ABSTRACT
Injera or Biddena commonly made from tef (Eragrostis tef (Zucc) Trotter) grain and a combination with other grains is a staple food of Ethiopian and neighboring countries. Millet and millet-based food products are rich in polyphenols and antioxidant activities. This study aimed to evaluate millet varieties for their injera making quality and polyphenol content and antioxidant activity. Five millet varieties and one tef variety called Quncho were collected, investigated and compared. A significant (p<0.05) variations were noticed in rollability, adhesiveness and overall acceptance of injera. Quncho was perceived differently and rated higher in its overall acceptance (8.04). Among millet injera’s, Kola-1 rated higher whereas Padet was perceived lower in overall consumer acceptance. The result showed that the total phenols, flavonoids and anthocyanin contents of flour and injera ranged from 18.63 to 27.29 µg GAE/g, 11.99 to 15.43 µg catechin equivalent per g of sample, and 5.11 to 53.23 mg/l for flours and 22.99 to 27.25 µg GAE/g, 13.47 to 14.49 µg catechin/g and 5.53 to 24.27 mg/l for injera. Tesema had the highest total phenols (27.29 µg GAE/g), total flavonoid content (15.43 µg catechin/g) and antioxidant activity (41.07%) against the inhibition of 2,2-diphenyl-1-picrylhydrazyl (DPPH). Kola-1 showed the lowest L* (59.76) and a* (-0.11) and the highest b* (4.21) flour color characteristics. Baking caused a non-significant (p>0.05) reduction in total phenols, total flavonoids contents and DPPH free radical scavenging capacity. Hence, it is recommended that Tesema and Kola-1 varieties could be used for functional food development and injera making quality, respectively.
Key words: Millet varieties, tef, injera, baking, polyphenols, antioxidant effects.
Injera or biddena is a staple food of Ethiopian which accounts for approximately 70% of dietary calories. It is made from quite a lot of cereal grains such as tef, sorghum, millet, maize, barley and wheat depending on the regions and availability. However, best injera is made from tef (Eragrostis tef (Zucc) Trotter) grain. Recently, because of its being a whole grain product and gluten free nature (the cause for celiac disease) tef injera is gaining popularity in the developed countries as well (Abiyu et al., 2013). Injera is also considered as good sources of energy, fiber, iron, calcium and vitamins although the fermentation process during preparation results in significant reduction of most of the nutrients found in the cereals flour (Mezemir, 2015). Injera from white tef is most preferred due to its softer texture, preferred taste and color, and can be rolled without cracking (Boka et al., 2013). Tef is commonly grouped as a small millet along with fonio, ï¬nger millet and proso millet and it belongs to the same subfamily and tribe (Eragrostideae). As the tef price goes up, even middle income households tend to mix tef flour with cheaper cereals such as millet, sorghum maize or rice in preparing injera (Di Marcantonio and Demeke, 2013). Reports have shown that millet is inexpensive and nutritionally comparable or even superior to major cereals (Pathak et al., 2000).
Nowadays, agricultural research institutes have been releasing new varieties with the aim of increasing crop yield. However, grain quality and flour functionality are the most important criteria for good dough handling properties and specific health benefits. Millets and millet based food products are rich in phytochemicals which exhibit antioxidant and free-radical scavenging activity. As these are also gluten-free it could be suitable for persons suffering from celiac disease. Free radicals and reactive oxygen species are fundamentally the core cause of several disorders in humans that are generated as an imbalance between formation and neutralization of pro-oxidants resulting in oxidative stress as they cause oxidative damage to lipids, proteins, and DNA. Numerous studies and epidemiological evidences had shown that whole-grain cereal based foods are rich in plant polyphenols which could protect the body against age-related diseases such as cancer, cardiovascular ailments, diabetes, metabolic syndrome, and Parkinson’s disease (Chandrasekara and Shahidi, 2012; Fardet et al., 2008; Manach et al., 2005; Scalbert et al., 2005). Antioxidants are thought to be important in reducing oxidative damage (Halliwell, 1994).
