ABSTRACT
The mastitis causing microorganisms resist beta-lactam antibiotics by releasing beta-lactamase and the enzyme can be traced in raw milk. This study was aimed at developing a novel platform test to detect beta-lactam antibiotics residues in raw milk based on the Hardy Diagnostic Beta-lactamase Test (HDBT) reagent. The HDBT ingredients modified were penicillin, sodium chloride, trisodium citric acid, trisodium phosphate and phenol red dissolved in distilled water. Pooled raw milk samples were obtained from 3 Friesians and 3 Ayrshires lactating cows identified to have subclinical mastitis and treated using beta-lactam antibiotics. The appropriate mixing ratios were investigated at nine levels. Investigation on the effect of breeds on the test method results was also carried out. Evaluations to determine the colour differences between beta-lactam positive and negative raw milk samples for all the experiments were carried out using trained panelists. The results indicate that gradual addition of trisodium phosphate and phenol red in the reagent showed significant difference (P ≤ 0.05) between a beta-lactam positive and negative raw milk sample. Ratio 5:5 was selected as the best and had significant difference (P ≤ 0.05) from the others. Conversely, the test method indicated no significant difference (P ≤ 0.05) between the Friesians and Ayrshires raw milk samples. This method can be used along the raw milk collection routes to accept, set aside or reject raw milk suspected to have residues. The colour observed for a beta-lactam negative sample was fuchsia purple while peach or pink signified a positive sample.
Key words: Raw milk, antibiotic residues, beta-lactam, beta-lactamase enzyme, trisodium phosphate
In the Kenyan value chain, rapid antibiotic residues testing are not up to standard (Orwa et al., 2017) and a need to improve the quality control procedures is paramount. As an appropriate control and preventive measure at the farm level, a critical control point (Ndungu et al., 2016b) development of highly sensitive detection tools to avoid the false negative results is vital (Sachi et al., 2019). Moreover, antibiotic residues quality control tests that are simple, economically sustainable and suitable for field situations, should be introduced (Prajwal et al., 2017). These platform tests should be simple, rapid and flexible for use in field-based tests to avoid time
wastage during raw milk collection (Ndungu et al., 2016a). They should serve as a basis for accepting or rejecting raw milk. Milk processors should be encouraged to assess raw milk on residues before acceptance to ensure food safety (Kurjogi et al., 2019; Mwagore et al., 2019) and avoid miss productions when manufacturing fermented products. Factors limiting such quality control tests should be investigated and addressed together with the chain actors.
Worldwide findings have revealed that among other antibiotics, beta-lactam, penicillins and cephalosporins, were the most used antibiotic drugs in disease management at a rate of 36.54% (Sachi et al., 2019). Similar findings have been reported in Kenya and it was associated with management of mastitis (Ahlberg et al., 2016; Ali et al., 2017) which is a prevalent disease. The bacteria associated with causing mastitis include: Staphylococcus aureus, Enterobacteriaceae species, Streptococcus agalacteae and Escherichia coli (Ondiek et al., 2013; Gomes and Henriques, 2016). These bacteria produce beta-lactamase enzyme to counter beta-lactam antibiotics bactericidal effect, which is the most common cause of resistance (Konaklieva, 2014).
The molecular classification for beta-lactamases is based on the amino acid sequence. This classification divides beta-lactamases into class A, C, and D enzymes which utilize serine for beta-lactam hydrolysis and class B metallo-enzymes which require divalent zinc ions for substrate hydrolysis (Palzkill, 2018). Serine beta-lactamases including molecular classes A and D, represent the largest group of beta-lactamases, due to the increasing identification of Extended Spectrum Beta-lactamases (ESBLs). Penicillinases belong to class A and represent a small group of beta-lactamases with a relatively limited spectrum of hydrolytic activity. They are the predominant beta-lactamases in Gram-positive cocci, including the staphylococci and occasionally enterococci. These enzymes preferentially hydrolyze benzylpenicillin and many penicillin derivatives, but have poor hydrolysis on cephalosporins, carbapenems, or monobactams (Bush, 2018).
