ABSTRACT
Source separated human feces can be used as a valuable source of nutrients if it is properly sanitized and its nutrient is conserved. In this study, the efficiency of fresh cabbage waste produced lactic acid treatment of human feces as a pretreatment to evaluate the sanitizing effect, urea stabilizing, and odor removal was investigated for 33 days. Four reactors were used for the treatment process containing different lactic acid to feces ratio (that is, 1:1, 1:2, 1:3 and control). Escherichia coli was used as the indicator of pathogen inactivation, whereas pH and ammonium were used as the main indicators of urea hydrolysis. The result showed that inactivation of the indicator, stabilization of urea and odor reduction improved in 1:1 reactor compared with other treatment reactors. Therefore, human feces is recommended to be treated by food waste produced lactic acid for 9 days for hygienic and stabilization purpose. Under this treatment condition, pH is maintained below 4.1 and ammonium content is maintained at approximately 5.0 mg/g. Moreover, the combination of lactic acid and activated biochar also played important role in odor control for effective treatment of feces.
Key words: Activated biochar, feces, lactic acid, Escherichia coli, urea stabilization.
Nowadays, the conventional urban water and sanitation systems are questionable in terms of its adequacy and long-term sustainability both in developed and developing countries. Issues of high energy and water demand, sludge disposal problems, and limited nutrient recycling in developed countries (Brands, 2014) and the high infrastructure costs in developing countries (Larsen et al., 2016) are prohibitive for implementation of the systems. As a result, source separation and control is getting attention since it provides the opportunity for resource recovery and minimizes dilution and contamination of human excreta (Wilsenach et al., 2003; Larsen et al., 2009). Source separation is believed to answer the concern about future fertilizer availability for better nutrient management, including comprehensive recycling of nutrients contained in human excreta to agriculture (Harder et al., 2019). Due to its nutrient contents, human feces are a natural fertilizer that could replace chemical fertilizers (Andreev et al., 2018) to increase the soil fertility for agricultural productivity (Kimetu et al., 2004).
However, the risk of infecting people with disease via contaminated crops and loss of nutrients are the main challenges (Yemaneh, 2015; Gold et al., 2018).
Different treatment techniques have been used to reduce the amount of pathogens in feces. Storage and composting (thermophilic composting and vermicomposting), which needs at least 1 to 2 years storage for pathogen removal (WHO, 2006). Desiccation along with high pH was proven to be efficient in pathogen destruction during the storage of feces by maintaining moisture content below 25% and a pH > 9, but rarely achieved (Niwagaba et al., 2009). Thermophilic composting that needs to be maintained at least for several days in stored feces is not economical since it requires additional energy source (Kone et al., 2010). Vermicomposting requires an extended time of 3 to 5 months to sanitize the feces during which a significant amount of nitrogen would be lost (Sinha et al., 2009). Lime treatment reduces pathogen present in feces. The concern in this process includes, lime scaling, ammonia odors and pathogen regrowth after a few days (Strande et al., 2014). Ammonia disinfection is effective in urine (Adamtey et al., 2009); however, its effectiveness to feces and disadvantages over the other technologies are not exhaustively studied (Magri et al., 2015).
Fermentation and acidification process is one of the most reliable methods for pathogen inactivation, nutrient loss reduction and odor control. Several studies (Factura et al., 2010; Otterpohl and Buzie, 2013; Bettendorf et al., 2014) showed the use of lactic acid fermentation for treatment of human feces within the terra preta sanitation approach. Lactic acid fermentation is a cheap and simple method that can be achieved through acidification at pH ≤ 4 (Anderson et al., 2015; Andreev et al., 2017). However, several lactic acid species do not produce effective lactic acids for pathogen inactivation in feces (Colehour et al., 2014; Sanni et al., 2013). As a result, effective, cheap and locally available lactic acid from wastes must be produced to sanitize feces, preserve its nutrient value as fertilizer and reduces its odor.
Lactic acid fermentation (LAF) is a cheap and simple method that can be achieved through acidification at pH ≤ 4 (Anderson et al., 2015; Andreev et al., 2017). However, not all lactic acid bacteria (LAB) species are able to produce lactic acid in feces and urine, making them ineffective for pathogen inactivation (Odey et al., 2018). In this study, we set out to have effective, cheap and locally available waste sources likely to contain LAB that could be fermented to produce lactic acid as a sanitizing agent for human feces prior to being used as a fertilizer. Specifically, cabbage waste was evaluated as organic source, for its ability to create LAB inocula that produced enough lactic acid to eliminate fecal indicator bacteria. The lactic acid produced from cabbage waste was evaluated for its ability to inactivate pathogen, inhibit urea hydrolysis and eliminate odor in feces. Escherichia coli was used as the indicator of pathogen inactivation, whereas pH and ammonium were used as the main indicators of urea hydrolysis.