Prior phytochemical profiling of millet indicated that it contained significant amounts of antioxidants such as carotenoids, phenolics, and tocopherols (Asharani et al., 2010). Several forms of phenolics which exist in the grain have been reported to render antioxidative and antiproliferative effects and are responsible for the control of cholesterol oxidation in vitro systems (Madhujith and Shahidi, 2007, 2009; Liyana-Pathirana and Shahidi, 2006). Health benefits imparted by cereal phenolics may be a result of additive and synergistic effects of multiple compounds present in the grains. Phenolic compounds are secondary plant metabolites and their type and content in the grains may depend on a number of factors such as the type of cereal, variety, and part of the grain, climatic conditions, and cultivation practices (Naczk and Shahidi, 2004). In addition, thermal treatments may reduce or increase the phenolic content and the antioxidant activities of cereals (Zielinski et al., 2006). Siroha and Sandhu (2017) revealed that toasting significantly increased the polyphenols content and antioxidant activity of pearl millet than cooking. Gull et al. (2018) noticed a significant reduction of antioxidant activity in cooked millet-pomace based pasta due to thermal degradation. Therefore, as injera is a staple food of Ethiopian, the investigation of millet varieties grown in several agro-ecological parts of the country and the effect of processing on the product making quality, and phenolic contents and antioxidant potential is unquestionable. Admassu et al. (2009) studied the chemical composition of local and improved finger millet varieties grown in Ethiopia. However, information on the polyphenol contents and antioxidant activity, and product making quality of Ethiopian millets are very limited. Thus, the objective of this study was to evaluate the polyphenol profiles, antioxidant activity and injera making quality of improved millet varieties grown in Ethiopia.
In this study five samples of released millet varieties (four finger millet and one pearl millet) namely Padet, Tessema, Tadesse, Aksum and Kola-1 grown in 2018/2019 season in moisture stress areas under similar but not the same agroecologies of Ethiopia were collected from Melkassa Agricultural Research Center, Ethiopia. One tef variety called Quncho was obtained from Debrezeit Agricultural Research Center and was used as a control. All millet varieties were grown in dry land areas and the tef grain was grown in moist and humid area. The grains were sorted, cleaned, washed, drained, sun dried and ground into flour. All the required chemicals were purchased from a company found in Can Tho City, Vietnam. The experiment was conducted at Food Science and Nutrition laboratory, Melkassa Agricultural Research center, Ethiopia and Food Technology laboratory of Can Tho University, Vietnam.
Injera processing and sensory evaluation
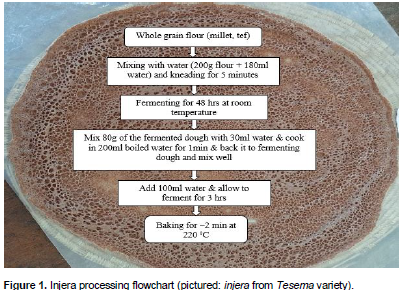
Injera was prepared using a standardized injera making procedure (Yetneberk et al., 2004). The procedure involved milling whole millet grain into a flour, preparation of a dough, and fermentation of the dough after adding yeast (a batter from a previous batch) and fermenting at room temperature for 48 h. After fermentation, 80 g of the fermented dough was thinned with 30 mL of water and cooked in 200 ml of boiling water for 1 min. The gelatinized batter was cooled to 45°C at room temperature and added back to the fermenting dough. After thorough mixing, 100 ml of water was added and the batter was fermented at room temperature for 3 h. Additional water (20 ml) was added to the fermented dough to bring to batter consistency. About 500 g of the fermented batter was poured in a circular manner on a 50 cm diameter hot clay griddle, covered, and baked for 2 min. The overall image of injera processing technique is shown in Figure 1.
A semi-trained panel (a panel briefed about the scoring of sensory attribute) of 40 people, who are consuming that particular product evaluated the samples. Accordingly, 20 consumer panelists were considered as replication one and the other 20 panelists as second replication. A rolled piece of injera was presented for panelist on a tray at room temperature within 2 h after baking. Panelists were served with a three digit coded samples on white plates in fluorescent-lighted rooms and was asked to evaluate some basic injera attributes (eye distribution, color, rollability, taste, adhesiveness, bitterness after taste and overall acceptability) using a nine point hedonic scale (1= extremely dislike, 9= extremely like).
