a major component of sesame oil contains phytoestrogen capable of producing estrogenic effect by binding to the estrogen receptors. Moreover, the assayed estradiol level was highly increased, about two to seven folds in the test diets-fed groups compared to the control, probably resulting from the effect of one or more of the phyto-components of the oils. Estrogens in general are known to promote mitotic activity in the uterine muscle and endometrium, cause rapid gene transcription in uterine tissues and influence the secretion of the gonadotrophic hormones in the anterior pituitary (Deb, 2011). The combined effects of the increased estradiol and the phytoestrogen may have caused the increased litter size, hence enhanced fertility.
The plasma concentration of LH a typical gonadotrophic hormone of the anterior pituitary was also selectively and significantly raised in tandem with the litter size and the raised estradiol level in the oil-treated female rats. In females, an acute rise in LH and FSH triggers ovulation and development of the corpus luteum which may lead to multiple pregnancies while in the male, they stimulate leydig cell production of testosterone (Louvet et al., 1975). The high plasma LH in groups fed 5 and 10% oils supplemented diets (both male and female rats) may justify the observed multiple births. The oils might have triggered multiple ovulations and development of the corpus luteum in the female rats and production of testosterone from the leydig cells; combined mechanisms that work in synergy to enhance multiple pregnancies/births.
At the percentages supplemented, the oils exerted a null effect on the relative organ (heart, kidney and liver) weights implying that on the basis of weight alone, the oils have no detrimental effect on these organs and thus may be safe for consumption at the levels used in this study.
Prolactin is known to stimulate lactogenesis or milk production after birth (Bowen, 2002) and it decreases the level of sex hormones, estrogen in females and testosterone in males (Kumar and Clark, 2005). In this study, measured prolactin level was significantly high in test groups fed the 5 and 10% supplemented diets compared to the control implying that the seed oils may influence prolactin release. Prolactin being a peptide hormone, it is possible that the seed oils have a modulatory effect on the prolactin release mechanism. Prolactin released, exerts a direct effect on the mammary gland via prolactin receptors which are located on the plasma membrane of the secretory cells thereby stimulating breast milk production. This seed oil-induced plasma prolactin increase may explain the mechanism by which milk production is increased and as such scientifically validates the claim that some seed oils particularly sesame oil; enhance the production of breast milk (
www.whyfood.com). It is also quite interesting and striking too, to note that in these treatment groups (groups fed 5 and 10% oils-supplemented diets) progesterone production was concomitantly decreased. Progesterone is secreted majorly by the corpus luteum of the ovary; it is responsible for preparing the inner lining of the uterus (endometrium) for pregnancy and prevents ovulation during this period (Chard, 1988); its concentration decreases with increased prolactin production (Lelte et al., 1992, Deb, 2011). This observed reciprocal action of the seed oils clearly corroborates our earlier submission on the impact of prolactin on breast milk production, as the oils simultaneously increased prolactin and decreased progesterone in favour of increased milk production. This effect on enhanced milk production mechanism was however not indicated at high supplementation levels (20% oils supplementation), suggesting that maximum efficacy in milk production is obtained only when the oils are used as minor component of the diet and not as a major component in which case cross interferences with other mechanisms may compromise the milk production enhancement function. The study oils by this mechanism have proven to be good agents for maximum lactation or increased milk production in lactating mothers. However, this requires further research to be firmly established.
In this study, testosterone, a hormone primarily secreted in leydig cells of testes, responsible for the development of the male sex organs and secondary sexual characteristics such as physique, strength, sex drive and performance man (Waterman and Keeney, 1992) was significantly increased in experimental animals fed oils-supplemented diets. The observed increase in testosterone concentration suggests consumption of sesame oil and lower melon and groundnut oils at 5 and 10% may boost sex drive and enhance fertility and potency in male via increase in testosterone production. It is plausible to think that this testosterone increase may be an effect of the zinc component of the seed oils. Sesame oil is reportedly rich in zinc (Kanu, 2011), and previous studies have also shown that zinc rich-diets and diet supplementation with zinc are extremely effective at raising testosterone levels and helping men to improve their body composition, sex drive and increase semen volume (Shafiei et al., 2011). The oils-incorporated diets have by this testosterone and LH productions also shown possible capacity to positively influence male fertility. Further and comprehensive research is however needed to fully establish this.
Overall, data from this study suggest that the consumption of these seed oils at 5 and 10%, especially sesame oil, may contribute effectively to increased incidence of multiple births, enhanced fertility in males and females as well as improves lactation.
The authors did not declare any conflict of interest.
REFERENCES
|
Aremu MO, Olaofe O, Akintaya TE (2006). A comparative study on the chemical and amino acid composition of some Nigerian underutilized legume flour. Pakistan J. Nutr. 5:34-38.
crossref
|
|
|
|
Bamigboye AY, Okafor AC, Adepoju OT (2010). Proximate and mineral composition of whole and dehulled Nigerian sesame seed. Afr. J. Food Sci. Technol. 1(3):071-075.
|
|
|
|
|
Bowen R. (2002). Prolactin. Colorado State University. Retrieved 12 November 2012. www.vivo.colostate.edu
|
|
|
|
|
Chard T (1988). "What is happening to plancental function tests"? Ann. Clin Biochem. 24:435-437.
crossref
|
|
|
|
|
Deb ACD (2011). Fundamentals of Biochemistry New Central Book agency (P) Ltd. London 10th edition; pp. 559-563.
