This work was aimed at investigating the effect of storage temperature on the physicochemical, nutritional, and microbial quality of soursop juice. Physicochemical quality was determined by measuring changes in pH, titratable acidity, total soluble solids and colour (L*a*b* values). Ascorbic acid levels, total phenolic content and total antioxidant capacity were analysed to determine the effect of storage temperature on nutritional quality. The changes in aerobic mesophilic and psychrophilic bacteria, lactic acid bacteria, Enterobacteriaceae, as well as yeast and moulds were enumerated to determine the effect of storage temperature on microbial quality. Soursop juice was pasteurised at 85°C for 5 min and stored at varying temperatures of 4, 10 and 25°C. Storage was carried out for 12 weeks at both 4 and 10°C, while at 25°C the juice was stored for 3.5 weeks when visible signs of spoilage was detected. Storing soursop juice at 4ºC did not affect quality, however, at 25°C, decreases in pH and total soluble solids with an increase in titratable acidity was observed. In addition, a faster rate of ascorbic acid degradation was observed at 25°C. The main group of microorganisms that were responsible for the spoilage of soursop juice stored at 25°C were lactic acid bacteria, and yeasts and moulds. The results of this work show that pasteurised soursop juice can be stored at refrigeration temperatures without changes in quality.
Extraction of juice from fruits can be used to improve the usage of fruits as the extracted juice can be easily transported and stored (Bhardwaj and Pandey, 2011). The extraction process, however, can lead to microbial contamination, which can limit the shelf life of the extracted juice. To improve the shelf life of the extracted juice, thermal processing methods such as pasteurization can be carried out to reduce the level of microbial contamination and other reactions (enzymatic and non-enzymatic) which can lead to spoilage (Rivas et al., 2006). However, due to the fact that pasteurization do not completely destroy all microorganisms and enzymes, changes in quality may occur in the juice during storage (Chia et al., 2012; Polydera et al., 2003; Touati et al.,
2016; Umme et al., 2001; Wibowo et al., 2015). Some of these changes may affect colour and flavor which in the end may reduce the sensory acceptability of the juice (Umme et al., 2001). The degradation of some bioactive compounds such as ascorbic acid may also occur during storage, thus, reducing the nutritional composition of the juice (Touati et al., 2016). The resuscitation and growth of microorganisms is also possible during storage. The temperature of storage usually influences the changes that occur during storage. It is, therefore, important to investigate the effect of storage temperature on the changes in fruit juice after pasteurization.
This study sought to specifically pasteurise soursop juice and determine how storage temperature impacted on physicochemical, nutritional and microbial safety. Soursop juice was pasteurised at 83°C for 5 min and stored at temperatures of 4, 10 and 25°C. The effect of storage temperature on the physicochemical quality of the juice was determined by measuring the changes in pH, total soluble solids, titratable acidity and colour (L*a*b*). The effect of storage temperature on the changes in nutritional components such as ascorbic acid levels, total phenolic content and total antioxidant capacity were determined. Additionally, a kinetic model was developed to explain the effect of storage temeprature on the changes in ascorbic acid levels. The microbial quality of the juice during storage was assessed by enumerating the changes in aerobic mesophilic and psychrophilic bacteria, lactic acid bacteria, Enterobacteriaceae, and yeast and moulds.
Pasteurization of soursop juice and shelf life studies
Mature and uniformly ripe soursop fruits (Annona muricata L.) were cleaned, disinfected and peeled. The seeds were removed from the pulp and the juice extracted. The extracted juice were pasteurised in sterilised glass tubes at and rapidly cooled in ice-cold water. Pasteurization was carried out on a water bath at 83°C for 5 min. To determine the initial microbial load immediately after pasteurization, some soursop juice samples were store at 4°C for 24 h to allow for the resuscitation of stressed microorganisms.
The pasteurised soursop juice were stored in incubators at different temperatures (4, 10 and 25°C) and changes in quality monitored. Storage was carried out for 12 weeks at both 4 and 10°C, while at 25°C the juice was stored for 3.5 weeks until spoilage was detected. Periodically, the soursop juice were sampled and physicochemical and microbial analyses carried out on the same day. The sampled juice was, however, stored at -80°C for subsequent nutritional analyses. The quality of both the pasteurised and unpasteurised juices were analysed before storage, and the pasteurised juice was used as control for comparative purposes.
