Full Length Research Paper
ABSTRACT
The study was designed to chemically characterize essential oils from Kenyan Cupressus lusitanica, Ocimum americanum and Lippia javanica and bio-prospect for new compounds as possible biocontrol agents of insect pests. Leaf essential oils of the three test plants were obtained by hydro-distillation. GC-MS analysis of leaf oils revealed that monoterpenes were the major group of chemical constituents in all plants. In C. lusitanica oil, 91 compounds were identified with α-pinene (13.8%), umbellulone (12.66%), δ- cadinene (7.47) and Limonene (6.64%) being major compounds. The O. americanum oil had 72 compounds with geraniol (18.72%), 1, 8- cineole (17.48%), elemicin (8.20%) and camphor (7.55%) being main chemical constituents. Results also in L. javanica oil, the 47 compounds identified were dominated by ipsdienone (26.07 %), ocimenone (14.32%), bicyclo [3.1.1] hept-3-en-2-one, 4,6,6-trimethyl-, (1S)-(10.91%) and myrcene (7.04%). The chemotypes of essential oils from the tested plants may be considered as α-pinene-umbellulone, geranial-1, 8-cineole and ipsdienone-ocimenone for C. lusitanica, O. americanicum and L. javanica respectively. The chemical constituents such as α-pinene, umbellulone, geraniol, 1, 8-cineole and myrcene are known to have insecticidal properties. Therefore, the essential oils have possible uses in production of natural pesticides of plant origin for sustainable management of insect pest.
Key words: Essential oil, Cupressus lusitanica, Ocimum americanum, Lippia javanica, botanical pesticide.
INTRODUCTION
Essential oils are secondary plant metabolites that defend plants directly or indirectly against microorganisms and herbivores (Isman et al., 2011; Regnault-Roger et al., 2012; Isman, 2020). Many researchers have reported that essential oils mainly consist of monoterpenes, sesquiterpenes, phenylpropanoids, alcohols, esters, aldehydes, ketones, among others (Castillo et al., 2009; Bett et al., 2016). Furthermore, plants belonging to the families of Annonaceae, Asteraceae, Apiaceae, Chenopodiaceae, Cupressaceae, Lauraceae, Lamiaceae, Meliaceae, Myrtaceae, Poaceae, Piperaceae, Rutaceae, Verbenaceae and Zingiberaceae were reported as promising sources of botanical insecticides (Souza et al., 2008; Castillo et al., 2009; Ogendo et al., 2012; Bett et al., 2016; Kiplagat et al., 2020). However, many important compounds have been identified from plants but many remain to be identified and tested for their activity against many organisms. This huge pool of natural chemicals is largely underutilized for development of pesticides. Different authors have reported the medicinal value of C. lusitanica, O. americanicum and L. javanica but there been few reports of the pesticidal properties of leaf essential oils from the plants (Viljoen et al., 2005; Mujovo et al., 2008; Chikukura et al., 2011; Teke et al., 2013; Kipkore et al., 2014).
Cupressus lusitanica has been reported to have phytochemical, ethno-botanical, agricultural, ornamental and industrial importance (Kamatenesi-Mugisha et al., 2013; Teke et al., 2013). In other research reports, the essential oil C. listanica has been shown to have antibacterial and antifungal activities against Bacillus cereus and Aspergillus niger, respectively (Hassanzadeh et al., 2010). On the other hand O. americanum is useful in medical practice in treatment of dysentery, leprosy, pruritus, parasitic infections helminthiasis, anorexia, dyspepsia, flatulence, vomiting, poisonous affections, fever among others (Yamada et al., 2013). The screening of L. javanica for pharmacological activity has indicated that its activities include antimalarial, antimicrobial, antioxidant, antiplasmodial, anticancer, antiamoebic, antidiabetic, and pesticidal properties (Viljoen et al., 2005; Van Wyk, 2011; Mujovo et al., 2008).