Beta-lactamases (penicillinases) enzyme can be traced in raw milk and urine after treatment with beta-lactam drugs and thus referred to as endogenous beta-lactamases. Its presence in raw milk indicates presence of beta-lactam antibiotic residues (Wang et al., 2013; Zhang et al., 2015; Canzani and Aldeek, 2017) which is a public health concern. This study aimed at developing a quality control test to detect beta-lactam residues in raw milk. The test development was based on Hardy Diagnostic Beta-lactamase Test (HDBT), an acidimetric test used in detecting production of beta-lactamase enzyme by microorganism to indicate antimicrobial resistance. HDBT reagent aimed at determining the resistance of Neisseria gonorrhoeae, Haemophilus and Staphylococcus species to β-lactam antibiotics through production of beta-lactamase enzyme which in turn breaks down penicillin drugs to penicilloic acid that is detected by the colour change of phenol red indicator. The ingredients that compose HDBT include penicillin, trisodium phosphate, trisodium citric acid, sodium chloride and phenol red indicator. This test method is based on the ability of mastitis causing microorganisms to resist beta-lactam antibiotics by releasing beta-lactamase enzyme which can be traced in raw milk. The ingredients used in making the HDBT reagent were modified to allow detecting of the enzyme in raw milk.
Study site
This research was carried out at Olenguruone Dairy Farmers Cooperative Society milk quality control laboratory. Richard’s farm located at Olenguruone, Nakuru county was identified and used for this study. The farm had both Friesians and Ayrshire breeds, a requirement that was key in the study.
Selection of experimental animals and treatment
The selected animals had daily milk production per cow of between 5 and 9 kg/day. The criteria used to select the animal that participated in this study involved: the breed type (pure friesians or Ayrshire); lactation stage; must have four functional udder quarters; no visible signs of mastitis. Fourteen cows were selected for milk sampling and analysis on subclinical mastitis in a laboratory. Composite milk samples from all the teats per cow were collected.
The laboratory results indicated that the cows had subclinical mastitis and the causative microorganisms identified were S. aureus and Enterobacteriaceae species. Six infected cows (3 Friesians and 3 Ayrshires) were selected for treatment using a qualified veterinary officer. For each cow and in their four teats, intramammary infusions were carried out. The drug used composed of: Procaine Penicillin G (60 mg), Streptomycin Sulphate (100 mg), Neomycin Sulphate (100 mg) and Prednisolone (10 mg). This was done only once in the first day of treatment. In the same day and time, the same cows were injected with 30 ml of a drug made up of 120 mg Procaine benzylpenicillin and 200 mg Dihydrostreptomycin. This was done for 3 consecutive days.
Raw milk sampling
The sampling procedure used was as per ISO 707; IDF 50, 2008. On the first day before treatment, a pooled antimicrobial-free (negative) raw milk sample of 5 L from 3 cows (either Friesians or Ayrshires) was separately obtained. This was to overcome the individual variations in raw milk composition. Similar sampling for the two breeds continued during the 3 days of treatment, 3 days of withdrawal period and 2 days after withdrawal period. Therefore, out of the 18 samples collected, 6 were negative raw milk samples collected during the 1st day before treatment and the 2 days after the withdrawal period was completed. These samples were stored in frozen conditions to retain their integrity.
Experimental design
The pooled raw milk samples, known to be beta-lactam antibiotic positive or negative were used in all experiments. Three laboratory experiments were carried out including: reagent development through modification of the HDBT ingredients, establishing the appropriate mixing ratios (milk: reagent) for better results and investigating the effect of breed on the test method results. For each experiment, sensory evaluation having trained panelist was used. Randomized Complete Block Design (RCBD) with three replications was used in reagent modification for test method development. Completely Randomized Design (CRD) with three replications was used to establish the mixing ratios (milk: reagent) and the effect of breeds on the test method results.