|
|
|
|
|
Fukuda Y, Nagata M, Namiki MJ (1986). Contribution of Lignan Analogues to Antioxidant Activity of Refined Unroasted Sesame Seed Oil. J. Am. Oil Chem. Soc. 63:1027-1031.
crossref
|
|
|
|
|
Hirose N, Inoue T, Nishihara K, Sugano M, Akimoto K, Shimizu S, Yamada H (1991). Inhibition of cholesterol absorption and synthesis in rats by sesame. J. Lipid Res. 32:629-638.
|
|
|
|
|
Ide T, Kushiro M, Takahashi Y, Shinohara K, Fukuda N Sirato-Yasumoto S (2003). Seamin, a sesame lignan, as a potent serum lipid-lowering food component (a review). Japan Intl. Res. Centre Agric. Sci. J. 37(3):211-232.
|
|
|
|
|
Jacklin A, Ratledge C, Welham K, Bilko D, Newton CJ (2003). The sesame oil constituent, sesamol, induces growth arrest and apoptosis of cancer and cardiovascular cells. Ann. New York Acad. Sci. 1010:374-380.
crossref
|
|
|
|
|
Jacks TJ, Hensarling TP, Yatsu LY (1972). Cucurbit seeds: Characterizations and Uses of Oils and Proteins. A review. Econ. Bot. 26(2):135-141.
crossref
|
|
|
|
|
Kang MH, Naito M, Tsujihara N, Osawa T (1998). Sesamolin inhibits lipid peroxidation in rat liver and kidney. J. Nutr. 128:1018-1022.
|
|
|
|
|
Kanu PJ (2011). Biochemical analysis of black and white sesame seeds from China. Am. J. Biochem. Mol Biol. 1:145-157.
crossref
|
|
|
|
|
Kritchevsky D (1988). Cholesterol vehicle in experimental atherosclerosis. A brief review with special reference to peanut oil. Arch. Pathol. Lab. Med. 112:1041-1044.
|
|
|
|
|
Kumar P Clark M (2005). Clinical Medicine 6th ed. Elsevier Saunders publishers, London.
|
|
|
|
|
Lazos ES (1986). Nutritional, Fatty acid, and Oil Characteristics of Pumpkin and Melon Seeds. J Food Sci. 51(5):1382.
crossref
|
|
|
|
|
Lelte V, Cosby H, Sobrinho LG, Fresnoza A, Santos MA, Frlesen HG (1992). Characterization of big, big prolactin in patients with hyperprolactinaemia. Clin. Endocrinol. 37(4):365-372.
crossref
|
|
|
|
|
Liu X, Jin Q, Liu Y, Huang J, Wang X, Mao W, Wang S (2011). "Changes in volatile compounds of peanut oil during the roasting process for production of aromatic roasted peanut oil". J. Food Sci. 76(3):404-412.
crossref
|
|
|
|
|
Louvet J, Harman S, Ross G (1975). "Effects of human chorionic gonadotropin, human interstitial cell stimulating hormone and human follicle-stimulating hormone on ovarian weight in estrogen-primed hypophysectomized immature female rats". Endocrinology 96(5):1179-1186.
crossref
|
|
|
|
|
Olanyanju TMA, Akinoso R, Oresanya MO (2006). Effect of wormshaft speed, moisture content and variety on oil Recovery from expelled Beniseed. Agric. Eng. Intl. 8:1-7.
|
|
|
|
|
RMRDC (2004). Raw Material Research and Development Council Survey Report of ten Selected Agro-Raw Material in Nigeria. pp. 1-4.
|
|
|
|
|
Rûgheimer S (1997). Chemical characterization of the oil extracted from Citrullus lanatus seeds. University of Stellenbosch, Zulu novels publisher, South Africa.
|
|
|
|
|
Sanders TH, Mcmichael Jr, RW, Hendrix KW (2000). Occurrence of resveratrol in edible peanuts. J. Agric. Food Chem. 48(4):1243-1246.
crossref
|
|
|
|
|
Sangha M, Gupta P, Tharpo V (2004). Storage studies on plant oils and their methyl esters. Ejournal. December Vol. 7.
|
|
|
|
|
Shafiei Neek L, Gaeini A , Choobineh S. (2011) Effect of zinc and selenium supplementation on serum testosterone and plasma lactate in cyclist after exhaustive exercise bout. Biological trace element research, 144 (1-3):454-462.
crossref
|
|
|
|
|
Stampfer J, Manson JE, Rimm EB, Hu FB, Colditz GA, Rosner BA, Sperzer FE, Hennekens CH, Willett WC (1998). Frequent nut consumption and risk of coronary heart disease study. Brit. Med. J. 317(7169):1341-5.
crossref
|
|
|
|
|
Tunde-Akintunde TY, Oke M O, Akintunde BO (2012). Sesame Seed, Oilseeds, Dr. Uduak G. Akpan (Ed.), ISBN: 978-953-51-0665-4, InTech, Availablefrom: http://www.intechopen.com/books/oilseeds/sesame-seed). www.whyfood.com- sesame seeds benefits to your health, eating benefits of sesame seeds retrieved 15, April, 2013.
|
|
|
|
|
Yildiz F (2005). Phytoestrogens in functional foods. Taylor and Francis Limited. pp. 3-5:210-211.
crossref
|
|