It is also a known fact that plants with medicinal properties are likely to have reproductive inhibition antifeedant, toxic, repellent properties against field and stored product insect pests (Pandey et al., 2017). Furthmore, reports on the qualitative and quantitative variations of chemical constituents among plants from same species and growing in different parts of the world may be associated to environmental conditions and genetic differences. Variations in analytical methods can also be responsible. Therefore, it is scientifically stimulating to screen essential oils from different plant species to determine whether they possess pesticidal properties. In addition, few research efforts have directed towards determining chemical constituents of aromatic plants of medicinal and pesticidal importance in the Eastern African region. Therefore, the main focus of the current study was to determine chemical constituents of essential oils of C. lusitnica, O. americanum and L. iavanica growing in Kenya.
MATERIALS AND METHODS
Plant materials collection and essential oil extraction
The fresh plant leaves were collected from different geographic regions in Kenya: C. lusitanica from Busia (0º25’20.02”N, 35º7’45.00”E, 1215 MASL), O. americanum from Homa Bay (0º22’03’’S, 35º16’59’’E, 2002 MASL) and L. javanica from Nakuru (0º20’23.52’’S, 35º56’34.67’’, 2250 MASL), Kenya in December, 2019. The fresh plant leaves were separately packed in paper bags and transported to the laboratory for essential oil extraction. The identification of plant species was carried out on sight based on professional expertise, literature materials and pictorial aids (Bett et al., 2016). The preserved samples of each plant species were presented to Plant Taxonomist, from Department of Biological Sciences, Egerton University for confirmation of identity. The preserved voucher samples of plant materials were then deposited at the herbarium of Egerton University. The fresh leaves of C. lusitanica, O. americanum and L. javanica weighing 500g were water extracted using a modified Clevenger-type apparatus. After 4 (h) of hydro-distillation the floating oil on top of the water was collected. The remaining water in the oil removed over anhydrous sodium sulphate (Na2SO4) and dried oil stored in the refrigerator at below 4 ºC until use (Bett et al., 2016).
GC-MS analysis and determination of essential oil constituents
The chemical constituents of essential oils from plant leaves were analysed using an HP-7890A Gas Chromatograph (GC) connected to an HP 5975 C Mass Spectrometer (MS) (Agilent, Wilmington, USA) at the International Centre of Insect Ecology and Physiology (ICIPE), Nairobi. From each sample 1mg was separately weighed and dissolved in 1 mL dichloromethane, dried using anhydrous sodium sulphate (Na2SO4) to make a stock solution (1 mg/mL). The experimental sample was obtained whose final concentration was 100 ng/µL. The samples were analyzed on an GC-MS in in full scan mode and a HP-5 MS low bleed capillary column (30 m × 0.25 mm i.d., 0.25 μm) (J and W, Folsom, CA, USA). The carrier gas was Helium (1.25 ml/min, constant flow mode) and the injection mode was split mode. The oven temperature was programmed at 35 ºC (for 5 min) to 280 ºC at 10 ºC/ min(5.5min) and then at 285ºC @50ºC/min (14.9 min) and the total run time was 50 min. The electron ionization mass spectra were acquired at 70 eV within a mass range of 38–550 Daltons (Da) during a scan time of 0.73 scans s-1 whereas the ion source was maintained at a temperature of 230 ºC. The essential oil constituents were identified by comparing mass spectra with library data (NIST05a and Adams MS HP, USA) and by comparison of the retention times with mass spectra (Adams et al., 1997).
RESULTS
The percentage yields (w/w) of essential oil extraction indicate that C. lusitanica leaves were richer (0.35 ± 0.2%) in essential oils as compared to O. americanum (0.27 ± 0.06%) and L. javanica (0.15 ± 0.09%). Tables 1 to 3 shows the retention time (min) identified chemical constituents and percentage concentration of oils obtained from C. lusitanica, O. americanum and L. javanica. The results of C. lusitanica, O. americanum and L. javanica essential oil extraction and GC-MS analysis showed that the oils were dominated by monoterpenes. However, each of the essential oils had different major chemical constituents. In C. lusitanica oil, 91 compounds were identified, corresponding to 99.8% of the total essential oil composition. The monoterpene hydrocarbons were 38.63%- dominated by α-pinene (13.8%), limonene (6.64%) and ortho-cymene (4.07%). On the other hand oxygenated monoterpenes were 15.77% with umbellulone (12.66%) and Terpinen-4-ol (1.37%) as major compounds.
