ABSTRACT
Bombax buonopozense is one of the underutilized plants in West African. Most of the parts of this plant are used by some populace in West Africa for medicinal and nutritional purposes while the sepals mostly go waste. The objective of this study is to evaluate the effect of different drying methods on some nutritional and anti- nutritional composition of fresh and dried sepals of B. buonopozense. The sepals were oven, solar and sun dried while the fresh sepals served as a control. Proximate, some mineral and anti- nutrient composition of the sepals were determined. The fresh samples were significantly different from all the dried samples (p<0.05). Ash content was relatively high with a range of 7.38 to 7.86%; protein, 9.99 to 10.76%; crude fiber, 14.63 to 15.39%; carbohydrate, 56.62 to 63.52%; iron, 1.55 to 3.22 mg/100 g; magnesium, 171.92 to 184.47 mg/100 g for the dried sepals. The various drying methods reduced the content of both oxalate and phytate of the sepals. The present findings show that drying of sepals of B. buonopozense tends to retain most of the nutrients as well as reduction in the anti- nutrients. Hence the dried sepals could be used as a potential ingredient in making foods such as soups and sauces.
Key words: Bombax buonopozense, antinutrients, drying methods, nutritional composition.
Plants play a vital role in maintaining the human health through the production of foods which provide nutrients for the body as well as medicinal purposes. Medicinal plants are defined as plants that contain substances useful for therapeutic purposes in one or more of its organs. These plants also serve as precursor for the synthesis of useful drugs (Chisom et al., 2014). Medicinal plants are known to have antioxidants, anti-inflammatory, anti-bacterial and anti- tumor activities etc. The World Health Organization (2010) estimates that 80% of population in Asian and African countries depends on traditional medicine for primary cure. General assumption of dietary constituents contributing to the protective effect of these plants is a secondary metabolite in the form of phytochemicals, vitamins and minerals. In addition to these secondary metabolites, these plants contain other compounds that moderate the effects of the active ingredients known as Anti- nutrients.
Drying of food materials has advantageous benefit such as; extending the shelf life, inhibiting microbial growth, less transport and storage cost. Moreover, drying causes reduction in the food materials bioactive compounds which may have beneficial health promotion properties such as antioxidants properties (Orphanides et al., 2013). Plants like moringa, dandelion, turkey berry and red silk cotton are mostly consumed for both nutritional and medicinal purposes. Drying of food materials also has side effect depending on the method of drying such as sun, oven, microwave, solara and freeze drying etc.
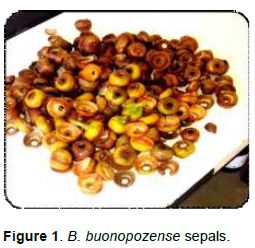
Red silk cotton plant (B. buonopozense) also known as Gold Coast Bombax belonging to the family Bombacaceae is a perennial plant which is locally called ‘Akonkode3 or Akata’ and ‘Vagba’ by the Akans and Dagbani respectively in Ghana. They are mostly found in rain forest zones of West African countries such as Sierra Leone, East Gabon some part of Nigeria (Beentje and Smith, 2001). In Ghana, red silk cotton plant is widely known for its medicinal purpose with a minute contribution to diet. The most utilized parts include the leaves, bark, root, stem, trunk etc. A greater percentage of the populace use it for medicinal purposes such as in the treatment of swellings, fever, convulsion, insanity as well as driving away evil spirits through the burning of its bark. Its seed covered with cotton are mostly harvested and used as stuffing for pillows and dresses. Report by Yi- Fang et al. (2002) indicated that its deep green leaves and deep yellow fruits provide high amount of ascorbic acid, carotene and micro minerals which play a vital role in nutrient metabolism and slowing down of degenerative diseases such as cancer and heart diseases. Its flowers having bright red to pink color attract birds and insects which contribute to pollination of the plant. With emphasis on it flowers some natives dry the sepals of the plant (Figure 1), ground them and add to food to prevent microbial growth. Chisom et al. (2014) discovered that various parts of this plant contain appreciable amount of nutrients such as carbohydrates, proteins calcium, magnesium, zinc as well as anti-nutrients such as oxalates, phytates and cyanide in minute quantity. Over the years, researchers have analyzed the nutrient and anti-nutrients composition of its leaves, stem, and bark, root of Red silk cotton plant; however, there is limited information on these parameters in relations to the sepals and the effects of drying methods on its nutritional and anti-nutrient composition. This research work will provide more information on some nutritional and anti-nutrient compositions of the sepals of B. buonopozense and also provide the most effective method of drying these sepals for the retention of higher amount of its nutrients.
Sources of the sepals and pre-treatment analysis
Flowers of B. buonopozense were obtained from Faculty of Agriculture, Kwame Nkrumah University of Science and Technology (KNUST). The sepals of the flowers were separated from the petals, sorted out, washed with clean water and allowed to drain. Samples were then divided into four portions together with the control (fresh sepals) by random sampling with equal weight of 200 g. Each of the three portions were solar dried (45 to 55°C) for 72 h, sun dried (25 to 37°C) for 37 h and oven dried (40°C) for 72 h. The dried samples were then pulverized with the blender in order to disintegrate the tissues and stop enzyme action to ensure prolonged storage. The pulverized samples were hermetically sealed in a zip lock bag and kept in refrigerator at 4°C for laboratory analysis.
