ABSTRACT
This study assessed the exposure of humans to Staphylococcus species expressing the Enterotoxins genes (SEs) through consumption of boiled-milk-served-hot and fermented milk in Mbeya, Tanzania. A survey involving 120 consumers revealed that 67.5% of the respondents were buying raw milk from milk shops for home consumption. About 76% of respondents boiled milk before consumption, 14.8% ferment the milk after boiling and 5.8% consumed fermented milk without boiling. Children (30%) consumed milk more frequently than other members in the family. Among consumers who buy milk from the milk shops, 71% were daily consumers of both boiled milk served hot and fermented milk. Approximately, 1197 L (90% CI, 987-1416) of ready to consume milk was sold per day. Of which 860 L (90% CI, 645-1071) and 337 L (90% CI, 168-530) were boiled-milk-served-hot and fermented milk, respectively. Out of the ready to consume milk, 490 L (90% CI, 464-516) of boiled-milk-served-hot was contaminated with SEs gene compared to 77.5 L (90% CI, 67-88) of fermented milk. Daily 2394 people were consumers of milk and their products. Exposure assessment shows that the probability of consuming boiled-milk-served-hot and fermented milk contaminated with SEs gene at a milk shop was 0.42 (90% CI, 0.071-0.838) and 0.17 (90% CI, 0-0.62), respectively. It was estimated that every day, 363 (90% CI, 341-385) and 58 (90% CI, 49-66) people were likely to consume boiled milk taken hot and fermented milk contaminated with SE gene, respectively. The finding shows that exposure to SEs gene was two times more likely to occur in people who consume boiled-milk-served-hot than in people who consume fermented milk (OR. 2.221 (90% CI, 0.6-6.16). Awareness creation on proper food handling among milk handlers to reduce contamination along the milk value chain is recommended.
Key words: Boiled milk served hot, foodborne disease, public health, fermented milk.
Foodborne disease is an important and growing public health concern in many countries around the globe (WHO, 2002; Le Loir et al., 2003). Animal source foods have been cited as an important cause of foodborne illness and Staphylococcus aureus is one of the pathogenic microorganisms most frequently linked with foodborne diseases (Le Loir et al., 2003). This bacterium is usually found in milk and milk products as a result of poor hygiene practices and animals with clinical or subclinical mastitis (Mdegela et al., 2009). Following the ingestion of staphylococcal enterotoxins (SEs) that are produced by enterotoxigenic strains of S. aureus, initial symptoms include nausea, vomiting (in spurts), abdominal pain, diarrhoea, dizziness, shivering and general weakness, sometimes associated with a moderate fever (Hennekinne, 2012).
Even though many people suffer from foodborne illness yearly, the accurate estimate of the incidences of food-borne disease is difficult to obtain in developing countries like Tanzania. People with symptoms like vomiting, diarrhoea and stomach cramps rarely go to the hospital, due to the limited access to the biomedicine and disease understanding especially in rural areas. In Tanzania, statistics show that 60 to 70% of the population seek healthcare from practitioners of traditional medicine (URT, 2000). Despite the presence of conventional medicine, traditional medicine is widely used and rapidly growing health care system in the country (Kayombo et al., 2012); therefore, the cases of foodborne diseases are under-reported.
Thus, ensuring the safety of milk from dairy farmer where animal husbandry practices differ widely presents a big challenge. Over 85% of milk consumed in Tanzania is from informal markets (Kurwijila, 2006). This causes the consumers of milk to be exposed to ingestion of milk containing pathogenic bacteria (S. aureus and their toxins) in cases where the milk is consumed without heat treatment or other processing capable of inactivating this enterotoxins which is heat resistant (Argudin et al., 2010). Unlike the producer organism, enterotoxins are remarkably heat resistant; as a result, they may be present in foods even when viable S. aureus are absent (Jørgensen et al., 2005). According European Commission for Health and Consumer Protection (ECHCP, 2003) inactivation of crude enterotoxins type A (SEA) in buffer was reduced from 21 to <1 μg/ml after heating at 100°C for 130 min and purified SEA (0.2 mg/ml) was completely inactivated in buffer after heating at 80°C for 3 min or 100°C for 1 min. Previous study showed that boiled hot milk ready to consume harboured pathogenic bacteria (S. aureus) with genes (SEs) responsible for toxins production (Gratian, 2012; Massawe et al., 2017). The SEs are resistant to inactivation by gastrointestinal proteases such as pepsin and trypsin and the toxins produced showed thermal stability (Argudin et al., 2010), making their elimination difficult to achieve (Le Loir et al., 2003). Thus, the aim of this study was to assess the consumption behaviour and the risk of exposure to milk with SE genes in Mbeya, Tanzania. The outcomes of this study will provide useful information and serves as a case study for future mitigation strategies to decrease the prevalence of S. aureus and SEs in the Mbeya milk value chain.