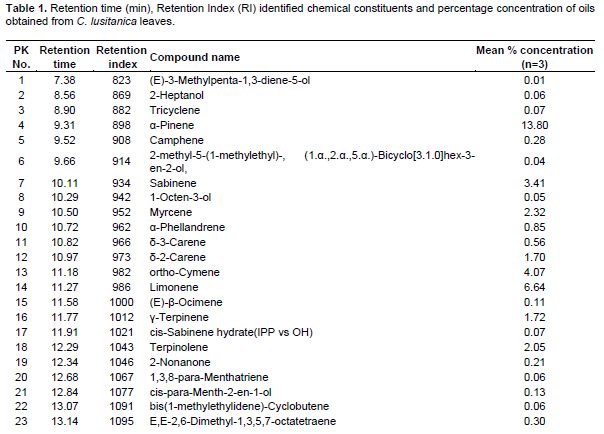


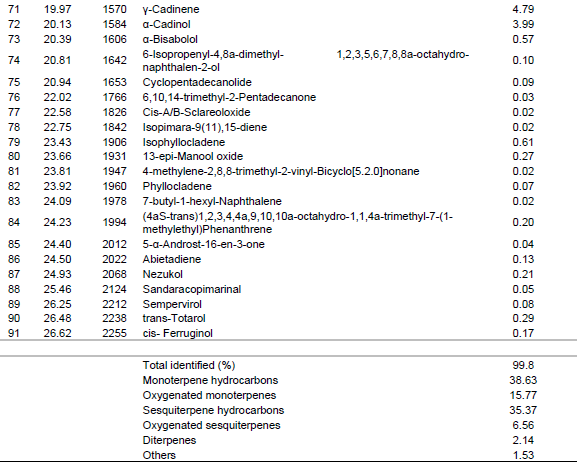
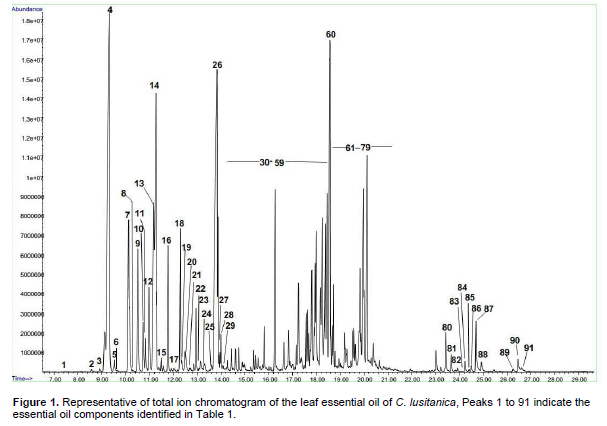
The sesquiterpene hydrocarbons and oxygenated sesquiterpenes were 35.37 % and 6.56% respectively. The major sesquiterpene hydrocarbons and oxygenated sesquiterpenes were cadinene (7.47%) and α-cadinol (3.99%), respectively (Table 1 and Figure 1).