Moisture content determination
Moisture content was determined using AOAC (1990) and the analysis was done in duplicate. Two grams of the samples were weighed using an analytical balance in clean, dry, weighed and labeled Petri dishes. The Petri dishes with the samples were put into thermostatically controlled oven (Binder heating and drying oven, Tuttingen, Germany) at 105°C for 4 h. The samples were cooled in a desiccator and weighed with the aid of an analytical balance (New classic MF electronic analytical balance, Mettler Toledo, Switzerland). The moisture content of the samples was expressed as;
Ash content determination
Ash was determined using AOAC (1990). Two grams of the samples were weighed into cleaned, dried, labeled and weighed crucibles which were heated in a muffle furnace for 2 h at 550 to 600°C. After 2 h, the crucible and its contents were cooled in a desiccator and then weighed. The weight of the ash was expressed as a percentage of the initial weight of the sample. The analysis was done in duplicates.
Crude fat determination
Crude fat was determined based on the soxhlet extraction method of AOAC (1990) using petroleum ether. A 250 mL round bottom soxhlet flask was cleaned, washed and dried in an oven at 103°C for 25 min and allowed to cool at room temperature before it was weighed. Two grams of the sample was weighed into a filter paper, folded and placed in a thimble. The thimble with the samples was placed into the extraction chamber which was attached to a round bottom flask containing 240 mL of petroleum ether. The round bottom flask was mounted on an electric thermal unit while the extraction chamber was connected to the reflux condenser with cold water. The extraction was allowed to run for 6 h after which the petroleum ether was distilled off. The flask containing the extracted fat was dried at 105°C for 1 h in an oven to remove the trapped petroleum ether. The flask and its content were transferred to the desiccator to cool and weighed. The analysis was duplicated. The weight of the fat obtained was expressed as percentage of the initial weight of the sample. Crude fat is calculated as;
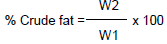
Where, W1 = weight of sample and W2 = weight of extract.
Crude fibre determination
Crude fibre was obtained using the method of (AOAC, 1990). The defatted sample which was obtained from crude fat determination was transferred into 750 mL Erlenmeyer flask and approximately 0.5 g of asbestos was added. Two hundred milliliters of boiling 1.25 % H2SO4 was added to the flask and immediately the flask was set on hot plate and connected to a condenser. The sample was boiled for 30 min and then filtered using a linen cloth in a funnel and was washed with large volume of boiling water until the residue was no more acidic. The sample was washed back into the flask using 200 mL of boiling 1.25 % NaOH. The flask was connected to the condenser again and boiled for 30 min after which it was filtered through linen cloth and washed thoroughly with boiling water until residue was no more alkaline. The residue was transferred to a crucible and the remaining was washed off using 15 mL ethanol into the crucible. The crucible and its content were dried for 30 min at 105°C and was cooled in a desiccator and weighed. The crucible was ignited in a muffle furnace at 550°C for 30 min, cooled and weighed. The loss in weight was crude fibre content and was expressed as percentage of the initial weight of the sample. The analysis was done in duplicate.
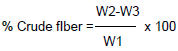
Where, W1= weight of defatted samples, W2= weight of Residue and W3 = weight of ash.
Crude protein
The crude protein was determined using the method of AOAC (1990). This process involves digestion, distillation and titration. Two grams of the sample was weighed into a digestion flask and 0.5 g of selenium catalyst was added. A blank sample was prepared alongside; 25 mL of concentrated H2SO4 was added and the flask shaken vigorously to mix the contents. The flask was inserted in the digestion chamber for digestion. Digestion was done for 3 h and the sample was prepared in duplicate. The sample was heated until boiling ceased and the resulting solution was clear.
After digestion, the digestion tubes were allowed to cool; contents were diluted with small quantity of distilled water and made up to 100 mL. Twenty-five milliliters of boric acid solution was pipetted into a conical flask and few drops of mixed indicator (bromoeresol green and methyl red) were added to the mixture. Ten milliliters of the digested samples were transferred into the kjedahl flask and 25 mL of 40% NaOH solution was added to the decomposition chamber of the distillation apparatus. The condenser tip of the distillation apparatus was dipped into the boric acid in the conical flask. The ammonia in the sample was distilled into the boric acid until it changes completely to bluish green.
The distillate was titrated with 0.1 N HCl solution until it became colorless. The percentage of total nitrogen and crude protein was calculated using a conversion factor 6.25.
Carbohydrate determination
Carbohydrate content was determined using AOAC (1990), where the value of the crude protein, crude fibre, crude fat, and moisture and ash contents of the samples were added and subtracted from 100.