The study was carried out in Mbeya and Mbozi district in Mbeya region which have a high population of dairy cattle. Description of the study area can be found in Massawe et al. (2017).
The study was carried out in three steps. In the first step, two questionnaires were administered to firstly milk consumers who were in the milk shop at the time of visits and willing to participate in the study. The information collected was on milk consumption pattern, frequency of consumption, amount consumed, type of milk preferred (boiled or fermented), whether they buy milk for home consumption, type of milk bought, amount, treatment performed before consumption and people in the family who consume milk and secondly milk shop owner to record information on procedure followed when receiving milk, access to training on milk handling, source of milk, amount of milk handled, amount sold, type of consumer, number of consumer, milk treatment conducted in their shops and types of quality check conducted. Personal observation was also used to get information on milk handling, type of serving utensils, cleanliness of the milk shop and personal cleanliness of owner and his/her staff. Data was collected during the wet (April 2015 to June 2015) and the dry season (August 2015 to November 2015). Samples were collected from 36 milk shops in the study area (18 sites from each district).
The second step involved sampling of milk (raw, boiled milk served hot and fermented milk) carried out concurrently with administration of questionnaire. The final step was laboratory analysis, where the isolation of S. aureus was performed using standard procedure and finally the detection of SE genes in the milk was determined by multiplex polymerase chain reaction (mPCR) (Rahimi, 2013) with modification of annealing temperature. The multiplex PCR was establish using nine pairs of primers (Table 1) allowing the detection of genes encoding staphylococcal enterotoxins genes sea, seb, sec, sed, see, seg, sei, seh and sej. The amplifications were performed in 0.2 ml reaction tubes in a final reaction volume of 25 μl. The PCR mixture consisted of 5 mM MgCl2, 200 μM dNTPs, buffer, 2 U of Taq polymerase, and 5 μl of DNA. DNA amplification was performed in a Takara thermal cycler (MJ Research, Inc. Tokyo Japan) using the following conditions :initial denaturation for 5 min at 94°C followed by 40 cycles of denaturation (94°C for 30 s), annealing (90 s at 57°C), initial extension for 72°C at 60 s. A final extension step (72°C for 10 min) was performed after the completion of the cycles. The amplified PCR products were visualized by standard gel electrophoresis in a 2% agarose gel stained by Gel red (5 μg/mL). The gel electrophoresis was run for 60 min at 110 V in order to achieve a visible separation of bands. The gels were photographed under ultraviolet light using the Gel-Doc 2000 system (Bio-Rad, USA). Samples that test positive for a particular gene were counted and their isolation rates calculated.
A stochastic model was developed for the exposure to the SE genes by consumers of boiled milk served hot and fermented milk using the following parameters: the number of milk shops (N), the total quantity of milk sold daily in the milk shops (Q), the average daily milk sold (XÌ„m), concentration of pathogens in contaminated milk (C), prevalence of SEs in ready to consume milk (PRV), the quantity of milk contaminated daily (Qc), the proportion of people consuming boiled hot milk (PB) and fermented milk (PF), the number of daily milk consumers (Dc), and the probability of consuming milk containing SEs (P) (Figure 1).
Milk consumption characteristics
The consumption characteristic of milk in the study area shows that most of the households (80%) consumed milk with other food (Table 2). About 70% of the respondents buy 0.5 to 2 L of milk (amount purchased depend on
income of the individual) and 74.2% of the respondents consume 0.25 to 1 L per day. Furthermore, 76.7% of the respondent’s boiled milk before consumption and 9.2% drink raw milk. Fifty percent of the respondents, whole family members consume the milk (6 people per family), 30% of the respondents only children consume the milk, while in 9.2% of the respondent’s only elders consumed milk.