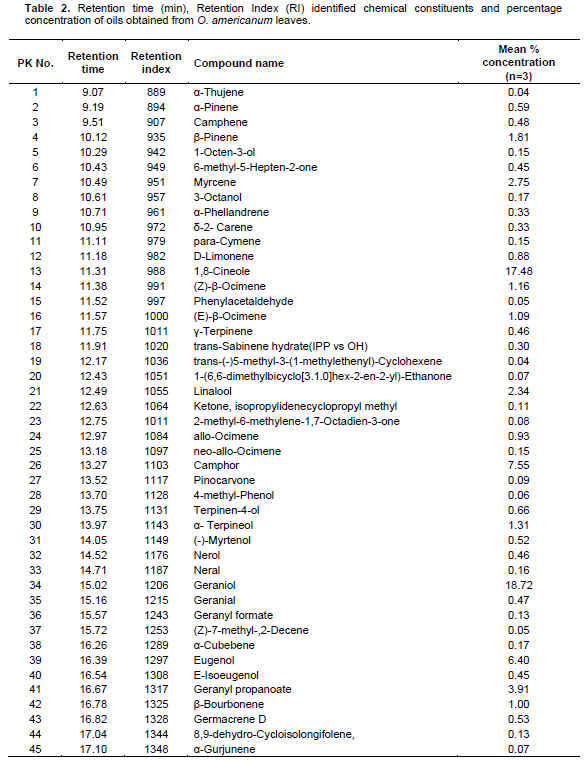
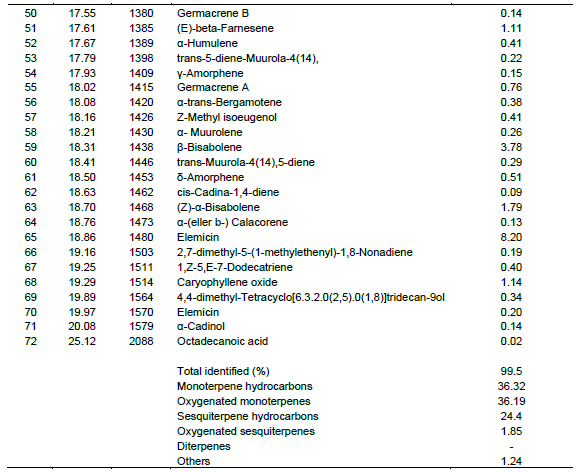
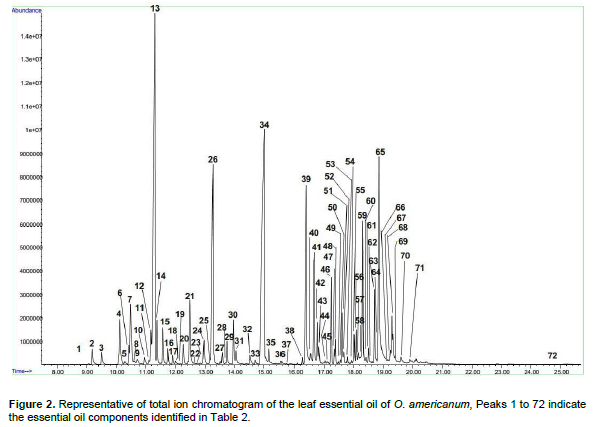
Table 2 and Figure 2 shows the retention time (min) identified chemical constituents and percentage concentration of oils obtained from O. americanum. In total, 72 compounds were identified, corresponding to 99.5 % of the total essential oil composition. The leaf essential oil contained 36.32, 36.19, 24.4 and 1.85% monoterpenes hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, and oxygenated sesquiterpenes, respectively.
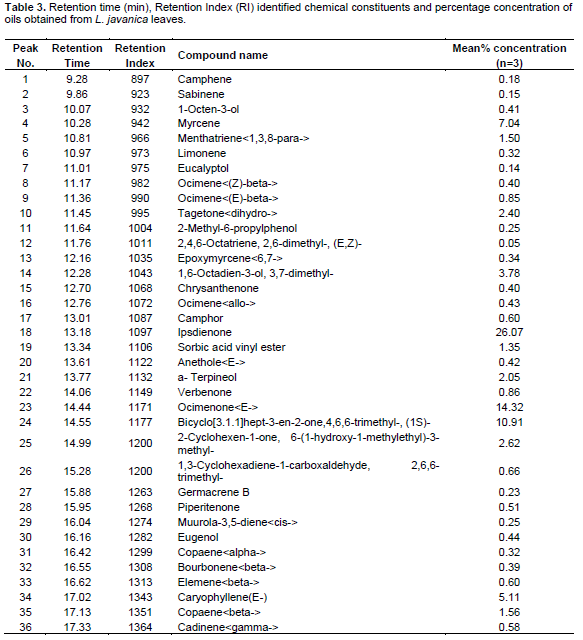
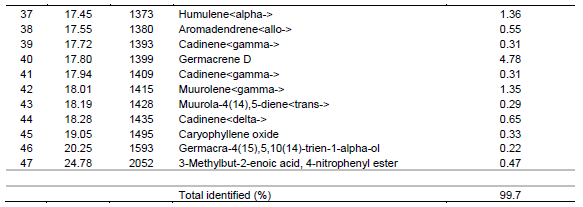
The major monoterpenes hydrocarbons were1, 8-cineole (17.48%), camphor (7.55%) and myrecene (2.75%) whereas oxygenated monoterpenes were geraniol (18.72%), Eugenol (6.40%), and geranyl propanoate (3.91%). On the other hand, sesquiterpenes hydrocarbons were represented by elemicin (8.20%), b-bisabolene (3.78%) and caryophyllene (E) (2.22%) whereas oxygenated sesquiterpenes were in trace amounts. The composition of the essential oil of L. javanica is listed in Table 3 and the peaks depicted in Figure 3. As shown, a total of forty-seven (47) compounds were identified, consisting of mainly of oxygenated monoterpenes (67.82%) followed by sesquiterpene hydrocarbons (19.35%), monoterpene hydrocarbons (10.95%) and oxygenated sesquiterpenes (1.34%). It’s noted that the major monoterpene hydrocarbon was myrcene(7.04%) whereas oxygenated monotwerpenes were mainly ipsdienone (26.07%), ocimenone (14.32%), and bicyclo [3.1.1]hept-3-en-2-one, 4,6,6-trimethyl-, (1S)-(10.91%), On the other hand sesquiterpene hydrocarbons were dominated by caryophyllene(E) (5.11%) and germacrene D (4.78%). When the chemical constituents of the three essential oils were compared, the highest amounts of monoterpenes were found in L. javanica (87.77%), followed by O. americanum (72.51%) and C. lusitanica (54.40%) in decreasing amounts, respectively. The converse was the situation with sesquiterpenes, with the highest percentage detected in C. lusitanica (41.93 %) followed by O. americanum (26.25%) and L. javanica (20.69 %), respectively. Diterpenes were found in trace amounts (2.14%) in C. lusitanica leaf essential oils.

DISCUSSION