Carbohydrate % = 100 – (crude protein + crude fat+ crude fiber+ ash content + moisture content)
Mineral determination using atomic absorption spectrometer
a) Chemical procedure
b) Aqua- regia was prepared with mixture of HCl and Nitric acid in the ratio of 3:1 respectively, that is 150 mL of HCl against 50 mL nitric acid.
c) Digestion and reading.
Approximately, 0.5 g of the sample was weighed into different 250 mL Kjeldahl flasks and 20 mL of Aqua –regia was prepared and added to the samples in the kjedahl flasks. The mixture was digested on a digestion block at a very low temperature. The mixture was then allowed to digest till it got to 5 mL. It was then allowed to cool at room temperature. Small amount of distilled water was added, shook and was filtered using Whatman No. 42 filter paper in 100 mL Volumetric flask. The filtrate was diluted to the mark on the volumetric flask with distilled water. The readings were taken using VGP 210 AAS (Atomic Absorption Spectrophotometer) at different wavelengths using each metal lamp. The AAS was auto calibrated to give readings in part per million (ppm). Reading was divided by 100 to convert it to milligram/100 g of the sample.
Anti - nutrient determination
Estimation of oxalates
The oxalates content of the sample was estimated according to the work done by Agbaire (2011) with slight modification. One gram of each sample was weighed and 75 mL of 1.5 N sulphuric acid solution was added; the mixture was carefully stirred intermittently with magnetic stirrer for 1 h and then filtered using whatman No. 1 filter paper. Twenty-five millimeters of the filtrate was collected and titrated hot (80- 90°C) against 0.1 N KMnO4 solution till the end point of a faint pink colour appears that persist for at least 30 min. Then the quantity of Oxalates in each sample was estimated and expressed in mg/g.
Estimation of phytate content
The phytates content was determined using the procedure cited in the work of Aina et al. (2012). Two grams of each samples was weighed into 250 mL conical flask. Each of the samples was soaked in 100 mL of 2% concentrated hydrochloric acid in the conical flask for 3 h and afterwards filtered through a double layer of hardened filter papers. Fifty millimeters of each filtrate was toped up with 107 mL of distilled water and 10 mL of 0.3% ammonium thiocyanate solution was added into each solution as an indicator. This was titrated against with standard iron (II) chloride solution, which contained 0.00195 g iron per ml. The end point was slightly brownish- yellowish and persisted for 5 min. the percentage phytic acid was calculated using the formula.
% Phytic acid = titre value ×0.00195× 1.19×100
Data analysis
All experiments were carried out in duplicates and the results were recorded as mean ± standard deviation. The significant difference among the drying methods and the control (fresh samples) was statistically analyzed using one- way analysis of variance (ANOVA) of SPSS version 20.
Proximate composition
Sepals of B. buonopozense are potentially endowed with essential nutrients required for the maintenance of good human health and could be utilized as adaptive technologies for food security. A high degree of browning occurred in all the dried sepals. Rate of enzymatic and non- enzymatic browning reactions increase during drying under favorable conditions which include moisture, temperature and presence of air (Wiriya et al., 2009). These reactions lead to oxidative bleaching through the formation of brown pigments and degradation of original pigments of food material.
The moisture content of the fresh and dried sepals in the present study is shown in Table 1. The results showed that there was significant difference (p Ë‚ 0.05) between the moisture content of the oven dried, solar dried and sun-dried sepals as compared to that of the fresh sepals where the latter recorded the highest moisture content (81.14 % ± 0.35) which was comparable to the moisture content of Ageratum conyzoides (83.20 % ± 0.02) reported by Nnamani et al. (2009). Comparing the moisture content of the dried sepals, the sun-dried sepals recorded a higher amount of moisture (9.18 % ± 0.10) with the least recorded for the oven dried sepals (2.81 % ± 0.28). These values of moisture contents for the sun, solar and oven dried sepals were lower than those reported by Chisom et al.(2014) on the leaves (14.34 % ± 2.0), stem (15.26 % ± 1.6) and the root (17.21 % ± 1.0) of B. buonopozense which was an indication that the dried sepals would have a longer shelf life because microbial growth is supported by higher moisture content which would tend to cause the spoilage of the leaves, stem and roots as compared to the dried sepals. Work done by Bassey and Khan (2015) on the leaves of B. buonopozense also recorded a higher moisture content of (12.50 % ± 0.01) as compared to that of the dried sepals. These moisture contents were also lower than bitter leaves (10.02%) and Indian spinach (11.57%) with that of sun dried higher than Moringa (6.30%) documented by both Bamishaiye et al. (2011) and Asaolu et al. (2012). High moisture provides greater activity of water-soluble enzymes and co- enzymes needed for metabolic activities of plants (Iheanacho and Udebuani, 2009) as well as a conducive environment for organs to function properly in human bodies (Iroka et al., 2014). Low moisture content of the sepals indicates their stability against microbial attack and potential longer shelf life.