Characteristics of milk shops in the study area
Characteristics and practices conducted in the milk shops are shown in Table 3. Sixty seven percent of the milk shops owners were male and their age ranged from 21 to 73 years old. Fifty eight percent of milk shop owners aged between 21 and 50 years.
Most of the respondents had primary education level (63.9%) and only 11.1% attended food handling training. The utensils used for milk handling were plastic buckets (86.1%) and aluminium cans (2.8%). Most of the milk shops (66.7%) had no cooling facilities. Test for milk quality was common to all milk shops and density in combination with clot on boiling was the most frequently method used by 69.4% of the respondents.
Factors associated with isolation of SEs gene in ready to consume milk
The age of the milk shop owner had influence on the rate of SEs isolation (Table 4). The samples collected from the milk shops owned by younger personnel had greater chance of SEs isolation (OR 1.83 (90% CI, 0.52-6.47) than those collected from shops of older personnel. Having a post secondary education reduces the odds (OR 0.57 (90% CI, 0.35-1.84) of SEs isolation. In addition, samples from milk shop owned by personnel who had little experience was at relatively higher chance (OR 1.31 (90% CI, 0.46-3.72) of isolating SEs gene. Absences of cooling facilities increase the odds (OR 1.72 (90% CI, 0.65-4.48) of SE isolation. Furthermore, the shops which conducted quality control reduce the odds of SEs isolation by 0.62 (90% CI, 0.22-1.83).
Hazard identification
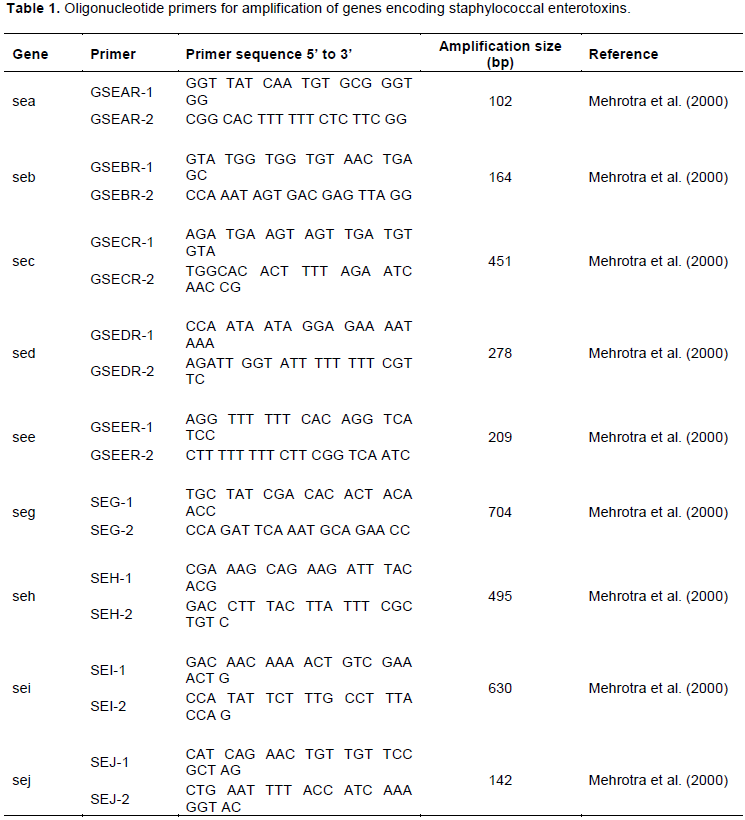
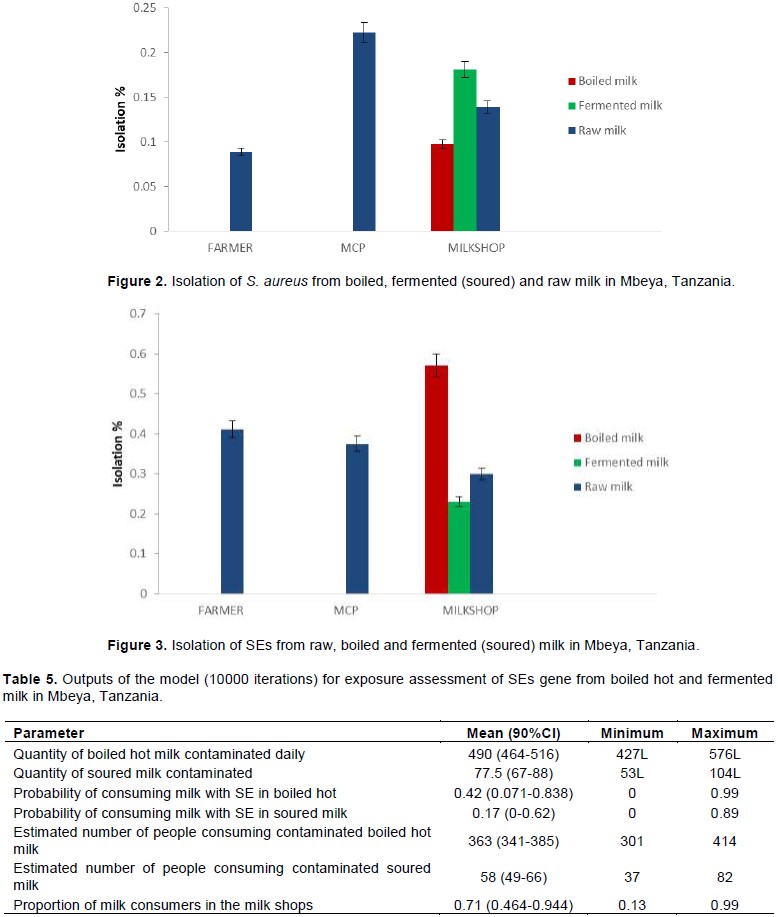
Analysis of milk samples showed that isolation rates of S. aureus from raw milk in farmer, MCP and milk shops were 8.9, 22.2 and 13.9%, respectively (Figure 2). The corresponding percentages for boiled milk served hot and fermented milk were 9.7 and 18.1%, respectively. Among the isolated S. aureus, 36.4% had SE coding genes. Thus, SEs is identified as a potential hazard and risk to milk consumers. The SE coding genes were isolated in the ready to consume milk (boiled hot 57.1% and fermented (23.1%) sold in the milk shops in the study area (Figure 3).
Isolation of SEs coding genes at the milk shops were highest in boiled milk (57.1%) followed by raw milk (30%) and fermented milk (23.1%) (Figure 3).
Exposure assessment
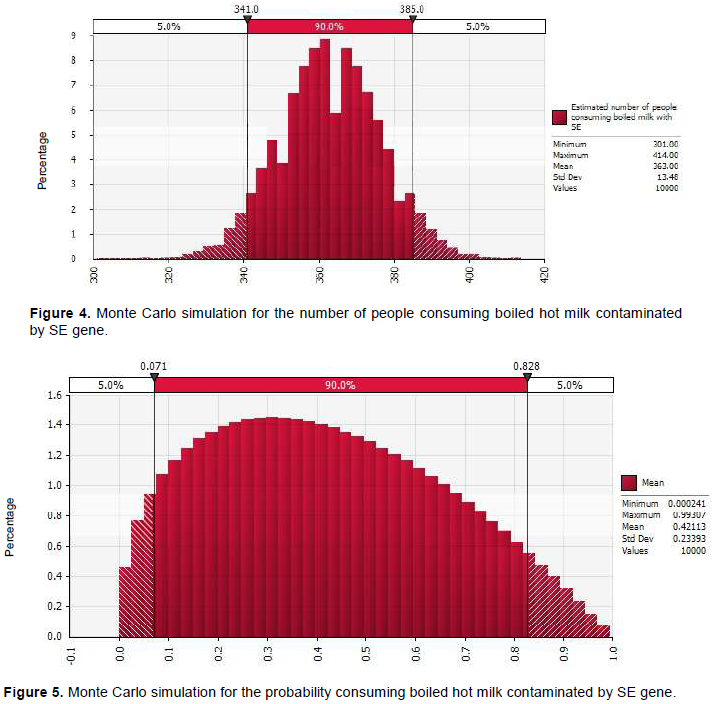