Preparing organic waste sources for fermentation
Fresh cabbage waste served as organic waste sources (heretofore called “substrates”); this substrate was collected in Addis Ababa, Ethiopia and was selected based on availability. It was collected from the big vegetable market in the city. The fresh cabbage waste was pulverized with a heavy-duty blender in the laboratory at Addis Ababa Institute of Technology, Addis Ababa University (AAU). Fifty grams of the substrate were mixed with 50 ml of distilled water and 50 ml of 10% sugar cane molasses to enhance lactic acid fermentation in the fermentation reactor, and sealed to make it air-tight and incubated at 37°C for seven days based on the method of Omar et al. (2009). Change in pH and the concentration of LAB and E. coli during the batch fermentation test were enumerated using the method described subsequently. The experiments were conducted in duplicate.
Preparation of lactic acid stock
Lactic acid was recovered for use in feces sanitization and urea hydrolysis inhibition using the methods of Mumtaz et al. (2008), Omar et al. (2009) and Phang et al. (2002). Specifically, the cabbage + sugar fermentation vessels were incubated at 37°C for seven days, then frozen at -30°C overnight followed by thawing in a drying oven at 60°C for 2 to 3 h. The solution was centrifuged and filtered with 0.8 µm cellulose acetate filter paper and a vacuum pump (KNF Neuberger, Germany). Finally, the water was evaporated at 50°C under vacuum for 8 h using a rotary evaporator (ROV 400, Czech Republic). After most of the liquid was evaporated, a clear brownish solution containing lactic acid was recovered (27.64 ± 3.03 ml). The solution pH was measured and found to be 3.90 ± 0.01. This acidic environment was anticipated to be used as an effective conditioner for pathogen inactivation and urea stabilization in feces.
Feces collection and experimental setup
Feces samples were collected from a volunteered family (a man – aged 36, three females – aged 41, 32, and 24) for a day and thoroughly mixed. At the end of the collection, it was stored in a tightly closed plastic container. Chemical analysis was performed and equally separated into four reactors. 130 g of feces sample was added to each of four 500 ml plastic containers (reactors). Three of the four reactors were mixed with different amount of lactic acid (lactic acid: feces) as 1:1, 1:2, and 1:3 (v/w). The fourth reactor was stored in parallel in tightly closed reactor without addition of lactic acid and named as control. All the bottles used for the experiments were disinfected and dried prior to filling. The treatment process was undertaken for 33 days for all reactors. The initial characteristics of the feces, pH and E. coli, were measured and found to be 6.89 ± 0.09 and 3.2 ± 0.00 × 106 CFU/g, respectively.
The feces treatment experiment was monitored by measuring the E.coli, ammonium concentration, and pH. The ability of lactic acid to inactivate the pathogens was determined by evaluating the survival rates of the E. coli using Compact Dry ECO plates (HyServe, Germany) and on the basis of the pH changes (measured by using Jenway 3505-UK pH meter) during the treatment process, whereas the ability to reduce nutrient loss was monitored by measuring the ammonium concentration throughout the experiments.
The pH value during the treatment process was determined by collecting 1 g of sample from each reactor, which was then dissolved in 10 ml distilled water. The dissolved portions were stirred for 15 min. After settling, the pH was measured with pH meter (Jenway3505, UK).
Inhibition of E. coli in each reactor was determined using dry chromogenic medium for the detection and enumeration of E. coli. Samples taken from each reactor was added to the plates containing the chromogenic medium and incubated for 24(±2) h at 35°C. E. coli colonies, present as blue colonies, were counted (HyServe, Germany).
The ammonium content was analyzed using the Spectroquant reagent kit 1.14752.0002 (Merck, 2019). Using a spectrophotometer (Spectro UV-VIS Double Beam PC (UVD-3200), Labomed INC) and 10 mm cells, the absorbance of wavelength 690 nm was measured. For the conversion from absorbance to concentrations a standard curve was prepared with concentrations 0.1, 0.5, 1, and 3 mg NH4/L prepared from standard solution (1000 mg NH4/L).