Modification of HDBT ingredients in test method development
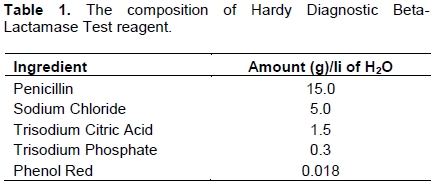
Five ingredients (penicillin, sodium chloride, trisodium citric acid, trisodium phosphate and phenol red) were used as described in the HDBT test. These ingredients were sourced from Chemoquip Limited Nairobi. Their certificate of analysis and seals were verified to ensure integrity before use. Table 1 shows the composition of the reagent as described in the HDBT test. To mix the ingredients, 1 L of distilled water was used.
The first experiment involved using HDBT reagent (reagent 1) in its original composition as described in Table 1. In this experiment, equal portions of raw milk (either beta-lactam positive or negative) and reagent were mixed and colour differences observed.
In the second experiment, four reagents were made using the ingredients amounts as described in Table 1, but excluding one of the ingredient at a time. This yielded four different reagents (reagent 2-5) such that reagent 2 had: trisodium phosphate, sodium chloride, penicillin and phenol red but excluded trisodium citrate; reagent 3 had: trisodium citrate, sodium chloride, penicillin and phenol red but excluded trisodium phosphate; reagent 4 had: trisodium citrate, trisodium phosphate, penicillin and phenol red but excluded sodium chloride; reagent 5 had: trisodium citrate, trisodium phosphate, sodium chloride and phenol red but excluded penicillin. Each reagent was mixed with equal quantities of known beta-lactam positive and negative raw milk samples and the colour differences observed.
In the third experiment, three times more of each ingredient amount at a time was used in making the reagents. This resulted into five different reagents (reagent 6-10). Reagent 6, 7, 8, 9 and 10 had excess of penicillin, trisodium phosphate, sodium chloride, phenol red indicator and trisodium citrate respectively. Each reagent was mixed with equal quantities of known beta-lactam positive and negative raw milk samples to determine the colour differences.
Finally, the exact ingredients composition that will exhibit much change was investigated at various levels. Table 2 shows the experiments involved in the test method development process including the activities and the expected outcomes.
In the four experiments, ten trained panelists were requested to identify the set of samples that gave largest differences between the beta-lactam positive and negative raw milk samples using the ranking as well as a line scale method as indicated by Sharif et al. (2017). For the ranking method, they were supposed to give the highest score (30) to their most preferred set, indicating the greatest difference, and arrange them in a descending order. For the line scale method, they were requested to place a mark on a 15 cm line to indicate how they perceive the difference. The line was marked “no difference” on the extreme left and “different” on the extreme right. A ruler was used to establish the measurement from the “no difference mark” to the point where the analyst had placed a mark. These measurements were used in statistical analysis where the reagent with the highest mean was selected as the most preferred.
Determination of reagent: Milk sample mixing ratio
The appropriate mixing proportions for reagent and raw milk were determined by mixing equal portions of reagent: milk at 9 levels. The ratios mixed included: 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2 and 9:1 independently. For each ratio, a pair consisting of known beta-lactam positive and negative raw milk sample was prepared and respective observations made. Selection of the best ratio was carried out by 12 trained panelists through ranking and line scale method, as described earlier. The ratio ranked first and with the greatest mean indicating the largest difference was considered the best and was applied in subsequent experiments.
Determination of breed’s effect on the test method outcome
Investigation on whether the test method was affected by breed differences was carried out. The breeds that were investigated included Friesians and Ayrshires. Pooled raw milk sample from 3 cows from each breed was taken for analysis, along the nine analysis days. The reagents as modified and the mixing ratio established were used in this experiment. The sensory analysis involved 10 trained panelists using the line scale method as described earlier. The measurements were used to analyze the data statistically and the means were used in determining the differences observed.
Statistical analysis
Data analysis was carried out using PROC GLM (general linear model) procedure of the statistical analysis system (SAS) version 9.4M6 (SAS Institute Inc.) for analysis of variance (ANOVA) and least significant difference (LSD) for mean separation where there were significant differences.