In this study, storage time, temperature profiles during harvesting, storage and transportation were not recorded. The quantity of milk contaminated and daily consumption of milk in the study area is shown in Table 5. The quantity of milk sold was estimated to be 1197 L (90% CI, 1109.7 -1316.4) per day. Among this, 860 L (90% CI, 797.3-922.7) was boiled hot and 337 L (90% CI, 312.4-361.6) fermented milk. Approximately, 490 L (90% CI, 464-516) and 77.5 L (90% CI, 67-88) of boiled hot and fermented milk, respectively were contaminated with SE coding gene. Furthermore, 363 (90% CI, 301-414) (Figure 4) and 58 (90% CI, 49-66) persons were estimated to consume contaminated boiled and fermented milk, respectively. The probability of consuming boiled hot milk and fermented milk contaminated with SE gene at a milk shop was 0.42 (90% CI, 0.071- 0.838) (Figure 5) and 0.17 (90% CI, 0-0.62), respectively. Odd ratio analysis showed that the exposure to SE gene was two times more likely to occur in people who consume boiled-milk-served-hot milk (P < 0.05) (OR: 2.21 (90% CI, 0.6-6.16) than in people who consume fermented milk.
The information on milk consumption in the study area revealed that milk was mostly consumed with other foods. Foods that were mentioned to be consumed with milk include tea, ugali (Ugali is made from maize/wheat/fingermillet flours mixed in boiling water and made into a thick porridge), porridge, banana and rice. In the family where keeping of cattle was not practiced, children, elderly and sick individuals were given priority more than other groups. The amount purchased and consumed depends on the economic status of individual/family. Similar findings were reported in Ghana (Aidoo et al., 2009) and Kenya (Njarui et al., 2011) that income of the households head influenced the milk consumption in a family. Boiling of milk before consumption was common practice in the study area. This practice should be encouraged because boiling reduces the microbial load into a level considered to be safe for human consumption, particularly that all pathogens are also destroyed. The finding concurs with Omore et al. (2005) that boiling of milk is a common practice in many households.
In order to safeguard the consumer’s health, knowledge on milk safety is very important. Lack of knowledge in milk handling may have a negative impact on consumer’s health. In this study, training of the milk