Color characteristics
Color measurements of flour and injera samples were carried out using a Hunter colorimeter (Model, NR60CP 3NH Technology Co., LTD) optical Sensor on the basis of L*, a*, and b* values as described by Kaur and Singh (2005). A glass cell containing flour was placed above the light source, covered with a white plate and L*, a*, and b* color values were recorded. The instrument was calibrated against a standard red-colored reference tile (Ls = 25.54, as = 28.89, bs = 12.03). Total color difference (∆E) was calculated by applying the equation:
where, the L* value indicates the lightness, 0–100 representing dark to light. The a* value gives the degree of the red-green color, with a higher positive a* value indicating redder. The b* value indicates the degree of the yellow-blue color, with a higher positive b* value indicating more yellow.
Polyphenol compounds and antioxidant activity
Sample preparation
Because of its high crude fat content, the flour from Kola-1 was defatted first by dissolving in hexane (1:5 w/v, 5 min for 3 times) to remove interfering lipids. A flour sample (2.5 g) was extracted with 25 ml acidified methanol (HCl/methanol/water, 1:80:10, v/v/v) for 2 h while shaking. The mixture was centrifuged at 3000×g for 10 min. The supernatant was used for determination of total phenols, total flavonoids and DPPH radical scavenging activity. The absorbance of the extracts was measured using Visible Spectrophotometer (Model 722, China). Note that both flour and injera were analyzed for the aforementioned parameters.
Anthocyanin content
The anthocyanin content (ACC) was determined by the pH differential method (Li et al., 2012). The diluted sample extracts (100 μL) in 25 mmol L−1 potassium chloride solution (pH 1.0, 5.0 mL) and 0.4 mol L−1 sodium acetate buffer (pH 4.5, 5.0 mL) were measured at 510 and 700 nm, respectively, after 15 min of incubation at 23°C. Finally, absorbance (A) variation was calculated as:
Total anthocyanin content of samples (mg cyanidin3- glucoside L−1 of sample extract) was calculated from the following equation:
Where A is absorbance value, M is molecular weight (449.2 g mol−1), DF is dilution factor (51), and e is the molar absorptivity of cyanidin3-glucoside (26,900 L mol−1 cm−1). The result was calculated in g cyanidin3-glucoside equivalents (CGE)/kg of sample wet weight.
Total phenolic content
The total phenolic content (TPC) was determined by following the Folin–Ciocalteu spectrophotometric method described by Gao et al. (2002). Aliquot of extract (250 µl) was added to 1.5 ml freshly diluted Folin–Ciocalteu reagent. The mixture was allowed to equilibrate for 5 min and then mixed with 1.5 ml of sodium carbonate solution (60 g/L). Then after incubation at 25ºC for 90 min, the absorbance of the mixture was read at 725 nm. Acidified methanol was used as a blank and the result was expressed as a µg of gallic acid equivalents (GAE)/g of flour.
Total flavonoids content
The total flavonoids content (TFC) was determined following the method as described by Zhishen et al. (1999). Extract (250 μl) was diluted with 1.25 ml distilled water. Sodium nitrite (750 μl of 5% solution) was added and the mixture was allowed to stand for 6 min. Then, 150 μl of a 10% aluminum chloride was added and the mixture was allowed to stand for 5 min. Finally, 0.5 ml of 1 M sodium hydroxide was added and solution was mixed well. The absorbance was measured immediately at 510 nm using a spectrophotometer. Catechin was used as standard and the result was reported as µg of catechin equivalents (CE)/g of flour.
DPPH free radical scavenging activity
Antioxidant activity by DPPH free radical scavenging capacity was measured following a modified version of the method described by Brand-Williams et al. (1995) with slight modification. The supernatant (250 µl) was reacted with 4 ml of a 6 × 10−5 mol/L of DPPH solution. Absorbance (A) at 515 nm was read at 0 and 30 min taking a methanol as a blank. DPPH free radical scavenging activity was calculated as % discoloration.
Statistical analysis
A duplicate data was used and analyzed using Minitab 16 statistical software package and Tukey’s multiple comparison tests was used to determine the significance of variation between treatments at 95% confidence level. Results were given as mean ± standard deviation.