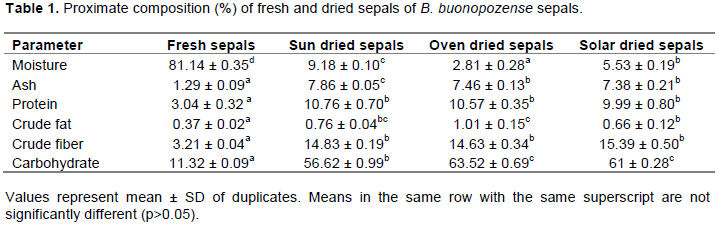
Plants foods that provide more than 12% of their calorific value from protein have shown to be a good source of protein (Ali, 2009). The results of the protein content in Table 1 shows a significant difference (p Ë‚ 0.05) between the fresh sepals and the dried sepals where the least was recorded for the fresh sepals. Comparing the dried sepals, there was no significant differences among these sepals where the highest was recorded for the sun dried (10.76 % ± 0.70) and the least recorded for the solar dried sepals (9.99 % ± 0.80). This indicated that the sepals dried by the sun were able to retain most of their proteins as compared to the oven and solar dried sepals although they were not significantly different. Work done by Chisom et al. (2014) on the leaves, stem and roots of B. buonopozense recorded a higher protein content of 13.18% ± 2.0 for the leaves, which is in line with work done by Bassey and Khan (2015) recording 13.85% ± 0.01 on the leaves of B. buonopozense. The stem and roots as documented by Chisom et al. (2014) recorded 8.94% ± 1.6 and 6.93% ± 0.5 respectively which were lower than that of the protein content of the sun dried, oven dried and solar dried but higher than the fresh sepal. Chisom et al. (2014) also worked on Ceiba pentandra leaves, stem and roots belonging to the same family as B. buonopozense and recorded a lower value of protein content in the stem (9.74 % ± 1.6) and roots (6.84% ± 1.58) of the plant. This indicates that the dried sepals would be a good substitute in place of the usage of the stem and roots when inculcated in cooking as it contains a higher amount of protein which is needed for body building, replacement of worn-out tissues, boosting immune system and help in cell division as well as growth (Okeke and Elekwa, 2006).
A documentation by Hanif et al. (2006) indicated that crude fat analysis of vegetables shows the deficiency of vegetables in fats making them good for health and not a source of lipid accumulation. Antia et al. (2006) also emphasized on the fact that lipid accumulation leads to aging as well as arteriosclerosis. From the results in Table 1, there was a significant difference (p Ë‚ 0.05)
between the crude fiber content of the dried and fresh sepals where the fresh sepals recorded the least (0.37 % ± 0.02). Comparing the dried sepals, the oven dried recorded the highest amount of crude fat (1.01 % ± 0.15), followed by the sun dried with (0.76 % ± 0.04) and the least recorded for solar dried (0.66 ± 0.12) although they were not significantly different (p˃ 0.05). The oven dried sepals were able to retain most of the fat content. A work documented by Bassey and Khan (2015) recorded 2.50 % ± 0.01 fat for B. buonopozense leaves. Also, Chisom et al. (2014) reported fat content of 2.18% ± 1.6, 8.94% ±1.6 and 6.93%± 2.5 for B. buonopozense leaves, stem and root respectively which were all higher than that recorded in this study. This shows that the sepals have a low composition of fat as compared to that of the leaves, stem and roots of B. buonopozense. Other documentations on fat content of medicinal plants which also have nutritional benefits such as; bitter leaves (9.05%) and Moringa (2.50%) were reported by Asalou et al. (2012) and Bamishaiye et al. (2011) respectively. This indicates that the B. buonopozense sepals had a higher fat content as compared to the spinach but lower than that of the bitter leaves and Moringa. Fats and oil help in the production of energy as well as the regulating of blood pressure of vital organs in the body (Iroka et al., 2014).
Carbohydrate content was the highest parameter among the dried sepals. Carbohydrates are hydrolyzed in the body to yield glucose which can be utilized immediately by the body or stored as glycogen in the muscle or liver. The least carbohydrate content was recorded for the fresh sample (11.32 % ± 0.09) where the highest was recorded for oven dried sepals (63.52 % ± 0.69). The fresh and dried sepals were significantly different (p Ë‚ 0.05). Comparing the dried sepals, the carbohydrates content of the sun dried, oven dried and the solar dried (Table 1) were not significantly different from each other (p ˃ 0.05). The oven dried sepals recorded the highest with least being the sun-dried sepals (56.62 ± 0.99). These values were higher than that recorded for B. buonopozense leaves by Bassey and Khan (2015) with an amount of 47.65% ± 0.01. Chisom et al. (2014) recorded 38.05% ± 0.9, 32.79% ± 2.55 and 31.42% ± 0.71 carbohydrate content for the leaves, stem and roots of B. buonopozense respectively. This indicates that the sepals are good source of carbohydrates which has primary usage of providing energy to the body especially the nervous system and brain due to the high levels as compared to the root, stem and leaves. Breakdown of carbohydrate leads to the production of glucose which has a pronounced effect on blood sugar level than fats and proteins whereas increase in carbohydrate intake leads to obesity (Bassey and Khan, 2015).