Determination of ingredients amounts
When the HDBT reagent in its original composition was used, the colour differences were not distinct and could hardly differentiate between a positive and a negative sample. Similarly, the reagents made in second experiment, where one of the ingredients was eliminated at a time, did not yield distinct differences too.
In the third experiment, where each ingredient was added three times more of the HDBT indicated amount, reagent 7, which had more of trisodium phosphate, gave clear differences between a positive and a negative raw milk sample (Figure 1). This was an expected outcome as described in Table 2. Subsequent experiment to establish the actual amount of trisodium phosphate was carried out.
Results shown in Figure 2 represent colour changes after gradual addition of trisodium phosphate and phenol red. After using 9 g of trisodium phosphate in making the reagent (reagent 14), the colour difference was not significant. However, use of 10.5 g in making reagent (reagent 15), better colour distinction between a beta-lactam positive and negative sample was observed, which was similar to reagent 7. Notable was the ceasing of colour differences observed with reagent 16, which had 12 g of trisodium phosphate. Figure 2 shows the observation made between a positive and negative sample with gradual addition of trisodium phosphate and with adjusted phenol red amounts. In Figure 1, each set of samples have a positive raw milk sample on the left test tube and a negative raw milk sample on the right test tube. In Figure 2, each set of samples have a positive raw milk sample on the right test tube and a negative raw milk sample on the left test tube.
The result as shown in Figure 2, reagent number 15, had the greatest mean and was ranked the best with a score of 300 points. Line scale method results similarly indicated that reagent number 15 had the highest mean and was significantly different (P ≤ 0.05) from the rest. Table 3 indicates the means for every reagent for the line scale method, where reagent number 15 having the highest mean was selected as the best. Reagent numbers 13 and 12 were not significantly different (P ≤ 0.05), similarly to reagent numbers 12 and 11. Reagent number 16 (Figure 2) had the lowest mean and was also significantly different (P ≤ 0.05) from the others.
Moreover, the difference between a positive and negative sample for reagent 16 was not distinguishable. Therefore, the experiment concluded the new reagent composition as indicated in Table 4.
Determination of reagent: Milk sample mixing ratio
Out of the nine suggested mixing ratios (milk: reagent), ratios 4:6, 5:5 and 6:4 could show difference between a beta-lactam positive and a negative sample. These three ratios were selected and further analyzed to determine the best out of the three. Figure 3 shows the photo of the results for the three ratios as described. Each set of samples have a positive raw milk sample on the left test tube and a negative raw milk sample on the right test tube.
The panelists were requested to use a line scale and the ranking method to select the best ratio. Ratio 5:5 was ranked as the best having followed by 4:6 and 6:4 was ranked last. Table 5 shows the line scale method results, where ratio 5:5 or otherwise 1:1 had the highest mean and was significantly different (p ≤ 0.05) from ratios 4:6 and 6:4. This means that for this test method to work efficiently, equal portions of raw milk and reagent should be mixed.
Determination of breed’s effect on the test method residue detection
This test was carried out using both beta-lactam positive and negative raw milk samples. Figure 4 shows the test results analysis for the raw milk samples from the two breeds. Each set of samples had the Friesian raw milk sample on the left and Ayrshire raw milk sample on the right. The results indicated no significance difference (p ≤ 0.05) between the results from Friesians and Ayrshire raw milk samples. Table 6 shows the means for six set of samples.