Sensory properties of injera
In this study, a nine point hedonic scale (1= dislike extremely, 2= dislike very much, 3= dislike moderately, 4= dislike slightly, 5= neither like nor dislike, 6= like slightly, 7= like moderately, 8= like very much, 9= like extremely) was used. Obviously injera with characteristics of white color, even eye distribution, less sourness and bitterness, rollable and less stick is preferred by consumers. The results of sensory characteristics of injera from five millet varieties and tef injera which was used as a control are shown in Table 1. In terms of aroma, taste and bitter aftertaste, no significant difference (p>0.05) were observed between and within millet varieties and control sample. Padet significantly (p<0.05) differed from Axum and rated lower in its injera eye evenness (6.25) and color (5.71). Injera eye is a honeycomb like structure of the top surface of the product and it is formed during baking.
A significant (p<0.05) variations were noticed in rollability, adhesiveness and overall acceptance of injera. Quncho was perceived differently and rated higher in its overall acceptance (8.04). Conversely, injera from Padet was rated lower in overall acceptance (6.21). In addition, among finger millet cultivars, Padet was perceived lower in its injera eye distribution and color; and a highest score of bitter aftertaste sensory attribute was for injera made from Axum (7.31) and Tesema (7.24) varieties with non-significant (p>0.05) variation in between. Bitter aftertaste could be due to the presence of polyphenols in the grain. Rollability is one the most important injera sensory attribute as it describes the ability of injera being rolled. The result showed that Quncho (7.99) and Tesema (7.04) had the highest and the lowest rollability with a significant difference among them and more preferred from others. This difference might be due to retro gradation of starch components.
Yetneberk et al. (2004) revealed that sorghum cultivar with floury endosperm were characterized by soft and rollable injera. The degree of adhesiveness of injera during consumption is desirable to consumer acceptance and it is a quality of being stick to human sense organs while eating. Tesema showed the lowest degree of stickiness among finger millet cultivars.
Polyphenols content and antioxidant activity of flours and injera
Anthocyanin content (ACC), total phenolic content (TPC), total flavonoid content (TFC) and DPPH radical scavenging capacity of whole grain flours and injera from different millet varieties are shown in Table 2. Importantly, antioxidants are known to limit the amount of free radicals produced in human bodies. Phenolics are one of the major antioxidants found in millets, which chemically act by donating hydrogen atoms via hydroxyl groups on benzene rings to electron-deficient free radicals, and form a resonance-stabilized and less-reactive phenoxyl radical. Phenolics from millets have also shown their ability as reducing agents, singlet oxygen quenchers, and metal chelators (Devi et al., 2014).
Polyphenols content and antioxidant activity of flours
Anthocyanin content: The anthocyanin content of millet flours varied significantly (p<0.05) among millet varieties and ranged from 5.11 to 53.23 mg/L, the lowest for Kola-1 and the highest for Tesema was noticed. Finger millet cultivars, Padet, Tadese, and Tesema were statistically similar in their anthocyanin content. Except from Axum, all finger millets varieties had the highest ACC than Kola-1 and showed significant variation. Tesema and Padet was found to possess the highest ACC among all the studied varieties which had nine times higher than the ACC of Kola-1. Axum had the lowest ACC (25.97 mg/g) among finger millet varieties. Quncho, which is under the same family of millets showed a significant difference with Axum and Kola-1. Anthocyanins are a group of naturally occurring and water soluble pigments that are responsible for the red-blue color of many grains, and can be found in glycosylated forms linked with sugars such as glucose, galactose, arabinose, xylose, rutinose. These compounds become increasingly popular due to their attractive colors and suggested benefits for human health (Pojer et al., 2013) and in addition, protect plants against various biotic and abiotic stresses (Ahmed et al., 2014).