Ishida et al. (2000) stated that sufficient intake of dietary fiber can lower the serum cholesterol risk of coronary heart diseases, hypertension, and constipation, diabetes, colon and breast cancer. The crude fiber content of B. buonopozense sepals was highest in the solar dried sepals (15.39 % ± 0.50). The sun-dried sepals and oven dried sepals recorded 14.83 % ± 0.19 and 14. 63 % ± 0.34, respectively, which was not significantly different. The fresh samples recorded the least for the crude fiber (3.21 % ± 0.04). The crude fiber contents of the sepals were lower than B. buonopozense leaves (17.20 %) documented by Bassey and Khan (2015) as well as documentation by Chisom et al. (2014) on the leaves (16.76 % ±1.0), stem (21.34 % ± 3.2) and root (20.65% ± 3.54). Although the crude fiber of the dried sepals was lower than that of the leaves, stem and the roots of B. buonopozense, its crude fiber was higher than spinach (7.83 %) and moringa (10.11 %) as documented by Bamishaiye et al. (2011) and Asaolu et al. (2012) respectively.
Ash content is the inorganic residue remaining after water and organic matter have been removed by heating (Bassey and Khan, 2015). Water and volatile materials are vaporized and organic compounds are burnt in the presence of oxygen in air to CO2, H2O and N2. The ash content of the fresh sepals was 1.29 % ± 0.09 which was significantly different from the dried sepals. Comparing the dried sepals, the sun-dried sepals, oven dried sepals and the solar dried sepals recorded 7.86% ± 0.05, 7.46% ± 0.13 and 7.38% ± 0.21 respectively. The sun-dried sepals recorded the highest with the least recorded for solar dried sepals which is an indication that after the incineration of the samples the sun-dried sepals had the highest amount of inorganic residue remaining. Ash contents of the sepals were higher than the leaves of B. buonopozense (6.30 % ± 0.01) documented by Bassey and Khan, (2015) as well as that reported for the leaves (7.26 % ± 2.6), stem (3.23 % ± 1.6) and root (1.83 % ± 0.7) of B. buonopozense by Chisom et al. (2014) although his report was not significantly different from the dried sepals. Bamishaiye et al. (2011) reported a higher amount of ash content for Moringa (8.00%) as compared to the sepals with the sepals recording a higher ash content than spinach (5.02%) documented by Asaolu et al. (2012).
Mineral composition
Table 2 Shows the mineral composition of B. buonopozense sepals, nutritional significant of minerals when compared with the standard Recommended Dietary Allowance (RDA) per 100 g. The sepals contained an ample amount of nutrient when compared to the standard RDA. The values of Iron and magnesium were comparable to the standard whereas the values for Zinc, Calcium and Potassium were moderately low.
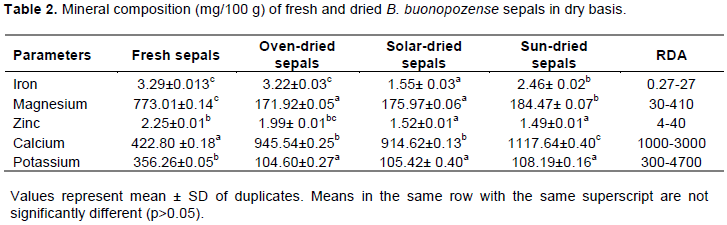
Iron is an essential trace element in the human body. It plays a crucial role of Haemopoiesic control of infection and cell mediated immunity (Bhaskaran, 2001). From Table 2, the fresh sepals recorded the highest for iron (3.29 ± 0.013 mg/100 g). Comparing the dried sepals, oven dried (3.22 ± 0.03 mg/100 g) recorded the highest followed by sun dried (2.46 ± 0.02 mg/100 g) with the least being solar dried sepals (1.55 ± 0.03 mg/100g) although they were not significantly different from each other (p Ë‚ 0.05). Values obtained were all within the range of iron by RDA (0.27 to 27 mg/ 100g). Bawa et al. (2017) recorded (3.12± 0.01) for B. buonopozense leaves which were higher than that recorded for the dried sepals but lower than the fresh sepals. Reduction of the iron content in the dried sepals can be attributed to the high intensity of heat used which causes the vaporization of some dissolved iron and amount of water during the drying process. Iron deficiency has been described as the most prevalent nutritional deficiency where anemia is estimated to affect more than one billion people worldwide (Trowbridge and Marlorell, 2002). In a research done by Dioxin et al. (2004), the consequence of iron deficiency causes reduction in the work capacity, impairment in behavior and intellectual performance and decrease in resistance to infections. Therefore, these sepals can be added to food in other to provide an appreciable amount of iron need daily.