Bacterial resistance may occur if the bacteria can produce beta-lactamase enzyme that is able to inactivate beta-lactam antibiotics. Beta-lactamase (BLs; Enzyme commission (EC) number 3.5.2.6) is an enzyme first identified in E. coli and has been described as penicillinase. Beta-lactamase specifically hydrolyzes beta- lactam ring present in antibiotics such as penicillin, cephalosporins, monobactam, and carbapenem, and confer resistance against these antibiotics (Shaheen, 2013). Penicillinases enzyme is a serine beta-lactamase that is specific for penicillin. In their action, these enzymes first associates non-covalently with the antibiotic to yield the noncovalent complex. The beta-lactam ring is then attacked by the free hydroxyl on the side chain of a serine residue at the active site of the enzyme, yielding a covalent acyl ester. Hydrolysis of the ester finally liberates active enzyme and the hydrolyzed inactive drug. This mechanism is followed by beta-lactamases of molecular classes A, C, and D, but class B enzymes utilize a zinc ion to attack the beta-lactam ring (Bonomo, 2017).
Li et al. (2014) carried out research on degradation of penicillin in raw milk by beta-lactamase enzyme using ultra-performance liquid chromatography coupled with time-of-flight mass spectrometry. In their study, beta-lactamase enzyme and penicillin were spiked in raw milk samples. The study indicated that in enzymatic degradation, two products are formed (penicilloic acid and penilloic acid). In another study carried out by Shaheen (2013), beta-lactamases was found to specifically hydrolyze beta-lactam rings present in beta-lactam antibiotics and confer resistance against the beta-lactam antibiotics. They indicated that the amide bond in a beta-lactam ring can be hydrolyzed by this enzyme to a corresponding penicilloic acid. Similarly, Harris (2015) indicated that when Beta-Lactam ring is broken by penicillinase, the resultant product is penicilloic acid without bactericidal effect. Canzani and Aldeek (2017) investigated the stability of penicillin G in various conditions including acidic, alkaline, natural acidic matrices after treatment of citrus trees infected with citrus greening disease. Penillic acid, penicilloic acid, and penilloic acid were found to be the most abundant metabolites of penicillin G. They indicated that iodometric method to determine penicilloic acids based on the decolourization of a chromophore has also been developed. Figure 5 shows the hydrolysis of beta-lactam antibiotics by beta-lactamase enzymes to yield penicilloic acid.
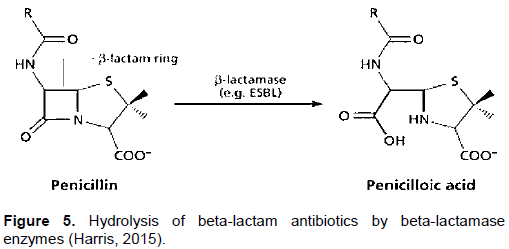
HDBT is an acidometric method recommended for use in testing beta-lactamase production by N. gonorrhoeae, Haemophilus and Staphylococcus spp. In HDBT test, the microorganisms were introduced into the reagent. With penicillin being one of the ingredient, and as the bacterial strive to resist its bactericidal effect by producing beta-lactamase enzyme, hydrolyses of penicillin by the enzyme occurs. This phenomenon, exhibited by phenol red indicator, showed colour changes from fuchsia purple to yellow (Hardy Diagnostic Catalogue, 2018). In this novel test method, when HDBT reagent was modified by increasing the quantity of trisodium phosphate, better results were observed. The beta-lactamase enzyme endogenically present in raw milk samples was utilized. Therefore, hydrolysis of the penicillin ring, available as an ingredient, yielding penicilloic acid was expected for beta-lactam positive raw milk samples. Production of penicilloic acid in the hydrolysis reaction causes a pH drop in the medium.
Subsequently, colour change occurs which is exhibited by an acid-base phenol red indicator, also available as an ingredient. This study agrees with previous findings that penicilloic acid is formed after hydrolysis of penicillin and this causes a drop in pH in the reaction as exhibited by phenol red indicator. This is because, the results of the experiment indicated the colour change from fuchsia purple for a beta-lactam negative test to peach/pink for a positive test. Although yellow colour expected for beta-lactam positive raw milk was not achieved, the colour difference observed between a beta-lactam negative and positive raw milk sample are enough to make judgement on the presence of residues in raw milk. These results could have been influenced by the amount of enzyme present in raw milk. As indicated by Li et al. (2014), the dosage of beta-lactamase influences the production of penicilloic acid. Hence, if the enzyme is deficient, more penicilloic acid will not be formed limiting anticipated the colour (yellow).