The chemical constituents of C. lusitanica, O. americanum and L. javanica essential oils revealed in the present study indicate a variation in yield, number and concentration of chemical constituents. However, the main chemical constituents in oils of the three plant species were dominated by monoterpene hydrocarbons. It is noted that in C. lusitanica oil, the major compounds were; ??pinene??umbellulone and Limonene??The finding of the current study are also comparable to previous researches which have shown chemical composition of C. lusitanica, O. americanum and L. javanica varied with countries and region. For instance Teke et al. (2013) found C. lusitanica to contain mainly germacrene D (18.5%), epi-zonarene (8.2%), cis-calamenene (8.2%), terpinen-4-ol (6.3%), linalool (6.0%) and umbellulone (6.0%). Similarly, the main components C. lusitanica oils has been found also to contain α-pinene (70.1%), δ-3-carene (45.4%), umbellulone (15.2%), β-phellandrene (10.8%)??terpinen-4-ol (9.7%) and myrcene (5.8%) (Bett et al., 2016). On the other hand, the main chemical constituents in O. americanum essential oil were Geraniol, 1, 8-cineole, elemicin and Camphor. In other studies, the main chemical constituents in O. americanum oil have been reported to include citral, linalool, geraniol, citronellol and 1, 8-cineole (Ilboudo et al., 2010; Yamada et al., 2013). In the same way, L. javanica oil was dominated by ipsdienone, ocimenone, and bicyclo [3.1.1] hept-3-en-2-one, 4, 6, 6-trimethyl-, (1S)-, myrcene, caryophyllene (E) and germacrene D.
Several other authors have indicated that Lippia javanica oil is rich in caryophyllene, carvone, ipsenone, ipsdienone, limonene, linalool, myrcene, myrcenone, ocimenone, p-cymene, piperitenone, sabinene, and tagetenone (Viljoen et al., 2005; Lukwa et al., 2009; Chagonda and Chelchat, 2015; Maroyi, 2017).
However, Viljoen et al. (2005) using cluster analysis, found that L. javanica chemotypes growing in South Africa and Swaziland, were rich in myrcenone (36 to 62%), carvone- (61 to 73%), piperitenone- (32 to 48%), ipsdienonerich (42 to 61%), and linalool (>65%). The variations observed in chemical constituents and concentrations may be influenced by climatic and soil conditions in different the regions, which directly affect the metabolic processes of the plant (Chéraif et al., 2007), but also may arise from interaction of different biotic components. In addition, it has also been found that chemical constituents of essential oils could differ with species of plant, season, geographic region, and age of the plant, and the method used to extract essential oil extraction (Brooker and Kleinig, 2006, Bernard et al., 2020; Baccaria et al., 2020).