Microbial analyses
The effect of pasteurization on the survival of microorganisms in soursop juice was determined by enumerating the changes in aerobic mesophiles and psychrophiles, lactic acid bacteria, Enterobacteriaceae, and yeast and moulds. After performing the appropriate decimal dilutions in peptone water, agar plates were inoculated with the juice samples. Both aerobic mesophilic and psychrophilic bacteria were enumerated on plate count agar (Oxoid Ltd., UK). The plates for aerobic mesophiles were incubated at 30°C for 48 h while the plates for aerobic psychrophiles were incubated at 10°C for 5 days. Lactic acid bacteria were enumerated on de Man-Rogosa-Sharpe agar (Oxoid Ltd., UK) were incubated at 30°C for 48 h. Yeast and moulds were enumerated on Sabouraud Dextrose Agar (Oxoid Ltd., UK) that were incubated at 35°C for 24 h while Enterobacteriaceae were enumerated on Violet Red Bile Glucose agar (Oxoid Ltd., UK) incubated at 37°C for 24 h (Suárez-Jacobo et al., 2010).
Physicochemical analyses
The effect of storage temperature on physicochemical quality was assessed by determining the changes in pH, total soluble solids, titratable acidity and colour. A pH meter and digital refractometer (MA871, Milwaukee Instruments USA) were used to determine the pH and the total soluble solids, respectively. The titratable acidity was determined by titrating the soursop juice against NaOH (AOAC, 2010), while a colour meter (CS-10, CHN Spec, China) was used to measure the changes in L*a*b values.
Nutritional analyses
Ascorbic acid was extracted with metaphosphoric-acetic acid solution and determined using the 2,4- dinitrophenylhydrazine based assay (Kapur et al., 2012). L-ascorbic acid was used as a standard and the results expressed as mg ascorbic acid per 100 g of soursop juice. Both the total phenolic content and total antioxidant capacity were analysed after extracting the juice with equal volumes of methanol. The Folin-Ciocalteu based assay was used to determine the total phenolic content (Meda et al., 2005) while the total antioxidant capacity was determined based on the scavenging activity of 2,2-diphenyl-1-picrylhydrazyl (Sánchez-Moreno et al., 1999). Gallic acid was used as the standard for both the total phenolic content and total antioxidant capacity.
Kinetic modelling of the effect of storage temperature on ascorbic acid levels
A first-order kinetic model (Equation 1) was developed to explain the effect of storage temperature on ascorbic acid levels.
Statistical analyses
Statistical analyses to determine the effect of storage temperature on the quality of soursop juice was carried out using analysis of variance (ANOVA). The effect of storage temperature was assessed by comparing the quality of the stored juice to the control (freshly pasteurised juice). When a significant effect was observed, the Tukey test was performed to determine which juice samples were different from the control. The same procedure was performed to determine the effect of storage temperature at a specific storage time. The difference among means were identified at a significance level of 0.05. All statistical analyses were performed at a significance level of 0.05 using SPSS (IBM, SPSS Statistics 20).
The statistical analyses of the obtained model parameters were estimated using an error based bootstrap resampling technique. By bootstrapping the complete dataset multiple times, the model was fitted to the obtained bootstrap datasets several times, hence obtaining the distribution around individual parameters from which the standard deviations were estimated.
Effect of pasteurization on the quality of soursop juice
The effect of pasteurization on the quality of soursop juice is shown in Table 1. The pH, total soluble solids, titratable acidity and colour of the juice were not significantly affected by pasteurization. Pasteurization resulted in reductions in ascorbic acid levels, total phenolic content and total antioxidant capacity; however, these reductions were not significantly different compared to the unpasteurised juice. The levels of aerobic mesophiles, aerobic psychrophiles, lactic acid bacteria, and yeast and moulds were significantly lower in the pasteurised compared to the unpasteurised juice. Enterobacteriaceae was not detected in both the unpasteurised and pasteurised soursop juice.
Ascorbic acid levels have been observed to decrease due to the effects of pasteurization (Ranu and Uma, 2012; Vikram et al., 2005). Pasteurization offers the possibility of extending the usage of fruit juices by reducing the action of microorganisms. Temperatures between 80-95°C for time durations between 1-10 min are usually employed in the pasteurization of fruit juices (Chia et al., 2012). In this study, pasteurization at 85°C reduced the microbial load of soursop juice. Fruit juice are usually expected to have between 3-5 log10cfu/ml of yeast (Vasavada and Heperkan, 2002) with the limit of microbial shelf life around 6 log10cfu/ml. The levels of microorganisms in both the unpasteurised and pasteurised juice were within acceptable limits.