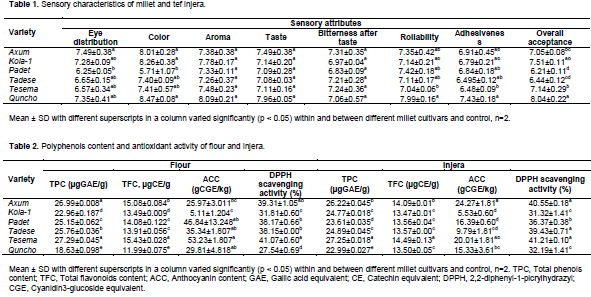
Total phenols and total flavonoids contents: The result showed that contents of total phenols and flavonoids differed significantly (p<0.05) between and within millet varieties and control sample as well. The total phenols and total flavonoids contents of flours ranged from 18.63 to 27.29 µg of GAE/g and 11.99 to 15.43 µg of CE/g, with the highest value for Tesema and the lowest content for Quncho, respectively. Kola-1 was significantly (p<0.05) different and had lower contents of total phenols (22.96 µgGAE/g) and total flavonoids (13.49 µgCE/g) compared to finger millet varieties. Among finger millet cultivars, Padet and Tadese showed the lowest TPC (25.15 µgCE/g) and TFC (13.91 µgGAE/g), respectivley. The lowest TPC and TFC were observed with Quncho. Chandrasekara and Shahidi (2011) revealed that the phenolic content of whole pearl and kodo millet varieties was 8.63 and 32.4 ferulic acid equiv μmol/g defatted meal, respectively. Contrarily, Ragaee et al. (2006) reported the highest total phenols content (1387 µg GAE/g) for pearl millet variety. This variation could be due to agronomic, environmental and varietal differences, and forms and level of sample processing.
DPPH free radical scavenging activity: The antioxidant activity assay by DPPH was significantly (p<0.05) different among millet varieties. The highest DPPH free radical scavenging activity was observed for Tesema (41.07%) and the lowest for Quncho (27.54%). From the studied millet varieties, Kola-1 was found to have the lowest (31.81%) antioxidant activity than finger millets and the greater antioxidant than Quncho with statistical difference. Tadese showed the lowest DPPH free radical scavenging activity (38.15%) with non-significant variation among finger millet cultivars. With the exception of Tesema, finger millet cultivars was not significantly (p>0.05) different. The DPPH free radical scavenging ability of all finger millet varieties was nearly 1.5 times the extracts from Quncho. An order of a free radical scavenging capacity, Tesema > Axum > Padet > Tadese > Kola-1 > Quncho was observed. DPPH radical is a synthetic organic radical that is widely used to evaluate free radical scavenging possessions of antioxidative compounds. Similar study by Siroha et al. (2016) noticed that the the antioxidant activity of pearl millet flours in the range of 31.8 to 46.7%. In addition, Ragaee et al. (2006) revealed that pearl millet had DPPH scavenging capacity of 23.83 µmol/g. Conversely, Chandrasekara et al. (2012) reported DPPH scavenging activities of 13.8 µmol ferulic acid equivalent/g for pearl millet flour. The antioxidant activity of grains could be influenced by genetic and environmental factors, and the level and method of grain processing.
Injera polyphenols content and antioxidant activity: After fermentation and baking, the polyphenol contents and antioxidant activity were ranged from 22.99 to 27.25 µgGAE/g for TPC, 13.47 to 14.49 µgCE/g for TFC, 5.53 to 24.27 mg/g for ACC and 31.32 to 41.21% for DPPH radical scavenging activity with a significant (p<0.05) difference among all varieties (Table 2). Tesema had the highest TPC, TFC and DPPH free radical scavenging capacity with a values 27.25 µgGAE/g, 14.49 µgCE/g and 41.21%, respectively. The lowest (even lower than injera made from Quncho) contents of total flavonoid, anthocyanin and antioxidant capacity was observed with Kola-1. Among finger millet varieties, Padet showed the lowest content in TPC (23.61 µgGAE/g) and TFC (13.56 µgCE/g). Except for the anthocyanin content, a non-significant (p>0.05) reduction or increment in total phenols, total flavononids and DPPH free radical scavenging activity were observed in injera compared to flours. This could be attributed to the partial degradation of phenols by microorganisms during fermentation (Bravo, 2009) and loss of some anthocyanin, which have been reported as labile to heat. An increment in DPPH free radical scavenging activity was noticed in finger millet cultivars and tef which is likely due to the amount reducing sugars that contribute to maillard reaction. Gélinas and McKinnon (2006) also observed that the crust of white bread contained more phenolic compounds than the crumb because of the Maillard reaction.