shop owner on milk handling had no significant influence on the quality of milk. Though not significant, the milk shop owners who attended training were more likely to sell good quality milk relative to those who lack training. In the present study, training reduces the risk of SEs isolation in milk. The lack of training in milk quality may be a contributing factor to unhygienic milk handling by the informal sector traders (Omore et al., 2005; Kitagwa et al., 2006).
Age of the milk shops owner varied from young to old age. It was observed that a younger age with little experience in milk business related with increased odds of selling milk with SEs in their shops. The finding concurs with the study conducted in India (Singh et al., 2015) which reported that experience in dairying and milk sales had positive and significant correlation with the milk quality. The practice for cold storage in most of milk shops was that the freezer/fridge was operating by day and switched off during the night purposefully for saving the cost of electricity. It was observed that the milk shops that had no cooling facilities were relatively at higher risk of having SEs genes in the milk than the milk shops with cooling facilities. Exposing the milk to ambient temperature creates a good environment for SEs coding genes to produce toxins (Paulin et al., 2012).
The exposure results show that there is a greater chance for consumers to be exposed to contaminated milk because ready to consume boiled hot milk sold in the milk shops contained SEs coding genes. Despite the fact that boiled milk is considered safe, its consumption could expose the consumers to the risk of consuming SEs coding genes which is heat resistant. This is because more than half of the boiled milk sold in the milk shops in the study area contained SEs coding genes at consumption. Although the study did not estimate the chances of human illness related to consumption of contaminated milk; still, consumption of SE contaminated milk is expected to result into illnesses. Regardless of the quantity of milk contaminated and consumed daily, no outbreaks of bacterial food-borne illness associated with consumption of milk has been reported in the study area. The reason could be that many cases are not reported, which may be due to the limited access to the healthcare system and understanding diseases especially in village areas where the healthcare system is not available and or not well established.