Beta-lactamase, from its action of breaking penicillin, can be categorized as a hydrolase enzyme that operates optimally under neutral to alkaline pH conditions. The major modification of the HDBT reagent is the change in trisodium phosphate quantity which makes it possible to observe the differences between a positive and a negative raw milk sample. Trisodium phosphate and trisodium citrate make up the Mcllvaine buffer which is used in colorimetric comparison and is prepared between a pH of 2.2 to 8.0. With increased trisodium phosphate (a strong alkali) quantity, the pH will tend towards alkaline (Mcllvaine, 1921). In addition, phenol red indicator, also known as phenolsulfonphthalein, is a pH indicator dye that exhibits a gradual transition from yellow to red over a pH range of 6.2 to 8.2. Above 8.2 the dye turns to a bright fuchsia colour (Held, 2018). Therefore, when penicilloic acid results, the pH drops and phenol red exhibits the colour changes. Li et al. (2014) also established that penicillin decompose in aqueous solution into penicilloic acid by the action of either an alkali or the bacterial enzyme. This could be another reason why better colour observation was made when the concentration of trisodium phosphate was increased as the medium became more basic. With continued increase of trisodium phosphate, the pH of the medium became more basic. Since with a pH of more than 8.2 the dye turns to bright fuchsia purple, the difference between a positive and negative raw milk sample could not be realized. As indicated in this study, at ratio 1:1, optimum pH conditions for the enzyme and the indicator was achieved contributing to favorable outcomes. Li et al. (2014) considered several factors influencing beta-lactam enzymatic degradation including beta-lactamase dosage, temperature, time and pH. They established that the enzyme dosage and pH are the greatest determinant in the hydrolysis reaction. When the pH was increased from 2 to 6 and in presence of beta-lactamase enzyme, response of penicilloic acid increased. Moreover, the degradation of penicillin was enhanced by increased quantities of the enzyme.
A similar test has been developed to detect the enzyme using phenol red indicator. Nordmann et al. (2012) developed a test for rapid identification of Extended-Spectrum-Beta-Lactamase (ESBLs) in Enterobacteriaceae. This test was based on detection of beta-lactam (cephalosporin) hydrolysis which can be reversed by adding tazobactam. According to their study, ESBL activity was evidenced by change in color change from red to yellow, exhibited by phenol red indicator which is similar to this study. Also, presence of penicilloic acid has been used to detect presence of penicillin in milk. As an example, Liu et al. (2011) developed a rapid, sensitive, and specific method for the determination of penicillin G, benzylpenicilloic acid, benzylpenilloic acid, and benzylpenillic acid in bovine milk using ultra-high performance liquid chromatography-tandem mass spectrometry. Their established method was successfully applied in the determination of penicillin and their major metabolites in bovine milk samples. They detected penicilloic acid in 20% of the bovine milk samples at an average concentration 320 ng/ml. Gaare et al. (2012), detected beta-lactam antibiotics in milk using indicator strain Bacillus cereus producing beta-lactamase enzyme through induction. The test ampoules containing spore were tested for induction with penicillin G in spiked milk and their findings indicated complete correlation with other reference methods. They indicated that the induction method can be applied in detection of beta-lactam antibiotic residues in dairy products.
This study aimed at developing a test that can be applied in detecting beta-lactam residues. The novel colour reaction test described will aid in raw milk acceptance, segregating raw milk suspected with residues as well as rejecting raw milk evidently established to have residues. It can be used as a preliminary test to detect presence of beta-lactam residues at the raw milk reception platform and along the collection chain. The test results are obtained immediately which facilitates faster milk collection. The testing procedure involves preparing the reagent as described earlier and completely mixing equal portions of the reagent and fresh raw milk, making observations and recording. The colours expected for a beta-lactam positive sample are peach or pink while for a beta-lactam negative sample is fuchsia purple. Advanced research aimed at improving its applicability in detecting other residues is recommended.