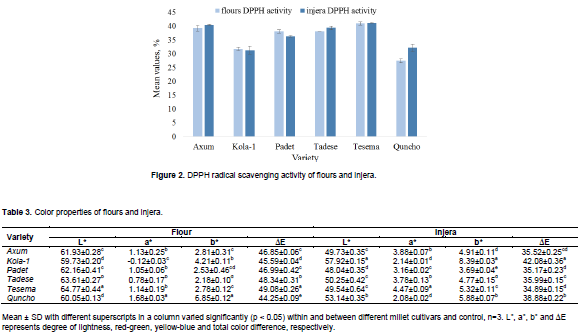
On the other hand, Salar et al. (2017) revealed that fermentation resulted in an increased TPC with fermented pearl millet grains showing higher values than unfermented pearl millet grains. According to these authors, as fermentation increased from 0 to 6 days, the TPC increased from 6.4 to 34.1 mg GAE/g dwb. However, beyond these fermentation days a reduction in TPC was noticed. In the present study, fermentation and then baking did not result in a significant (p>0.05) reduction and/or increment of DPPH radical scavenging activity of the millets extracts, except for Quncho which showed an increment (Figure 2). A strong correlation (r=0.98) was noticed between total phenolic content and DPPH radical scavenging activity. In addition, total flavonoids content and DPPH activity were highly correlated (r=0.94).
Color characteristics
Table 3 shows color characteristics (L*, a*, b* and ∆E) of flours and injera which were evaluated using hunter color lab. The L* value of flours ranged from 59.73 to 64.77, the more darken and light colors for Kola-1 and Tesema, respectively. After baking, the L* value of injera ranged from 48.04 to 57.92 and except for Kola-1 (which showed an increment) was significantly reduced in comparison with flours. Kola-1 and Quncho, and among finger millet varieties, Axum and Padet in both flours and injera did not show significant (p>0.05) variation in their L* value. The variation observed might be due to the chemical compositions of the grain mainly because of protein, starch and reducing sugar contents that affects Maillard reaction. The degree of a red-green color (a*) ranged from -0.12 to 1.68 for flours and 2.14 to 4.47 for injera, and baking significantly increased a* value compared to flours. The highest and the lowest a* value with a significant difference was noticed with Quncho and Kola-1 in flours, but had the lowest and exhibited insignificant variation in injera. Kola-1 was statistically different and showed the minimum redness and maximum greenness color in flours and injera, respectively, whereas Tesema exhibited the maximum a* value in injera indicating more redness. In comparison with the flour from pearl millet variety, flours from finger millet varieties showed the maximum redness with non-significant (p<0.05) difference among them. A significant difference was observed among flours of millet varieties in their b* value (yellow-blue color) which ranged from 2.18 to 4.21, the highest for Kola-1 (which exhibited maximum blueness) and the lowest for Tadese (attained maximum yellowness). Quncho showed the highest b* value, which indicated a less yellowness and more blueness. Baking increased the b* value in injera and ranged from 3.69 to 8.39 with the highest and lowest for Kola-1 and Padet, respectively. The total color difference (∆E) ranged from 44.25 to 49.08 for flours and from 34.89 to 42.08 for injera, with Tesema having the highest and the lowest in flour and injera, respectively. Significant reduction of ∆E was noticed after baking. Among finger millet cultivars, the lowest ∆E was noticed in Axum and Padet varieties with statistically non-significant difference in between. The total color difference of flours and injera was negatively correlated (r=-0.74), whereas a* value was positively correlated with the anthocyanin content in both flours (r=0.62) and injera’s (r=0.59) and were statistically different. Siroha and Sandhu, (2017) revealed that toasting significantly reduced the L* value and increased a* and b* values in flours from pearl millet cultivars.