The estimated number of people consuming milk with SE gene is higher especially for boiled-milk-served-hot. This result is alarming because if the isolated genes produce toxins this could affect large number of peoples who consume boiled-milk-served-hot in the milk shops. A study conducted in the Ivory Coast by Sylvie et al. (2012) reported that 652 people were estimated to ingest milk contaminated with S. aureus which is higher compared to current study. In their study, only total S. aureus was considered, without estimating SE producing strains.
The probability of consuming milk with SEs gene for the people who consume boiled-milk-served-hot and fermented milk in this study indicated that the risk for consumers of boiled-milk-served-hot is higher than consumers of fermented milk. The result shows that the probability of isolating SE gene in boiled-milk-served-hot was more than two times compared to fermented milk. Studies conducted by Gratian (2012) and Sylvie et al. (2012) in Tanzania and Ivory Coast, respectively reported the probability of ingesting milk with S. aureus to be 29.9 and 22.7%, without estimating SE producing strains.
The contaminations of milk can occur at any point from the production to selling points if proper hygienic measures are not followed. This was evident by the presence of SEs from production to selling point. The results showed decreasing trend of SEs gene isolation (raw milk) from production through selling point. The higher rate of isolation of SEs from raw milk in production level could be due subclinical mastitis and unhygienic milking procedure. In the milk collection points and milk shops, the effect of dilution and failure of S. aureus to compete with other bacteria could be the reason for low isolation rates of SEs. This is because S. aureus fails to reach the maximum concentration (>105 cfu/ml) for SEs to be detected, thus there is possibility that large number of S. aureus isolates with lower concentration of organisms will not reach its growth potential for SEs gene to be detected. It is worth to mention that S. aureus bacteria can be destroyed during food processing without destroying SEs; hence, their rate of isolation may differ between food products. Similar result was reported by Noha et al. (2011) that samples collected at farm had higher isolation of SEs followed by street distributors and milk shops. Furthermore, the results showed higher isolation rates of SEs coding gene in boiled milk served hot at consumption which is a potential risk to the consumers. The presence of SEs gene in boiled milk indicates that most of the SEs detected genes could produce toxins responsible for foodborne disease and probably by the time milk was boiled, already milk had heat stable toxins produced by SEs genes. Based on the results of the current study, large numbers of people who consume milk at milk shops could become sick if SEs genes in milk produces toxin. According to Le Loir et al. (2003), the exposure to SEs gene responsible for toxin production exists due to recontamination of food products and difficulties to eliminate SE toxins in the food by normal boiling. Thus, proper milk handling practices along the entire value chain should be a rule of thumb in order to safe guards the health of ready to consume milk in the study area.
CONCLUSIONS AND RECOMMENDATION
(1) Ready to consume milk sold at milk shops contained SE coding gene and pose a potential risk to the health of consumers.
(2) Higher numbers of consumers of boiled-milk-served-hot in the milk shops are exposed to consumption of milk with SE coding genes.
(3) Hygiene training to reduce the contamination of SEs on the ready to consume milk is recommended.
The authors have not declared any conflict of interests.
The authors sincerely appreciation the Commission for Science and Technology, Tanzania (COSTECH) for the financial support during this study. The livestock owners are thanked for allowing their animals resource (milk) to be used. The technical support by Mr. G. Makingi and his staff is highly acknowledged.
REFERENCES
|
Aidoo R, Nurah GK, Fialor SC, Ohene-Yankyera K (2009). Determinants of Dairy consumption Expenditure in Urban communities of Southern Ghana. Journal of Science and Technology 29(1):87-96.
|
|
|
|
Argudín MÁ, Mendoza MC, Rodicio M R (2010). "Food poisoning and Staphylococcus aureus enterotoxins." Toxins 2(7):1751-1773.
Crossref
|
|
|
|
|
European Commission Health and Consumers Protection (ECHCP) (2003). Opinion of the scientific committee on veterinary measures relating to public health on Staphylococcal enterotoxins in milk products, particularly cheeses, 73 p. Available at:
View.
|
|
|
|
|
Gratian KK (2012). Food Safety in Milk Markets of Smallholder Farmers in Tanzania: A case of peri urban wards in Temeke Municipality Dissertation for Award of MSc. Degree at Sokoine University of Agriculture, Morogoro, Tanzania 103 p.
|
|
|
|
|
Hennekinne JA, Byser MLD, Dragacci S (2012). Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiology Review 36:815-836.