Odor evaluation
The odor strength of the feces during treatment with lactic acid and combination of lactic acid and activated biochar was evaluated by eight people. The activated biochar was prepared from Prosopis wood based on the method laid out by Nahata et al. (2017). The potency of the perceived odour was evaluated by using a scale rank that has been used previously (Andreev et al., 2017). The scale rank categories are: 0 (no odor), 1 (very faint odor), 2 (faint odor), 3 (distinct odor), 4 (strong odor), 5 (very strong odor), and 6 (extremely strong odor).
Culture enumeration
Bacterial culturing was conducted in duplicate by applying 0.1 mL of serially diluted (1/10) sample in sterile distilled water. Lactic acid bacteria were cultured at 37°C for 24 h on MRS agar (Standard Method 9215), a Lactobacillus selective culture medium. E. coli was enumerated at 37°C for 24 h with Compact Dry ECO plates (HyServe, Germany) that use a dry chromogenic medium that is reconstituted when the liquid sample is applied; E. coli colonies present as blue. The method has an accuracy of ±0.5 log10 and a detection limit of 1 CFU/ml.
Statistical analysis
The experimental data were statistically processed via a Tukey test of multiple comparisons within one-way analysis of variance (ANOVA) and using Minitab 17 statistical software. The means of pH and ammonium concentration in all treatment reactors were compared for significance differences (at 95% significance, based on p values and confidence interval CI).
Fermented cabbage waste produced LAB and inactivated E. coli
The waste source produced low pH response to fermentation (Figure 1). Since it reflects the acidification in the experiment, pH variation is one of the most important parameters that must be observed during fermentation. The initial pH of the substrate was 5.5. The pH stabilized at average value of 3.98 ± 0.02 by day five and remained there until day seven. This favored to the rapid growth of LAB during the fermentation process. As a result, LAB metabolized molasses to obtain energy and produced lactic acid as the end product. The increase in LAB concentration resulted in a pH drop. This result shows that cabbage waste produced the lowest sustained pH during the experiment.

Throughout the experiment, the waste resulted in a multifold increase in LAB counts (0.8 × 108 ± 0.12 to 5.0 × 108 ± 0.22 CFU/100 ml). Furthermore, the fermented cabbage waste eliminated detectable E. coli counts by day three suggesting that acidic pH values lead to E. coli inactivation. The average result of duplicate samples is converted into log10 CFU/100 ml (Figure 1).
The fermentation test result indicates that fresh cabbage waste can produce conditions favorable for inactivating pathogens and stabilizing urea in feces. The waste creates acidic conditions so more quickly and rapidly inactivated E. coli, an enteric pathogen indicator. While the complete mechanism of inactivation was not determined, acidic pHs are outside of the ideal range for optimum growth (Desmarchelier and Fegan, 2003) and likely contributed to its inactivation.
Finally, the waste showed the potential to generate lactic acid given the proliferation of LAB and decrease in pH under all conditions. Consequently, fresh cabbage can be used to generate lactic acid for feces processing and pathogen inactivation. Cabbage is naturally rich in LAB and support rapid fermentation (Yang et al., 2010), which was seen in this study.
Fermented waste-generated lactic acid inactivated E. coli and improved urea stabilization
Addition of fermented waste-generated lactic acid solution (pH 3.98) stabilized the pH in human feces present at a higher volume. The pH value of the control reactor was initially 6.89, which then increased to 7.28 on the fifth day of the treatment. At the end of the treatment process, the reactor had a final pH of 7.94, which is consistent with what happens when urea in the feces is hydrolyzed to ammonia (Chang et al., 2015). The pH rapidly decreased for 1:1 reactor (4.01) followed by 1:2 (4.05) and 1:3 (5.16) reactors in the first five days of the treatment process and remained nearly constant for three of the reactors from day 9 to the final day of the treatment as shown in Figure 2A. The final pH value for 1:1, 1:2 and 1:3 was 4.08, 4.27, and 5.28, respectively. The observed pH value in 1:1 reactor is considered to have nearly the desired characteristics for feces acidification as described by Thimann (1963) and mentioned by Odey et al. (2018) that lactic acid treatment must have a final pH of [4 for feces hygienization. This result implied that food waste produced lactic acid is an effective conditioner for pathogen inactivation in feces, because pathogens rarely survive in acidic environment (Glaser and Guggenberger, 2001).