The chemical constituents found in oils in current study have been found in previous studies to have insecticidal properties against insect pests (Lee et al., 2003; Rosman et al., 2007 and Nivea et al., 2013). This supports the findings of Ilboudo et al. (2010); Olivero-Verbel et al. (2013) who reported that 1, 8-cineole, limonene and α-pinene were associated with contact toxicity of essential oils against insect pests. Similarly, Rozman et al. (2007) reported essential oils constituents containing, eugenol, 1,8-cineole, camphor and linalool to cause mortality of 85-100, 80-100 and 0-13% mortality of adult Sitophilus oryzae, Rhizopherta dominica and Tribolium castaneum, respectively.
The repellent properties of the major chemical constituents of essential oils of plant leaves for instance 1, 8-cineole, terpineol and α-pinene have also been reported in different researches (Tapondjou et al., 2005; Toloza et al., 2006; Bett et al., 2016). Furthermore, constituents of essential oils such as 1, 8-cineole, p-cymene, and γ-terpinene and α-pinene have earlier been reported to possess reproductive inhibition properties against insect pests (Sedaghat et al., 2011; Alzogaray et al., 2011 and Gomah et al., 2015).

For instance essential oils of Cupressus arizonica been found to have larvicidal activity against larvae of Anophhleles stephensi. The main essential oils constituents in the oil C. arizonica include limonene (14.44%), umbellulone (13.25%) and α-pinene (11%) (Sedaghat et al., 2011). Hence, the findings of this study is a proof that C. lusitanica, O. americanum and L. javanica and others possess pesticidal properties that may be exploited sustainably and cheaply for use in agriculture and related applications.

CONCLUSION
From the results of this study, it may be concluded that essential composition of C. lusitanica, O. americanum and L. javanica composition and classification were into various chemotypes varied with plant species. The essential oil constituents consisted mainly of monoterpenes that are known to have antifeedant, repellent, reproductive inhibition and insecticidal properties against insect pests of field and stored products insect pests. The presence of potentially insecticidal bioactive compounds in C. lusitanica, O. amicanum and L. javanica provides scientifically stimulating since natural insecticides are ecologically and environmentally friendly for the management of insect pests.
RECOMMENDATIONS
The authors recommended more studies to be carried out on the bioactivity of the essential oils against various field and stored insect pests’ product. Therefore, the results of present study may lead to the formulation of new natural insecticides for management of insect pests.
ACKNOWLEDGEMENTS
The authors wish to appreciate Egerton University, through the Division of Research and Extension, for their financial and technical support towards this study. They wish also to thank the International Centre of Insect Physiology and Ecology (ICIPE) for assisting with GC-MS analysis apparatus and Mr Xavier Cheseto for providing technical support during GC-MS analysis process of essential oils.
CONFLICT OF INTERESTS
The authors have not declared any conflicts of interests.
CONFLICT OF INTERESTS
The authors have not declared any conflicts of interests.