Even though tef injera is more popular than millet, it contains less health-promoting compounds in relation to its phytochemicals content and less effective on oxidative stress and probably associated health disorders. The present study revealed that among millet varieties, injera made from Kola-1 and Tesema varieties were more preferred in its overall consumer acceptance and the result was comparable to the most preferred tef injera. Finger millet varieties had the highest contents of total phenols, total flavonoids and DPPH radical scavenging activity than pearl millet variety. Among finger millet variety, Tesema showed the highest in polyphenols content and antioxidant activity. A lighter flour color was observed with Tesema. Kola-1 had the highest total color difference both in flour and injera.
The authors gratefully appreciate the VLIR and Can Tho University Improvement Project, VN14-P6 supported by Japanese ODA loan, and Can Tho University, for the financial and administrative support and Postharvest, Food Biotechnology, and Product Development and Food Safety Laboratories, Can Tho University, Vietnam, for their kind assistance during the experimental period.
The authors have not declared any conflict of interests.
REFERENCES
|
Abiyu HT, Woldegiorgis AZ, Haki GD (2013). Preparation of injera from pre-fermented flour: Nutritional and sensory quality. International Journal of Science Innovations and Discoveries 3(1):165-175.
|
|
|
|
Admassu S, Teamir M, Alemu D (2009). Chemical composition of local and improved finger Millet (Eleusine Corocana (L.) Gaetrtin] varieties
|
|
|
|
|
grown in Ethiopia. Ethiopian Journal of Health Sciences 19(1).
|
|
|
|
|
Ahmed NU, Park JI, Jung HJ, Yang TJ, Hur Y, Nou IS (2014). Characterization of dihydroflavonol 4-reductase (DFR) genes and their association with cold and freezing stress in Brassica rapa. Gene 550(1):46-55.
Crossref
|
|
|
|
|
Asharani VT, Jayadeep A, Malleshi NG (2010). Natural antioxidants in edible flours of selected small millets. International Journal of Food Properties 13(1):41-50.
Crossref
|
|
|
|
|
Boka B, Woldegiorgis AZ, Haki GD (2013). Antioxidant properties of Ethiopian traditional bread (Injera) as affected by processing techniques and teff grain [Eragrostis teff (Zucc.)Trotter] varieties. Canadian Chemical Transactions 1:7-24.
Crossref
|
|
|
|
|
Brand-Williams W, Cuvelier ME, Berset C (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology 28(1):25-30.
Crossref
|
|
|
|
|
Bravo L (2009). Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutrition Reviews 56(11):317-333.
Crossref
|
|
|
|
|
Chandrasekara A, Naczk M, Shahidi F (2012). Effect of processing on the antioxidant activity of millet grains. Food Chemistry 133(1):1-9.
Crossref
|
|
|
|
|
Chandrasekara A, Shahidi F (2011). Bioactivities and antiradical properties of millet grains and hulls. Journal of Agricultural and Food Chemistry 59(17):9563-9571.
Crossref
|
|
|
|
|
Chandrasekara A, Shahidi F (2012). Bioaccessibility and antioxidant potential of millet grain phenolics as affected by simulated in vitro digestion and microbial fermentation. Journal of Functional Foods 4(1): 226-237.
Crossref
|
|
|
|
|
Devi PB, Vijayabharathi R, Sathyabama S, Malleshi NG, Priyadarisini VB (2014). Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: A review. Journal of Food Science and Technology 51(6):1021-1040.
Crossref
|
|
|
|
|
Di Marcantonio F, Demeke M (2013). Analysis of incentives and disincentives for teff in Ethiopia.
|
|
|
|
|
Fardet A, Rock E, Rémésy C (2008). Is the in vitro antioxidant potential of whole-grain cereals and cereal products well reflected in vivo? Journal of Cereal Science 48(2):258-276.
Crossref
|
|
|
|
|
Gao L, Wang S, Oomah BD, Mazza G (2002). Wheat quality: Antioxidant activity of wheat millstreams. Wheat Quality Elucidation 219-233.
|
|
|
|
|
Gull A, Prasad K, Kumar P (2018). Nutritional, antioxidant, microstructural and pasting properties of functional pasta. Journal of the Saudi Society of Agricultural Sciences 17(2):147-153.
Crossref
|
|
|
|
|
Halliwell B (1994). Free radicals and antioxidants: a personal view. Nutrition Reviews 52(8):253-265.