Magnesium as an essential mineral plays a vital role in the human body. It is needed for enzymes that utilize adenosine triphosphate which contributes to DNA and RNA synthesis during cell proliferation (Wardlaw et al., 2004). The fresh sepals recorded the highest of (773.01 ± 0.14 mg/ 100g) which was above the range for RDA (30- 410 mg/100 g). Comparing the dried sepals, sun dried sepals recorded the highest magnesium content (184.47 ± 0.07 mg/100 g) with the least recorded for oven dried sepals (171.92 ± 0.06 mg/100g); however, they were not significantly different. Values obtained were within the range of RDA (30 - 410 mg/100 g). Work done by Bawa et al. (2017) reported magnesium content of 37.51± 0.02 mg/100 g for the leaves of B. buonopozense which was lower than that recorded for the sepals of B. buonopozense. This is an indication that the sepals studied have a high importance in treating some deficiency of Magnesium such as convulsion, irritability and even death when substituted in diets. Magnesium is important for the release of insulin and insulin action on cells. It also decreases blood pressure by dilating the arteries and preventing abdominal heart rhythms (Wardlaw et al., 2004).
Zinc as an essential micro nutrient is needed for human growth and immune function (Black, 2003). Hotz and Brown (2004) estimated that 20% of the world’s population is reported to be at risk of inadequate zinc intake. From Table 2, the fresh sepals recorded the highest of zinc content (2.25 ± 0.01 mg/100 g) which was below the standard RDA (4 to 40 mg/100 g). For the dried sepals, the oven dried sepals were able to retain a high amount of the zinc in the sepals compared to the solar dried and the sun-dried sepals with an amount of (1.99± 0.01 mg/100 g). The solar dried sepals and sun-dried sepals recorded 1.52± 0.01 and 1.49± 0.01 mg/100 g respectively. The amount of zinc recorded was moderately lower as compared to the standard RDA (4 to 40 mg/100 g). Bawa et al. (2017) reported an amount of zinc present in the leaves of B. buonopozense as 1.87 ± 0.01 mg/100 g which was also lower as compared to the standard but not significantly different from the sepals. Sepals of B. buonopozense can therefore be combined with foods containing an appreciable amount of zinc in order to provide the daily RDA needed. Research done by Dioxin et al. (2004) indicated that about 20% of children less than 5 years, 28.1% of mothers and 43.9% of pregnant women in Nigeria are affected by zinc deficiency which include loss of appetite, hair loss, diarrhea, hair and skin lesions.
Presence of calcium, magnesium and potassium is known to reduce hypertension and blood pressure (Wardlaw et al., 2004). From Table 2, the fresh sepals recorded the least for calcium (422.80± 0.18 mg/100 g) which was higher than work done by Bawa et al. (2017) which recorded 87.28 ± 0.01 mg/100 g for B. buonopozense leaves; however, was below the standard for calcium by RDA (1000 to 3000 mg/100 g). According to National Academy of Sciences (NAS, 2010), the Tolerable Upper Intake of calcium are; Infants – (1000-1500 mg/day), Children – (2500-3000 mg/day), Adults (18 to 30 years) – (2500 mg/day), 31 to 50 years – (2500 mg/day) and 51+ years (2000 mg/day).
The oven dried, solar dried and sun-dried sepals recorded 945.10 ±0.25, 914.62 ± 0.13 and 1117.64± 0.40 mg/100 g, respectively. The sun drying of the sepals which is mostly used by the populace was able to retain the highest amount of calcium content and was within the range provided for RDA (1000 to 3000 mg/100 g). Oven dried sepals and solar dried sepals were moderately lower than the standard RDA. In improvising for the daily calcium for individuals, sepals of B. buonopozense can be combined with other foods which contain an appreciable amount of calcium to provide the recommended dietary allowance need for the body daily. Imbalance of calcium- phosphorus tends to cause Oesteoporosis, pyorrhea, rickets and tooth decay (Asaolu et al., 2012).
Potassium is an extracellular cation which plays an important role in humans. Its function as an electrolyte helps in the maintenance of a healthy balance of fluid in the body (Akpanyung, 2005). It is crucial for heart functioning and plays a vital role in the contraction of skeletal and smooth muscles, making it normal for digestive and muscular function. From Table 2, the fresh sepals recorded the highest potassium content (356.26 ± 0.05 mg/100g) which was within the standard RDA (300 to 4700 mg/100 g). The dried sepals recorded 104.60±0.27 mg/100 g, 105.42± 0.40 mg/100g and 108.19 ±0.16 mg/100 g for oven dried, solar dried and sun dried respectively with the oven dried retaining the highest amount of potassium. Work done by Bawa et al. (2017) reported an amount of 162± 0.01 mg/100 g of potassium present in the leaves of B. buonopozense which was higher than the dried sepals but lower than the fresh sepals. Some effects of low potassium intake include palpitation, abdominal cramping, bloating nausea and constipation.