REFERENCES
|
Adams RP, Zanoni TA, Lara A, Barrero AF, Cool LG (1997). Comparisons among Cupressus arizonica Greene, C. benthamii Endl, C. lindleyi Klotz. ex Endl. and C. lusitanica Mill. using essential oils and DNA fingerprinting. Journal of Essential Oil Research 9(3):303-309. |
|
|
Alzogaray RA, Lucia A, Zerba EN, Masuh HM (2011). Insecticidal Activity of Essential Oils from Eleven Eucalyptus spp. and Two Hybrids: Lethal and Sublethal Effects of Their Major Components on Blattella germanica. Journal of Economic Entomology 104(2):595-600. |
|
|
Baccaria W, Znatia M, Zardi-Bergaouia A, Chaieb I, Flaminic G, Ascrizzi R, Jannet HB (2020). Composition and insecticide potential against Tribolium castaneum of the fractionated essential oil from the flowers of the Tunisian endemic plant Ferula tunetana Pomel ex Batt. Industrial Crops and Products 143:1-7. |
|
|
Bernard K, Groden E, Drummond FA (2020). Evaluation of Four Plant Extract Repellents for Management of the European Red Ant Myrmica rubra (Hymenoptera: Formicidae). Journal of Economic Entomology 113(4):1609-1617. |
|
|
Bett PK, Deng AL, Ogendo JO, Kariuki ST, Kamatenesi-Mugisha M, Mihale JM, Torto B (2016). Chemical composition of Cupressus lusitanica and Eucalyptus saligna leaf essential oils and bioactivity against major insect pests of stored food grains. Industrial Crops and Products 82:51-62. |
|
|
Brooker MIH, Kleinig DA (2006). Field Guide to Eucalyptus. vol.1. South-eastern Australia, Third edition, Bloomings: Melbourne. |
|
|
Castillo L, González-Coloma A, González A, Díaz M, Santos E, Alonso-Paz E, Bassagoda, MJ, Rossini C (2009). Screening of Uruguayan plants for deterrent activity against insects. Industrial Crops and Products 29(1):235-240. |
|
|
Chagonda LS, Chalchat JC (2015). Essential oil composition of Lippia javanica (Burm.f.) spreng chemotype from Western Zimbabwe. Journal of Essential Oil Bearing Plants 18(2):482-485. |
|
|
Chéraif I, Ben J, Jannet H (2007). Chemical composition and antimicrobial activity of essential oils of Cupressus arizonica Greene. Biochemical Systematics and Ecology 35(12):813-820. |
|
|
Chikukura L, Mvumi B, Chikonzo R, Chenzara C (2011) Evaluation of selected indigenous pesticidal plant powders against stored maize and cowpeas insect pests. African Crop Science Conference Proceedings 10:189-192. |
|
|
Gomah EN, Sahar IA, Ibrahim SIA, Basma A, Al-Assiuty BA (2015). Chemical composition, insecticidal activity and persistence of three Asteraceae essential oils and their nanoemulsions against Callosobruchus maculatus (F.). Journal of Stored Products Research 61:9-16. |
|
|
Hassanzadeh SL, Tuten JA, Vogler B, Setzer WN (2010). The chemical composition and antimicrobial activity of the leaf oil of Cupressus lusitanica from Monteverde, Costa Rica. Pharmacognosy Research 2:19-21. |
|
|
Ilboudo Z, Dabiré LCB, Nébié RCH, Dicko IO, Dugravot S, Cortesero AM, Sanon A (2010). Biological activity and persistence of four essential oils towards the main pest of stored cowpeas, Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Journal of Stored Products Research 46:124-128. |
|
|
Isman MB (2020). Botanical insecticides in the twenty-first century-fulfilling their promise? Annual Review of Entomology 65:233-249. |
|
|
Isman MB, Miresmailli S, Machial C (2011). Commercial opportunities for pesticides Phytochemistry Reviews 10(2):197-204. |
|
|
Kamatenesi-Mugisha M, Buyungo JP, Ogwal P, Kasibante A, Deng AL, Ogendo JO, Mihale JM (2013). Oral acute toxicity study of selected botanical pesticide plants used by subsistence farmers around the Lake Victoria Basin. African Journal of Environmental Science and Technology 7:93-101. |
|
|
Kipkore W, Wanjohi B, Rono H, Kigen G (2014). A study of the medicinal plants used by the Marakwet Community in Kenya," Journal of Ethnobiology and Ethnomedicine 10(1):1-22. |
|
|
Kiplagat A, Maina C, Bett P (2020). Chemical composition of oils of Azadirachta indica A. Juss and Ricinus communis Linn seed in Marigat, Baringo County, Kenya. East African Journal of Science, Technology and Innovation 1(2):1-11. |
|
|