Crossref
|
|
|
|
|
Kaur M, Singh N (2005). Studies on functional, thermal and pasting properties of flours from different chickpea (Cicer arietinum L.) cultivars. Food Chemistry 91(3): 403-411.
Crossref
|
|
|
|
|
Li X, Zhang JY, Gao WY, Wang Y, Wang HY, Cao JG, Huang LQ (2012). Chemical composition and anti-inflammatory and antioxidant activities of eight pear cultivars. Journal of Agricultural and Food Chemistry 60(35):8738-8744.
Crossref
|
|
|
|
|
Liyana-Pathirana CM, Shahidi F (2006). Importance of insoluble-bound phenolics to antioxidant properties of wheat. Journal of Agricultural and Food Chemistry 54(4):1256-1264.
Crossref
|
|
|
|
|
Madhujith T, Shahidi F (2007). Antioxidative and antiproliferative properties of selected barley (Hordeum vulgarae L.) cultivars and their potential for inhibition of Low-Density Lipoprotein (LDL) cholesterol oxidation. Journal of Agricultural and Food Chemistry 55(13):5018-5024.
Crossref
|
|
|
|
|
Madhujith T, Shahidi F (2009). Antioxidant potential of barley as affected by alkaline hydrolysis and release of insoluble-bound phenolics. Food Chemistry 117(4):615-620.
Crossref
|
|
|
|
|
Manach C, Mazur A, Scalbert A (2005). Polyphenols and prevention of cardiovascular diseases. Current Opinion in Lipidology 16(1):77-84.
Crossref
|
|
|
|
|
Mezemir S (2015). Probiotic Potential And Nutritional Importance of Teff (Eragrostis tef (Zucc) Trotter) Enjerra - A review. African Journal of Food, Agriculture, Nutrition and Development 15(2):9964-9981.
|
|
|
|
|
Naczk M, Shahidi F (2004). Extraction and analysis of phenolics in food. Journal of Chromatography A, 1054(1-2):95-111.
Crossref
|
|
|
|
|
Pathak P, Srivastava S, Grover S (2000). Development of food products based on millets, legumes and fenugreek seeds and their suitability in the diabetic diet. International Journal of Food Sciences and Nutrition 51(5):409-414.
Crossref
|
|
|
|
|
Pojer E, Mattivi F, Johnson D, Stockley CS (2013). The case for anthocyanin consumption to promote human health: A review. Comprehensive Reviews in Food Science and Food Safety 12(5):483-508.
Crossref
|
|
|
|
|
Ragaee S, Abdel-Aal ESM, Noaman M (2006). Antioxidant activity and nutrient composition of selected cereals for food use. Food Chemistry 98(1):32-38.
Crossref
|
|
|
|
|
Salar RK, Purewal SS, Sandhu KS (2017). Fermented pearl millet (Pennisetum glaucum) with in vitro DNA damage protection activity, bioactive compounds and antioxidant potential. Food Research International 100:204-210.
Crossref
|
|
|
|
|
Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L (2005). Dietary polyphenols and the prevention of diseases. Critical Reviews in Food Science and Nutrition 45(4):287-306.
Crossref
|
|
|
|
|
Siroha AK, Sandhu KS (2017). Effect of heat processing on the antioxidant properties of pearl millet (Pennisetum glaucum L.) cultivars. Journal of Food Measurement and Characterization 11(2):872-878.
Crossref
|
|
|
|
|
Siroha AK, Sandhu KS, Kaur M (2016). Physicochemical, functional and antioxidant properties of flour from pearl millet varieties grown in India. Journal of Food Measurement and Characterization 10(2):311-318.
Crossref
|
|
|
|
|
Yetneberk S, De Kock HL, Rooney LW, Taylor JRN (2004). Effects of Sorghum Cultivar on Injera Quality. Cereal Chemistry 81(3):314-321.
Crossref
|
|
|
|
|
Zhishen J, Mengcheng T, Jianming W (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry 64(4):555-559.
Crossref
|
|
|
|
|
Zielinski H, Michalska A, Piskuła MK, Kozłowska H (2006). Antioxidants in thermally treated buckwheat groats. Molecular Nutrition and Food Research 50(9):824-832.
Crossref
|
|