Anti-nutrients
According to the report by Bawa et al. (2017), anti- nutrients reduce the bioavailability of nutrients which causes reduction in the ability of the body to use nutrients when absorbed from diet. The oxalate contents of the solar and sun-dried sepals were statistically similar. The values ranged from 72.19 – 117.83 mg / 100 g. The fresh sepals had the highest amount whereas the sun-dried had reduced amount of the oxalate among the dried sepals (Table 3). Oxalate contents in the dried sepals were higher than that recorded for the leaves of B. buonopozense (14.55 ± 0.01 mg/ 100g) reported by Bawa et al. (2017). According to Youssef and Mokhtar (2014), drying methods tend to decrease anti- nutrient levels in food. It was observed that the different drying methods were able to decrease the amount of oxalate present in the sepals due to the high heat intensity which disrupts the cells of the sepals therefore causing the oxalate present to vaporize as compared to the fresh sepals. The physiological tolerance level of oxalate is 2 to 5 g/day (Gafar et al., 2012) which indicated that the oxalate contents in the sepals were within acceptable level. Presence of oxalate in food would tend to bind to minerals in the gastrointestinal tract and result in crystals secreted in urine in minute crystals.

The phytates content of the fresh and dried sepal ranged from 1.47 to 10.26 g/100 g (Table 3). The phytates content of the fresh sepals was higher than that of the oven, sun and solar dried sepals. Phytate is reported to have high binding affinity to minerals such as calcium, potassium, iron and zinc as well as macro nutrients such as carbohydrate, protein and lipids making them unavailable for digestion (Konietzny and Greiner, 2003). It was observed that the drying methods were able to reduce the Phytate level in the fresh to an appreciable level as stated by Youssef and Mokhar (2014) making them less adverse to human health. The phytate level in the sepals was higher than that in the leaves of B. buonopozense (10.86 mg/100 g). Although phytate level in the sepals was high they were within the range stated by Reddy (2002). According to Reddy (2002), daily intake of phytate for humans with vegetarian diet should be within the range of 2000 to 2600 mg while inhabitant of rural areas in a developing country with mixed diet should take 150 to 1400 mg per day. This is because high quantities of phytate in the food can make the nutrients in the food bio-unavailable to the human body. Anti- nutrients in the present study were higher than that of the leaves of B. buonopozense therefore further processing such as cooking can be used in reducing the high levels of anti- nutrients as they are affected by heat.
The dried sepals of B. buonopozense had considerably high amount of carbohydrate, ash, magnesium and calcium content as compared to the stem, leaves and roots of the plants. High amount of carbohydrate, ash content, magnesium and calcium could help improve the nutritional and health benefits in the body. Drying had a significant effect on the dried sepals especially the color and moisture content of the sepals. The different drying methods were able to retain most of the nutrients however there was less variation on the proximate and mineral composition of the dried sepals comparably to the fresh sepals. Although the fresh sepals had a higher amount of mineral, their higher content of anti- nutrients is an indication that consuming these sepals in the fresh state could hinder the bioavailability of nutrients in the body as the anti- nutrients cause the formation of insoluble compounds therefore drying the sepals is appropriate. Notwithstanding, the, solar drying method was the best in terms of quality, nutritional and cost factor.
The authors have not declared any conflict of interests.
REFERENCES
|
Agbaire PO (2011). Nutritional and Anti-nutrient levels of some local vegetables (Vernomia anydalira, Manihot esculenta, Teiferia occidentalis, Talinum triangulare, Amaranthus spinosus) from Delta state, Nigeria. Journal of Applied Science and Environmental Management 15(4):625-628.
|
|
|
|
Aina VO, Binta S, Amina Z, Hauwa Haruna, MS, Hauwa U, Akinboboye RM, Adama M (2012). Determination of Nutritional and Anti-Nutrient Content of Vitis vinifera (Grapes) Grown in Bomo (Area C) Zaria, Nigeria. Advance Journal of Food Science and Technology 4(6):445-448.
|
|
|
|
|
Akpanyung EO (2005). Proximate and mineral composition of Bouillon cubes produced in Nigeria. Pakistan Journal of Nutrition 4:327-329.
Crossref
|
|
|
|
|
Ali A (2009). Proximate and mineral composition of the marchubeh (Asparagus officinalis). World Dairy and Food Science 4(2):142-149.
|
|
|
|
|
Association of Official Analytical Chemists (AOAC) (1990). Official methods of analysis. 15th Edition, AOAC International Publisher, Washington DC.
|
|
|
|
|
Asaolu SS, Adefemi OS, Oyakilome Ajibulu KE, Asaolu MF (2012). Proximate and mineral composition of Nigerian leafy vegetables. Journal of Food Research 1(3):233-237.
Crossref
|
|
|
|
|
Antia BS, Akpan EJ, Okon PA, Umoren IU (2006). Nutritive and anti- nutritive evaluation of sweet potatoes (Ipomoea batatas) leaves. Pakistan Journal of Nutrition 5:166-168.