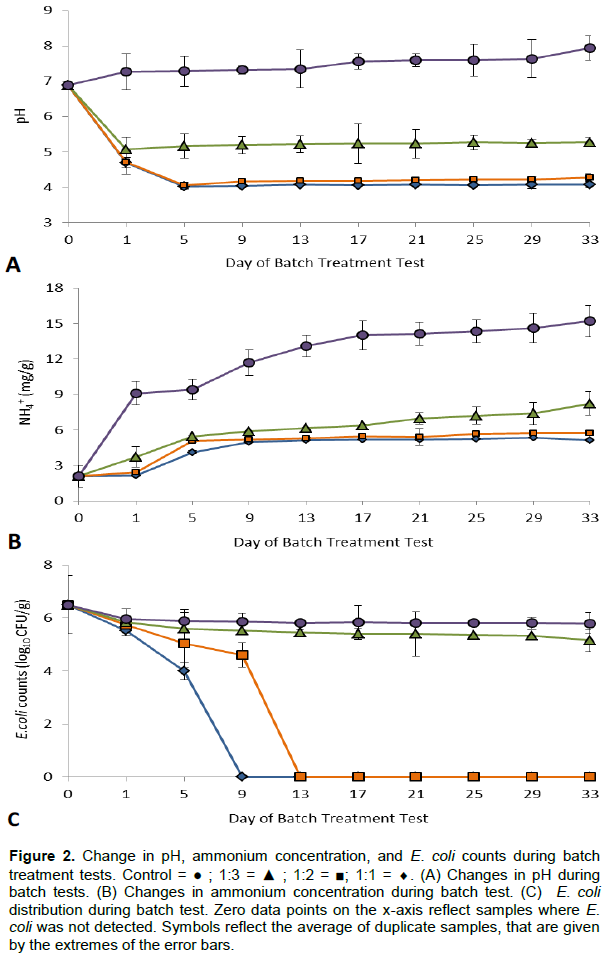
A one way ANOVA analysis for pH between the four test reactors shows that there was no statistically significant difference between the means of the pH value of the 1:1 , 1:2, and 1:3 reactors (p value < 0.05).
However, there was statistically significant difference between the mean of the pH value of the control reactor from the rest of the reactors.
In this study, lactic acid treatment of feces has been hypothesized to prevent urea from hydrolyzing into ammonia/ammonium. Urea hydrolysis is catalyzed by urease from urea producing bacteria. The effect of lactic acid, however, inactivates urea producing bacteria, thus inhibiting urease production during lactic acid treatment. In this study, the initial ammonium content of the feces was 2.11 mg/g. Ammonium concentration of 1:1 and 1:2 reactors on the first day was 2.14 and 2.4 mg/g, respectively, and showed sharp increment on the fifth day (4.13±0.11 in 1:1 reactor and 5.08±0.12 in 1:2 reactor). On day 5 onwards, the ammonium concentration in 1:1 and 1:2 reactors remained nearly constant until it reached 5.16 mg/g in 1:1 reactor and 5.74 mg/g in 1:2 reactor at the end of the treatment process. Although 1:1 and 1:2 reactors showed slight difference in inhibition of urea hydrolysis, both influenced urea hydrolysis nearly equally, as shown in Figure 2B. This was supported by one way ANOVA, which showed that there is no significant difference in ammonium concentration between 1:1 and 1:2 reactors (p value < 0.05). On the other hand, the ammonium concentration in 1:3 and control reactors keep increasing until the end of the treatment process. The final ammonium concentration in the 1:3 and control reactors was 8.32 and 15.2 mg/g, respectively as shown in Figure 2B. The control reactor showed the highest ammonium concentration over the others, indicating that there is continues urea hydrolysis. This was supported by one way ANOVA, which showed ammonium concentration in control reactor is significantly different from the other reactors (p value < 0.05). Therefore, considering urea hydrolysis in feces, both 1:1 and 1:2 reactors after 5 days lactic acid treatment produces the optimal urea stabilization.