Lee S, Peterson CJ, Coats JR (2003). Fumigation toxicity of monoterpenoids to several stored product insects. Journal of Stored Products Research 39:77-85. |
|
|
Lukwa N, Mølgaard P, Furu P, Bøgh C (2009). "Lippia javanica (Burm. f.) Spreng: its general constituents and bioactivity on mosquitoes," Tropical Biomedicine 26(1):85-91. |
|
|
Maroyi F (2017). Lippia javanica (Burm.f.) Spreng.: Traditional and Commercial Uses and Phytochemical and Pharmacological Significance in the African and Indian Subcontinent. Evidence-Based Complementary and Alternative Medicine 2017:1-34. |
|
|
Mujovo AF, Hussein AA , Meyer JJM, Fourie B, Muthivhi T, Lall N (2008). Bioactive compounds from Lippia javanica and Hoslundia opposite. Natural Product Research 22(12):1047-1054. |
|
|
Nivea MSG, de Oliveira JV, Navarro MAF, Dutra KA, da Silva WA , Wanderley MJA (2013). Contact and fumigant toxicity and repellency of Eucalyptus citriodora Hook., Eucalyptus staigeriana F., Cymbopogon winterianus Jowitt and Foeniculum vulgare Mill. essential oils in the management of Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae, Bruchinae). Journal of Stored Products Research 54:41-47. |
|
|
Ogendo JO, Deng AL, Birech RJ, Bett PK (2012). Plant-Based Products as Control Agents of Stored-Product Insect Pests in the Tropics, In Bhat R, Alias AK and Paliyath G (Eds.), Progress in Food Preservation, Wiley & Sons: London, pp. 581-601. |
|
|
Olivero-Verbel J, Tirado-Ballestas I, Caballero-Gallardo K, Stashenko EE (2013). Essential oils applied to the food act as repellents toward Tribolium castaneum. Journal of Stored Products Research 55:145-147. |
|
|
Pandey S, Singh S, Kumar N, Botany R (2017). Antiviral, antiprotozoal, antimalarial and insecticidal activities of Ocimum gratissimum L. Asian Journal of Pharmaceutical Research and Development 5:1-9. |
|
|
Regnault-Roger C, Vincent C, Arnason JT (2012). Essential oils in insect control: Low-risk products in a high-stakes world. Annual Review of Entomology 57:405-424. |
|
|
Rosman V, Kalinovic I, Korunic Z (2007). Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. Journal of Stored Products Research 43:349-355. |
|
|
Sedaghat MM, Dehkordi AS, Khanavi M, Abai MR, Mohtarami F, Vatandoost H (2011). Chemical composition and larvicidal activity of essential oil of Cupressus arizonica E.L. Greene against malaria vector Anopheles stephensi Liston (Diptera: Culicidae). Pharmacognosy Research 3(2):135-139. |
|
|
Souza AP, Marques MR, Mahmoud TS, Caputo BA, Canhete GM, Leite CB, de Lima DP (2008). Bioprospecting insecticidal compounds from plants native to Mato Grossodo Sul, Brazil. Acta Botanica Brasilica 22(4). |
|
|
Tapondjou AL, Adler C, Fontemc DA, Bouda H, Reichmuth C (2005). Bioactivities of cymol and essential oils of Cupressus sempervirens and Eucalyptus saligna against Sitophilus zeamais Motschulsky and Tribolium confusum du Val. Journal of Stored Products Research 41:91-102. |
|
|
Teke GN, Kemadjou NE, Kuiate JR (2013). Chemical composition, antimicrobial properties and toxicity evaluation of the essential oil of Cupressus lusitanica Mill. leaves from Cameroon. BMC Complementary and Alternative Medicine 13:130. |
|
|
Toloza A, Czygadlo J, Cueto GM, Biurrun F, Zerba E, Picollo M (2006). Fumigant and repellent properties of essential oils and component compounds against permethrin-resistant Pediculus humanus capitis (Anoplura: Pediculidae) from Argentina. Journal of Medical Entomology 43:889-895. |
|
|
Van Wyk BE (2011). The potential of South African plants in the development of new food and beverage products. South African Journal of Botany 77(4):857-868. |
|
|
Viljoen AM, Subramoney S, Van Vuuren SF, Ba?er KHC, Demirci B (2005). The composition, geographical variation and antimicrobial activity of Lippia javanica (Verbenaceae) leaf essential oils. Journal of Ethnopharmacology 96:271-277. |
|
|
Yamada AN, Grespan R, Yamada AT, Silva EL, Silva-Filho ES, Damião MJ (2013). Anti-inflammatory Activity of Ocimum americanum L. Essential Oil in Experimental Model of Zymosan-Induced Arthritis. The American Journal of Chinese Medicine 41(4):913-926. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0