Effect of storage temperature on the microbial of soursop juice
Figure 1 shows the effect of storage temperature on the growth of aerobic mesophiles. At 4°C, no significant changes in aerobic mesophiles was observed between the control and the stored samples. A similar observation was made in the changes in yeast and moulds, and lactic acid bacteria. However, storage at 4°C resulted in a gradual but not significant increase in aerobic psychrophiles.
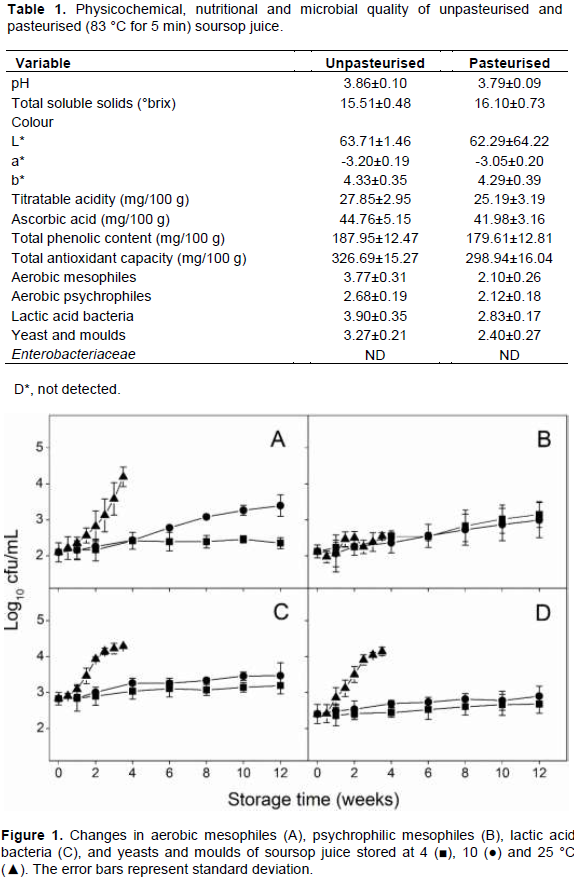
Storage at 10 and 25°C resulted in growth of aerobic mesophiles with significant differences between the control and the stored sample observed after 6 and 1.5 weeks of storage, respectively (Figure 1). In addition, growth of aerobic psychrophiles, lactic acid bacteria (Figure 1), and yeasts and moulds (Figure 1) was observed. Lactic acid bacteria, and yeast and moulds were the predominant group of microorganisms that were able to grow in the soursop juice. The growth of these two groups of microorganisms might be due to low pH of soursop juice, which favors the growth of acidophilic microorganisms. Indeed the spoilage of most juices has been attributed to the presence and growth of yeasts (Alwazeer et al., 2002; Elez-Martínez et al., 2005). The inhibition of microbial growth at 4ºC means that the storage of soursop juice can be extended beyond 12 weeks. In pasteurised pineapple juice, the microbial population remained unchanged during refrigerated temperature storage for 12 weeks (Chia et al., 2012).
Effect of storage temperature on pH, titratable acidity and total soluble solids of soursop juice
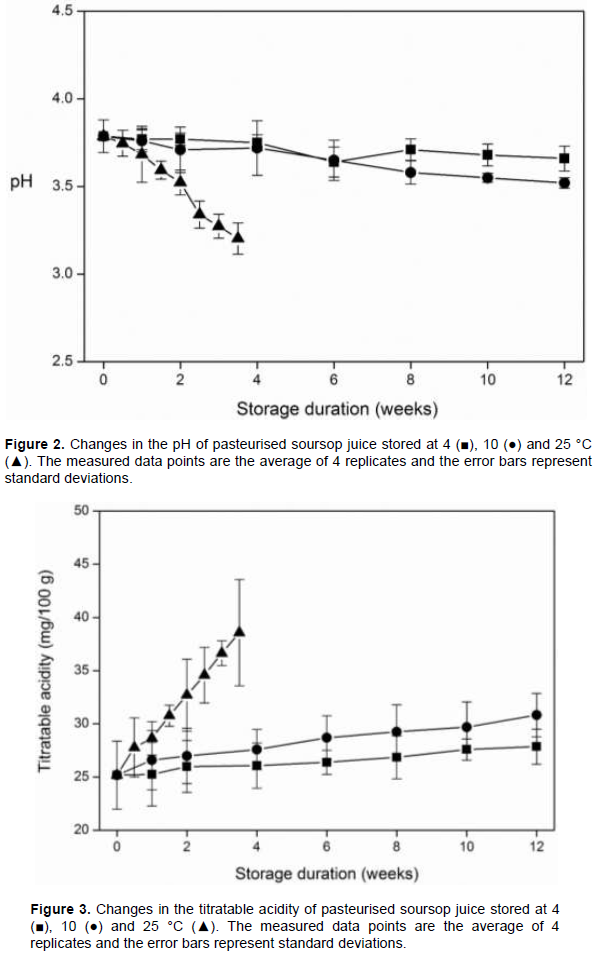
The effect of storage temperature on the pH of soursop juice is shown in Figure 2. Compared to the control (freshly pasteurised juice before storage), storage at refrigeration temperature (4°C) did not have a significant effect on the pH of soursop juice. A similar effect have been observed in other fruit juices stored a refrigeration temperatures. In thermo-sonicated soursop nectar, the pH did not change significantly during storage at 4°C for 45 days (Anaya-Esparza et al., 2017). Also, no significant changes in pH were observed during refrigerated storage of thermally pasteurised pineapple juice (Chia et al., 2012), thermally treated juice blend of orange and carrot (Rivas et al., 2006) and heated orange juice (Bull et al., 2004; Yeom et al., 2000). Higher temperatures of storage have been observed to affect the quality of fruit juices. At 10°C and 25°C, significant decreases in pH were observed between the control and the stored samples after 10 and 1.5 weeks of storage, respectively (Figure 2). This decrease is similar to the observation made by Touati et al. (2016) in thermally treated grape, orange and pear nectars and in soursop juice (Abbo et al., 2006).
Figure 3 shows the effect of storage temperature on the titratable acidity of soursop juice. There was a general increase in titratable acidity during storage, however, no significant differences were observed between the control and the juice stored at 4°C. During storage at 10 and 25°C, significant differences in titratable acidity were observed between the control and the stored juice after10 and 2 weeks, respectively. An increase in titratable acidity has also been observed in other fruit juices during storage (Anaya-Esparza et al., 2017; Bull et al., 2004; Chia et