E. coli was used in this treatment process as an indicator organism to assess the pathogen inactivation efficiency of fresh cabbage waste and sugar cane molasses produced lactic acid in feces. Accordingly, the result showed that reduction in the concentration of E. coli was observed in the 1:1 and 1:2 reactors, whereas the concentration of E. coli remained high in the 1:3 and control reactors. On day 5 onwards, E. coli was not detected in the 1:1 reactor and on the ninth day of the treatment process in the 1:2 reactor as shown in Figure 2C. On the other hand, slight E. coli concentration reduction was observed in 1:3 and control reactors. While the complete mechanism of inactivation was not determined, acidic pHs are outside of the ideal range for optimum growth (Desmarchelier and Fegan, 2003) and likely contributed to its inactivation. According to various studies, pH range of 3.51 to 4.2 could efficiently eliminate various pathogens. Odey et al. (2018) reported that the addition of lactic acid produced from fermented rice flour and brown sugar led to an effective acidification of the treatment process to pH of 3.51 to 3.52 and fecal coliform inactivation. Anderson et al. (2015) found that lactic acid fermentation could reduce the E. coli count in fecal sludge to below the detection limit at pH 4.2. As it was also found in this study, the addition of lactic acid produced from the fermentation of fresh cabbage waste and sugar cane molasses showed removal of E. coli at pH range of 4.04 to 4. 18. This is also consistent with the result reported by Soewondo et al. (2014) who recorded approximately pH 4.5 after 7 days of lacto-fermentation treatment of fecal matter by EM4 and 5% glucose.
In this study, lactic acid treatment of feces in 1:1 and 1:2 reactors reduced the pathogenic microorganism load (E. coli) in a more efficient and faster way. Thus, acidification through lactic acid can eliminate pathogen in feces while conserving nutrients that could be lost during collection, transportation and treatment. Therefore, considering disinfection effect and urea hydrolysis, 1:1 treatment produces the optimal pathogenic bacteria inactivation and urea stabilization. However, further investigations are required to see the efficiency of food waste produced lactic acid to remove dangerous pathogens such as Salmonella, Ascaris and Schistosoma before the practical application of lactic acid treatment of feces.
Food waste produced lactic acid and biochar combined treatment of feces reduced the odor to the acceptable level
The presence of feces odor and its acceptability as a pretreatment of feces to be used in collection system was compiled from the qualitative responses of eight panelists. According to the panelists, lactic acid treatment of 1:1 and 1:2 reactors suppressed the odor and changed it with a lactic acid smell compared to the 1:3 and control reactors. In the 1:1 reactor, the odor was ranked as very faint odor by four panelists, faint odor by three panelists, and a distinct odor by one panelist. In the 1:2 reactor, the odor was ranked as a faint odor by five panelists and a distinct odor by three panelists. In the 1:3 reactor, the odor was ranked as strong odor by all panelists. However, the odor in the control reactor was reported by all panelists to be extremely strong odor as shown in Figure 3A.
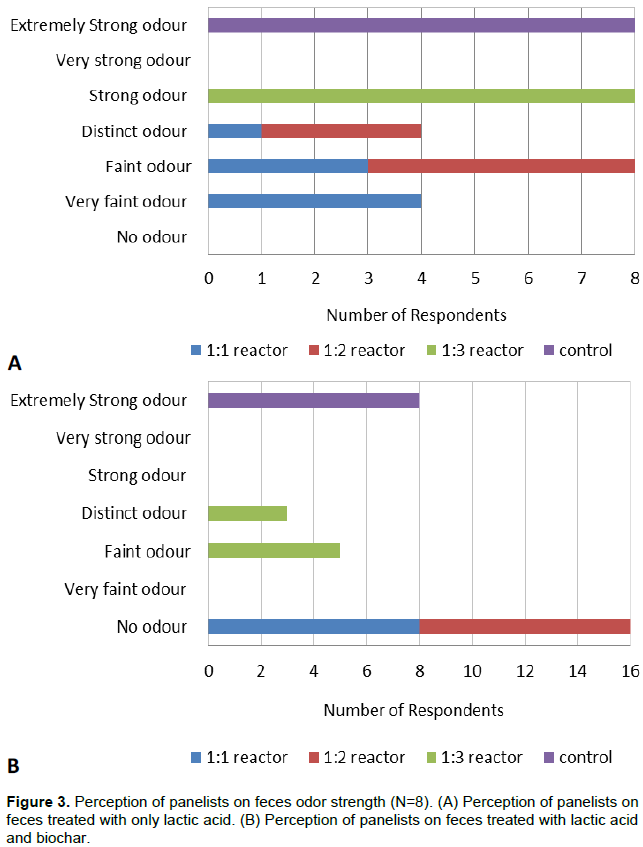
Other studies also reported the removal of offensive feces odor through application of lactic acid produced from other organic wastes. Wang et al. (2001) reported suppression of odor through lactic acid fermentation of kitchen biowaste and fish waste. Huang et al. (2006) found odor suppression through lactic acid fermentation of swine manure added with lactic acid bacteria. Odey et al. (2018) reported odor suppression through fecal sludge treatment using fermented rice flour with brown sugar. In this study, the odor reduction observed in 1:1 reactor could make the sanitized feces acceptable during collection of the feces for direct application in agriculture as a soil amendment or further treatment to recover nutrients.