Crossref
|
|
|
|
|
Kayombo E, Uiso F, Mahunnah R (2012). Experience on Healthcare Utilization in Seven Administrative Regions of Tanzania. Journal of Ethnobiology and Ethnomedicine 8:5.
Crossref
|
|
|
|
|
Kitagwa WGI, Bekker JL, Onyango RO (2006). The influence of knowledge, attitudes and practices of food handlers on food kiosk hygiene. Eldoret, Kenya. Environment and Health International 8(2):19-29.
|
|
|
|
|
Kurwijila LR (2006). Hygienic milk handling, processing and marketing: reference guide for training and certification of small-scale milk traders in Eastern Africa. ILRI (International Livestock Research Institute), Nairobi, Kenya.
|
|
|
|
|
Le Loir Y, Baron F, Gautier M (2003). Staphylococcus aureus and food poisoning. Genetic and Molecular Research 2:63-67.
|
|
|
|
|
Massawe HF, Makingi GI, Shija DS, Mdegela RH, Kurwijila LR (2017). Prevalence of the Staphylococcal Enterotoxins Genes in Raw and Milk Products along the milk value chain. Journal of Natural Science Research 7(18):47-48.
|
|
|
|
|
Mdegela RH, Ryoba R, Karimuribo ED, Phiri EJ, Løken T, Reksen O, Mtengeti E, Urio NA (2009). Prevalence of clinical and subclinical mastitis and quality of milk on smallholder dairy farms in Tanzania. Journal of the South African Veterinary Association 80(3):163-168.
Crossref
|
|
|
|
|
Mehrotra M, Wang G, Johnson WM (2000). Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. Journal of Clinical Microbiology 38:1032-1035.
|
|
|
|
|
Njarui DMG, Gatheru M, Wambua M, Nguluu SN, Mwangi DM, Keya GA (2011). Consumption Pattern and Preference of Milk and Milk Products among Rural and Urban Consumers on Semi-Arid Kenya. Ecology of Food and Nutrition 50(3):240-262.
Crossref
|
|
|
|
|
Noha A, Afifi A, Sadek MG, Aggour AA, El-Tamawy MM, Nemr (2011). Molecular Characterization of Staphylococcus Aureus Enterotoxins in Milk and Some dairy Products. Egyptian Journal of Medical Microbiology 20(1):107-116.
|
|
|
|
|
Omore A, Lore T, Staal S, Kutwa J, Ouma R, Arimi S, Kang'the E (2005). Addressing the public health and quality concerns towards marketed milk in Kenya. SDP Research and Development Report No.3 Nairobi (Kenya): Smallholder Dairy (R and D) Project pp. 1-45.
|
|
|
|
|
Paulin S, Horn B, Hudson JA (2012). Factors Influencing Staphylococcal Enterotoxin Production in Dairy Products 78 p.
|
|
|
|
|
Singh V, Gupta J Ponnusamy K (2015). Socio-economic factors affecting quality of raw milk in dairy value chain. Indian Journal of Dairy Science 68(5):502-506.
|
|
|
|
|
Statistical Analysis Systems (SAS) (2004). Statistical Analysis Systems. User's guide, version 9.3, SAS Institute, INC, Cary. NC. USA.
|
|
|
|
|
Sylvie M, Kouammle KO, Kohei M, Solenne C, Delia G, Adjehi DadiiiMarcellin D, Bassirou B (2012). Hazard identification and exposure assessment for bacterial risk assessment of informally marketed milk in Abidjan, Côte d'Ivoire. Food and Nutrition Bulletin 33(4):223-234.
Crossref
|
|
|
|
|
United Republic of Tanzanian Ministry of Health (URT) (2000). The National Birth Attendants Implementation Policy Guidelines, Tanzania. Dar es Salaam.
|
|
|
|
|
World Health Organization (WHO) (2002). WHO global strategy for food safety. Geneva.
|
|