RESEARCH PERMIT AND ETHICAL APPROVAL
This study was approved by Egerton University Board of Post Graduate Studies. Ethical clearance approval was obtained from Egerton University Ethics Review Committee (EUREC) (approval number; EUREC/APP/ 097/2020) and the research license obtained from National Commission for Science, Technology and Innovation (NACOSTI) (license number; NACOSTI/P/20/ 5861).
The aim and procedures of the study were explained to the study participants who were required to give written informed consent prior to their voluntary participation in the study. Confidentiality of research information and data was observed and maintained through forms issued and signed before research commenced, password protected computers and observing good professional conduct.
The authors have not declared any conflict of interests.
This research was carried out with financial support from the Centre of Excellence in Sustainable Agriculture and Agribusiness Management (CESAAM), Egerton University. The authors are thankful to those farmers who kindly provided milk samples and cows treatment data. The authors are also grateful to Olenguruone Dairy Farmers Cooperative Society for providing the laboratory where the study took place and to the staff for participating in sensory evaluation.
REFERENCES
|
Ahlberg S, Korhonen H, Lindfors E, Kang'ethe E (2016). Analysis of antibiotic residues in milk from smallholder farms in Kenya. African Journal of Dairy Farming and Milk Production 3(4):152-158.
|
|
|
|
Ali T, Rahman S, Zhang L, Shahid M, Han D, Gao J, Zhang S, Ruegg PL, Saddique U, Han B (2017). Characteristics and genetic diversity of multi-drug resistant extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli isolated from bovine mastitis. Oncotarget 8(52):90144.
Crossref
|
|
|
|
|
Bonomo RA (2017). β-Lactamases: a focus on current challenges. Cold Spring Harbor Perspectives in Medicine 7(1):a025239.
Crossref
|
|
|
|
|
Bush K (2018). Past and present perspectives on beta-lactamases. Antimicrobial Agent's Chemotherapy 62:e01076-18.
Crossref
|
|
|
|
|
Canzani D, Aldeek F (2017). Penicillin G's function, metabolites, allergy, and resistance. Journal of Nutrition Human Health 1(1):28-40.
Crossref
|
|
|
|
|
Gaare M, Kumar N, Raghu HV, Khan A, Singh VK (2012). Specific detection of beta-lactam antibiotics in milk by spore based assay. International Research Journal of Microbiology 3:168-178.
|
|
|
|
|
Gomes F, Henriques M (2016). Control of bovine mastitis: old and recent therapeutic approaches. Current Microbiology 72(4):377-382.
Crossref
|
|
|
|
|
Hardy Diagnostic Beta-lactamase Test website (2018). Acidometric test to detect Beta lactamase producing microorganisms. Accessed on 12th October 2018.
|
|
|
|
|
Harris PN (2015). Clinical management of infections caused by Enterobacteriaceae that express extended-spectrum beta-lactamase and AmpC enzymes. In Seminars in Respiratory and Critical Care Medicine. Thieme Medical Publishers 36(01):056-073.
Crossref
|
|
|
|
|
Held P (2018). Using phenol red to assess pH in tissue culture media. BioTek Appl. Note1, 1-7.
View
|
|
|
|
|
Konaklieva M (2014). Molecular targets of β-lactam-based antimicrobials: Beyond the usual suspects. Antibiotics 3(2):128-142.
Crossref
|
|
|
|
|
Kurjogi M, Issa Mohammad YH, Alghamdi S, Abdelrahman M, Satapute P, Jogaiah S (2019). Detection and determination of stability of the antibiotic residues in cow's milk. PLoS ONE 14(10):e0223475.
Crossref
|
|
|
|
|
Li L, Guo C, Ai L, Dou C, Wang G, Sun H (2014). Research on degradation of penicillins in milk by beta-lactamase using ultra-performance liquid chromatography coupled with time-of-flight mass spectrometry. Journal of Dairy Science 97(7):4052-4061.