In an experiment conducted with the addition of lactic acid and 10 %( w/w) of activated biochar into all reactors, complete suppression of odor in 1:1 and 1:2 reactors were reported by all panelists. Accordingly, in 1:1 and 1:2 reactors, the odor was ranked as no odor by all panelists. In the 1:3 reactor, the odor was ranked as a faint odor by five panelists and a distinct odor by three panelists. In the control reactor, the odor was ranked as extremely strong odor by all panelists as shown in Figure 3B.
As the observation of the researcher, combination of lactic acid and 10% (w/w) activated biochar showed complete feces odor removal. This finding is similar with the study result obtained by Yemaneh et al. (2012) who conducted feces treatment involving LAB microbial inoculant, 10% (w/w) molasses and 10% (w/w) charcoal. According to the study, the combined LAB microbial inoculant, 10% (w/w) molasses and 10% (w/w) charcoal suppressed completely the odor of the feces than the treatment with only LAB microbial inoculant and 10% (w/w) molasses.
Replication tests
Based on the aforementioned experimental results, food waste produced lactic acid was applied to human feces for 9 days in two duplicate tests to test the reproducibility of the selected operational conditions (pH, NH4+ and E. coli). The experimental results, for the lactic acid treatment of the two feces samples are presented in Figure 4.
No variation in the rate of E. coli inactivation was observed between the two test samples. E. coli in the two feces samples was completely inactivated on the 9th day of the treatment process. This result is consistent with the previous experimental results. Similar results were obtained for urea hydrolysis that no large variation was found in the pH and ammonium concentration of the two feces samples during the 9 days of the treatment process. Both pH and ammonium content stabilized starting from the 9th day of the treatment process, which is consistent with the previous experimental result. The pH value of the two feces samples on the 9th day of the treatment were 4.03 and 4.07, whereas the ammonium concentrations were 4.96 and 4.99 mg/g. These results further supported the efficiency and reproducibility of the previous experimental results.
Thus, considering the potential of food waste produced lactic acid for hygienization as well as reduction of nutrient loss and odor emissions, this treatment technique can be applied in separately collecting sanitation systems. Moreover, it laid foundation to utilize food waste for feces sanitization. One-third of the food produced globally for human consumption (approximately 1.3 billion tons) is lost. Industrialized and developing countries dissipate roughly the same quantities of food 670 and 630 million tonnes, respectively (News, 2011). Using part of these wastes for the lactic acid treatment of feces will be an economic advantage.
Lactic acid treatment of source separated feces can be a cost effective method during collection and further treatment. In this study, 1:1 reactor showed better performance in terms of pathogen inactivation, nutrient lose reduction via inhibition of urea hydrolysis, and odor removal over the other reactors. The addition of lactic acid produced from fresh cabbage waste and 10% sugarcane molasses led to an effective acidification of the process to a pH of 4.01 to 4.08 in the 1:1 reactor and E. coli inactivation during the treatment process. Ammonium concentration remained constant after fifth day of treatment in 1:1 reactor, thereby showing the efficiency of lactic acid for inactivation of urea hydrolysis. Moreover, in 1:1 reactor lactic acid treatment also played important role in odor control for effective treatment of feces. The combined lactic acid and 10% (w/w) activated biochar showed complete odor removal. Therefore, this study showed that fresh cabbage waste and 10% sugarcane molasses produced lactic acid and 10% activated biochar can be used for feces treatment in terms of pathogen inactivation, urea stabilization, and odor control. The capacity of lactic acid to remove dangerous pathogens such as Salmonella, Ascaris and Schistosoma need further investigation.
The authors have not declared any conflict of interests.
The authors are thankful to School of Civil and Environmental Engineering, Addis Ababa Institute of Technology (AAiT), Addis Ababa University for providing necessary infrastructure to complete this work.
REFERENCES
|
Adamtey N, Cofie O, Ofosu-Budu, GK, Danso, SKA, Forster D (2009). Production and storage of N-enriched co-compost. Waste Management 29(9):2429-2436.
Crossref
|
|
|
|
Anderson C, Malambo DH, Perez MEG, Nobela HN, de Pooter L, Spit J, Hooijmans CM, de Vossenberg JV, Greya W, Thole B, Lier VJB, Brdjanovic D (2015). Lactic acid fermentation, urea and lime addition: promising fecal sludge sanitizing methods for emergency sanitation. International Journal of Environmental Research and Public Health 12(11):13871-13885.