Crossref
|
|
|
|
|
Bamishaiye EI, Olayemi FF, Awayu EF, Bamishaiye OM (2011). Proximate and phytochemical composition of Moringa Oleifera leaves at three stages of maturation. Advance Journal of Food Science and Technology 3(4):233-237.
|
|
|
|
|
Bassey EE, Khan ME (2015). Proximate composition and phytochemical analysis of Bombax buonopozense Leaves (Gold coast Bombax). International Journal of Current Research in Chemistry and Pharmaceutical Science 2:51-56.
|
|
|
|
|
Bawa A, Bassey EE, Daniel J, Umar YD (2017). Biomedical Significance of the Elemental and Anti- nutritional Composition of Bombax buonopozense leaves. International Digital Organization for Scientific Research 2(2):18-28.
|
|
|
|
|
Beentje H, Smith S (2001). Plant systematic and Phytogeography for the understanding of African Biodiversity. Systematic and Geography of plants 71(1):234–286.
|
|
|
|
|
Bhaskaran P (2001). Immunobiology of mild nutrient deficiency. British Journal Nutrition 85: S75-S80.
Crossref
|
|
|
|
|
Black RE (2003). Zinc deficiency, infectious disease and mortality in developing world. Journal of Nutrition 133:S1485-S1489.
Crossref
|
|
|
|
|
Chisom FI, Okereke C, Okeke CU (2014). Comparative phytochemical and proximate Analyses on Ceiba pentandra (L) Gaertn and Bombax buonopozense (P) Beauv. International Journal of Herbal Medicine 2(2):162-167.
|
|
|
|
|
Dixon MB, Akinyele IO, Oguntona EB, Nokoe S, Sanusi RA, Harri E (2004). Nigeria food consumption and nutrition survey, 2001-2003. International Institute of Tropical Agriculture (IITA).
|
|
|
|
|
Gafar MK, Itodo AU, Senchi DS (2012). Nutritive and Anti – Nutritive Composition of Chanca Piedra (Stone Breaker). Food and Public Health 2(2):21-27.
Crossref
|
|
|
|
|
Hanif RZ, Iqbal M, Iqbal S, Hanif RM (2006). Use of vegetables as nutritional food as nutritional food: role in humans' health. Journal of Agricultural and Biological Science 1:18-22.
|
|
|
|
|
Hotz C, Brown KH (2004). Assessment of the Risk of Zinc Deficiency in Populations and Options for Its Control. International Zinc Nutrition Consultative Group (IZINCG) eds. Food and Nutrition Bulletin 25: S91-S204.
|
|
|
|
|
Iheanacho K, Ubebani AC (2009). Nutritional composition of some leafy vegetable consumed in Imo- State, Nigeria. Journal of Applied Science and Environmental Management 13(3): 35-38.
|
|
|
|
|
Iroka CF, Okereke CN, Okeke CU (2014). Comparative phytochemical and proximate analyses on Ceiba pentandra (L) Gaertn. and Bombax buonopozense (P) Beauv. International Journal of Herbal Medicine 2(2):162-167.
|
|
|
|
|
Konietzny U, Greiner R (2003). Phytic acid: Nutritional impact. In Caballero B, Trugo L, Finglas P (Eds.), Encyclopaedia of Food Science and Nutrition pp. 4555-4563.
Crossref
|
|
|
|
|
Nnamani CV, Oselebe HO, Agbatutu A (2009). Assessment of Nutritional Values of Three Underutilized Indigenous Leafy Vegetables of Ebonyi State, Nigeria. African Journal of Biotechnology 8:2321-2324.
|
|
|
|
|
Okeke CU, Elekwa I (2006). Proximate and Preliminary Photochemical Analyses of Avocado Pea Persea gratissima Cacrtn. F (Family Lauracea). Nigeria Journal of Botany 9(1):159-162.
|
|
|
|
|
Orphanides A, Goulas V, Gekas V (2013). Effect of drying on the phenolic content and antioxidant capacity of spearmint. Czech Journal of Food Science 31(5):509-513.
Crossref
|
|
|
|
|
Reddy NR (2002). Occurrence, distribution, content, and dietary intake of phytate. In Reddy NR, Sathe SK (Eds.). Boca Raton, Florida: CRC Press. Food Phytates pp. 25–51.
|
|
|
|
|
Trowbridge F, Martorell M (2002). Forging effective strategies to combat iron deficiency. Summary and recommendations. Journal of Nutrition 85:875-880.
Crossref
|
|
|
|
|
Wardlaw GM, Hampl JS, DiSilvestro RA (2004). Perspectives in nutrition. 6th ed. New York: McGraw Hill.
|
|
|
|
|
Wiriya P, Paiboon T, Somchart S (2009). Effect of drying air temperature and chemical pretreatments on quality of dried chili. International Food Research Journal 16:441-454.
|
|
|
|
|
Yi–Fang C, Jie S, Xia –Hong Wu, Rui- Hai L (2002). Antioxidant and anti-proliferative activities of common Vegetables Review. Journal of Agricultural and Food Chemistry 50:6910-6919.
Crossref
|
|
|
|
|
Youssef MK, Mokhtar SM (2014). Effect of drying methods on the Anti-oxidant capacity, Color and Phytochemicals of Portulaca oleracea L. Leaves. Journal of Nutrition and Food Science 4(6):1-6.
Crossref
|
|