Crossref
|
|
|
|
|
Liu C, Wang H, Jiang Y (2011). Rapid and simultaneous determination of amoxicillin, penicillin G, and their major metabolites in bovine milk by ultra-high-performance liquid chromatography-tandem mass spectrometry. Journal of Chromatography B Anal Technol Biomed Life Science 879(7-8):533-540.
Crossref
|
|
|
|
|
Mcllvaine TC (1921). A buffer solution for colorimetric comparison. Journal of Biological Chemistry 49(1):183-186.
Crossref
|
|
|
|
|
Mwagore D, Koge J, Ng'ang'a M (2019). Milk quality seminar; workshop proceedings.
View
|
|
|
|
|
Ndungu TW, Muliro PS, Omwamba M, Oosterwijk G (2016b). Hygienic practices and critical control points along the milk collection chains in smallholder collection and bulking enterprises in Nakuru and Nyandarua Counties, Kenya. African Journal of Food Science 10(11):327-339.
Crossref
|
|
|
|
|
Ndungu TW, Muliro PS, Omwamba M, Oosterwijk G, Jansen A (2016a). Quality control of raw milk in the smallholder collection and bulking enterprises in Nakuru and Nyandarua Counties, Kenya. African Journal of Food Science 10(5):70-78.
Crossref
|
|
|
|
|
Nordmann P, Dortet L, Poirel L (2012). Rapid detection of extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Journal of Clinical Microbiology 50(9):3016-3022.
Crossref
|
|
|
|
|
Ondiek JO, Ogore PB, Shakala EK, Kaburu GM (2013). Prevalence of bovine mastitis, its therapeutics and control in Tatton Agriculture Park, Egerton University, Njoro District of Kenya. Basic Research Journal of Agricultural Science and Review 2(1):15-20.
|
|
|
|
|
Orwa JD, Matofari JW, Muliro PS, Lamuka P (2017). Assessment of sulphonamides and tetracyclines antibiotic residue contaminants in rural and peri urban dairy value chains in Kenya. International Journal of Food Contamination 4(1):5.
Crossref
|
|
|
|
|
Palzkill T (2018). Structural and Mechanistic basis for extended spectrum drug resistance mutationsin altering the specificity of TEM, CTX-M and KPC beta-lactamases. Frontiers in Molecular Biosciences 23(5):16.
Crossref
|
|
|
|
|
Prajwal S, Vasudevan VN, Sathu T, Irshad A, Nayankumar SR, Pame K (2017). Antibiotic residues in food animals: Causes and health effects, The Pharma Innovation Journal 6(12):01-04.
|
|
|
|
|
Sachi S, Ferdous J, Sikder MH, Hussani SMAK. (2019). Antibiotic residues in milk: Past, present, and future, Journal of Advanced Veterinary and Animal Research 6(3):315-332.
Crossref
|
|
|
|
|
Shaheen BW, Nayak R, Boothe DM (2013). Emergence of a New Delhi metallo-beta-lactamase (NDM-1)-encoding gene in clinical Escherichia coli isolates recovered from companion animals in the United States. Antimicrobial Agents and Chemotherapy 57(6):2902-2903.
Crossref
|
|
|
|
|
Sharif MK., Butt MS, Sharif HR, Nasir M (2017). Sensory evaluation and consumer acceptability. Handbook of Food Science and Technology 361-386.
|
|
|
|
|
Wang W, Liu L, Xu L, Ma W, Kuang H, Xu, C (2013). Detection of beta-lactamase residues in milk by sandwich ELISA. International Journal of Environmental Research and Public Health 10(7):2688-2698.
Crossref
|
|
|
|
|
Zhang H, Zhou Y, Guo S, Chang W (2015). High prevalence and risk factors of fecal carriage of CTX-M type extended-spectrum beta-lactamase-producing Enterobacteriaceae from healthy rural residents of Taian, China. Frontiers in Microbiology 6:239.
Crossref
|
|