Crossref
|
|
|
|
|
Andreev N, Ronteltap M, Boincean B, Wernli M, Zubcov E, Bagrin N, Borodin N, Lens PNL (2017). Lactic acid fermentation of human urine to improve its fertilizing value and reduce odour emissions. Journal of Environmental Management 198(1):63-69.
Crossref
|
|
|
|
|
Bettendorf T, Stoeckl M, Otterpohl R (2014). Vermi-composting of municipal solid organic waste and faecal matter as part of Terra Preta Sanitation - a process and product assessment In: Bettendorf T.W., C; Otterpohl R. (Ed.). 1st International Conference on Terra preta sanitation. DBU, Hamburg, Germany.
|
|
|
|
|
Brands E (2014). Prospects and challenges for sustainable sanitation in developed nations: A critical review. Environmental Reviews 22:346-363.
Crossref
|
|
|
|
|
Chang Y , Deng C, Dore AJ, Zhuang G (2015). Human excreta as a stable and important source of atmospheric ammonia in the megacity of Shanghai. Plos One 10(12):1-13.
Crossref
|
|
|
|
|
Colehour AM, Meadow JF, Liebert MA, Cepon-Robins TJ, Gildner TE, Urlacher SS, Bohannan BJM, Snodgrass JJ, Sugiyama LS (2014). Local domestication of lactic acid bacteria via cassava beer fermentation. PeerJ 2 , e479.
Crossref
|
|
|
|
|
Desmarchelier PM, Fegan N (2003). Enteropathogenic Escherichia coli. Ch 9 In: Hocking AD (ed) Foodborne microorganisms of public health significance. 6th ed. Australian Institute of Food Science and Technology (NSW Branch), Sydney pp. 267-310.
|
|
|
|
|
Factura H, Bettendorf T, Buzie C, Pieplow H, Reckin J, Otterpohl R (2010). Terra Preta sanitation: re-discovered from an ancient Amazonian civilization - integrating sanitation, bio-waste management and agriculture (eng). Water science and technology: a journal of the International Association on Water Pollution Research 61(10):2673-9.
Crossref
|
|
|
|
|
Glaser BHL, Guggenberger G (2001). The "Terra Preta" phenomenon: a model for sustainable agriculture in the humid tropics. Naturwissenschaften 88(1):37-41.
Crossref
|
|
|
|
|
Gold M, Harada H, Therrien JD, Nishida T, Cunningham M., Semiyaga S., Fujii S, Dorea C, Nguyen V.A, Strande L (2018). Cross-country analysis of faecal sludge dewatering. Environmental Technology 39(23):3077-3087.
Crossref
|
|
|
|
|
Harder R, Wielemaker R, Larson TA, Zeeman G, Oberg G (2019). Recycling nutrients contained in human excreta to agriculture: Pathways, processes, and products. Critical Reviews in Envirnmental Sceince and Technology 49(8):695-743.
Crossref
|
|
|
|
|
Huang C, Li J, Kang WL, Tang XY (2006). Effect of adding Lactobacillus plantarum and soluble carbohydrates to swine manure on odorous compounds, chemical composition, and indigenous flora. International Journal of Environmental Science and Technology 18(1):201-206.
|
|
|
|
|
Kimetu JM, Mugendi DN, Palm CA, Mutuo PK, Gachengo CN, Bationo A, Nandwa S, Kungu JB (2004). Nitrogen fertilizer equivalencies of organics of differing quality and optimum combination with inorganic nitrogen source in Central Kenya. Nutrient Cycling in Agroecosystems 68(2):127-135.
Crossref
|
|
|
|
|
Kone D (2010). Making urban excreta and wastewater management contribute to cities economic development: a paradigm shift. Water Policy 12(4):602-610.
Crossref
|
|
|
|
|
Larsen TA, Alder AC, Eggen RIL, Maurer M, Lienert J (2009). Source separation: Will we see a paradigm shift in wastewater handling? Environmental Science and Technology 43:6121-6125.
Crossref
|
|
|
|
|
Larsen TA, Hoffmann S, Luthi C, Truffer B, Maurer M (2016). Emerging solutions to the water challenges of an urbanizing world. Science 352(6288):928-933.
Crossref
|
|
|
|
|
Magri ME, Fidjeland J, Jonsson H, Albihn A, Vinneras B (2015). Inactivation of adenovirus, reovirus and bacteriophages in fecal sludge by pH and ammonia. Science of the Total Environment 520:213-221.
Crossref
|
|
|
|
|
Nahata M, Chang YS, Krishnakumar P, Schwank J (2017). New approaches to water purification for resource-constrained settings: Production of activated biochar by chemical activation with diammonium hydrogenphosphate. Frontiers of Chemical Science and Engineering 12(1):194-208.
Crossref
|
|
|
|
|
News F (2011). Shocking-food-waste-starts/Food New.
|
|
|
|
|
Niwagaba C, Kulabako RN, Mugala P, Jonsson H (2009). Comparing microbial die-off in separately collected faeces with ash and sawdust additives. Waste Management 29(7):2214-2219.
Crossref
|
|
|
|
|
Odey EA, Li Z, Zhou X, Yan Y (2018). Locally produced lactic acid bacteria for pathogen inactivation and odor control in fecal sludge. Journal of Cleaner Production 184:798-805.
Crossref
|
|
|
|
|
Omar FN, Rahman NA, Hafid HS, Yee PL, Hassan MA (2009). Separation and recovery of organic acids from fermented kitchen waste by an integrated process. African Journal of Biotechnology 8(21):5807-5813.
Crossref
|
|
|
|
|
Otterpohl R, Buzie C (2013). Treatment of the solid fraction. In: Larsen, T., Udert, K., Lienert, J. (Eds.), Source Separation and Decentralization for Wastewater Management. IWA Publishing, London pp. 259-273.
|
|
|
|
|
Phang LY, Wakisaka M, Shirai Y, Hassan MA (2002). Freezing and thawing technique for the removal of suspended solids and concentration of palm oil mill effluent (POME). Journal of Chemical Engineering of Japan 35(10):1017-1019.
Crossref
|
|
|
|
|
Sanni A, Franz C, Schillinger U, Huch M, Guigas C, Holzapfel W (2013). Characterization and technological properties of lactic acid bacteria in the production of "sorghurt", a cereal-based product. Food Biotechnology 27(2):178-198.
Crossref
|
|
|
|
|
Sinha RK, Herat S, Bharambe G, Brahambhatt A (2009). Vermistabilization of sewage sludge (biosolids) by earthworms: converting a potential bihazard destined for landfill disposal into a pathogen-free, nutritive and safe biofertilizer for farms. Waste management and Research 28(10):872- 881.
Crossref
|
|
|
|
|
Soewondo P, Febriana A, Handajani M, Firdayati M (2014). Faeces Treatment by lactic acid fermentation process and future perspectives of Terra Preta sanitation concept in Indonessia. In Terra Preta sanitation; Bettendorf, TWC, Otterpohl R. Eds. Deutsche Bundesstiftung Umwelt: Hamburg, Germany.
|
|
|
|
|
Strande L, Ronteltap M, Brdjanovic D (2014). Faecal Sludge Management: systems Approach for Simple Implementation and Operation. IWA Publishing 13(5):1-14.
Crossref
|
|
|
|
|
Thimann KV (1963). The Life of Bacteria: Their Growth, Metabolism, and Relationships. second ed. Macmillan.
|
|
|
|
|
Wang Q, Yamabe K, Narita J, Morishita M, Ohsumi Y, Kusano K, Shirai Y, Ogawa HI (2001). Suppression of growth of putrefactive and food poisoning bacteria by lactic acid fermentation of kitchen waste. Process Biochemistry 37(4):351-357.
Crossref
|
|
|
|
|
WHO (2006). Guidelines for the Safe Use of Wastewater, Excreta and Greywater in Agriculture and Aquaculture. WHO, Geneva.
|
|
|
|
|
Wilsenach JA, Maurer M, Larsen TA, van Loosdrecht MCM (2003). From waste treatment to integrated resource management. Water Science and Technology 48(1):1-9.
Crossref
|
|
|
|
|
Yang J, Cao Y, Cai Y, Tereda F (2010). Natural populations of lactic acid bacteria isolated from vegetable residue and silage fermentation. Journal of Dairy Science 93:3136-3145.
Crossref
|
|
|
|
|
Yemaneh A (2015). Evaluation of lactic acid fermentation process in terra preta sanitation system and application in Arba Minch, Ethiopia. Hamburg University. Hamburg Technical University, Hamburg.
|
|
|
|
|
Yemaneh A, Bulbo M, Factura H, Buzie C, Otterpohl R (2012). Development of System for Waterless Collection of Human Excreta by Application of Lactic Acid Fermentation Process in Terra Preta SanitationSystem. 4th International Dry Toilet Conference.
|
|