al., 2012). The changes in total soluble solids of soursop juice during storage is shown in Figure 4. Storage at 4°C did not have a significant effect on the total soluble solids of the juice. Anaya-Esparza et al. (2017) made a similar observation. According Bhardwaj and Pandey (2011), the retention or slight increase in total soluble solids of fruit juices during storage is desired for quality preservation. Storage at low temperature, therefore, can help retain the total soluble solids content of soursop juice. At 10 and 25°C, however, significant decreases in total soluble solids were observed after 8 and 1.5 weeks of storage, respectively, compared to the control. Comparing the three storage temperatures, significant differences in total soluble solids were observed on weeks 3 and 3.5, where the juice samples stored at 25°C recorded significantly lower total soluble solids content. The changes in pH, titratable acidity and total soluble solids of soursop juice during storage, especially at the higher temperatures, can be attributed to the growth of microorganisms. The utilisation of sugars by microorganisms can led to the production of organic acids, which can lead to reduction in pH and total soluble solids, and an increase in titratable acidity (Rivas et al., 2006).
Effect of storage temperature on the colour of soursop juice
Figure 5 shows the changes in colour (L*a*b*) of soursop juice store at different temperatures. There was a general decrease in L* values. No significant difference in L* value were observed between the control and soursop juice stored at 4 and 10°C after one week. However, the soursop juice stored at 25°C recorded a significantly lower L* value compared to the control after one week. At 4°C, significant differences in L* values were observed between the control and stored samples on weeks 6, 8, 10 and 12. Similarly, at 10°C, significant differences in L* values were observed between the control and stored samples on weeks 4, 6, 8, 10 and 12. During storage at 25°C, significant differences in L* values between the control and the stored samples were observed after 0.5 weeks of storage. Comparing the different storage temperatures, significant difference in L* values were observed between the juice samples stored at 4 and 10°C on weeks 10 and 12. Significant differences were also observed between the samples stored at 25 °C and the lower storage temperatures (4 and 10°C) on all sample weeks except on week 0.5 (Figure 2). There was a slight increase in a* values of soursop juice during storage, however, no significant differences in a* values were observed between the control and all the stored samples. There was a general increase in b* values of soursop juice during storage (Figure 2B). At 4°C, no significant differences in b* values were observed between the control and the stored samples. At 10°C, however, significant differences were observed compared to the control on weeks 10 and 12. During storage at 25°C, significant differences in b* values were observed after 1.5 weeks of storage. Comparing the different storage temperatures, a significant difference in b* values were observed between the juice samples stored at 4 and 10°C only on week 10. Significant differences were, however, observed between the juice samples stored at 25°C and the lower storage temperatures (4 and 10°C) after 2 weeks of storage (Figure 2B).
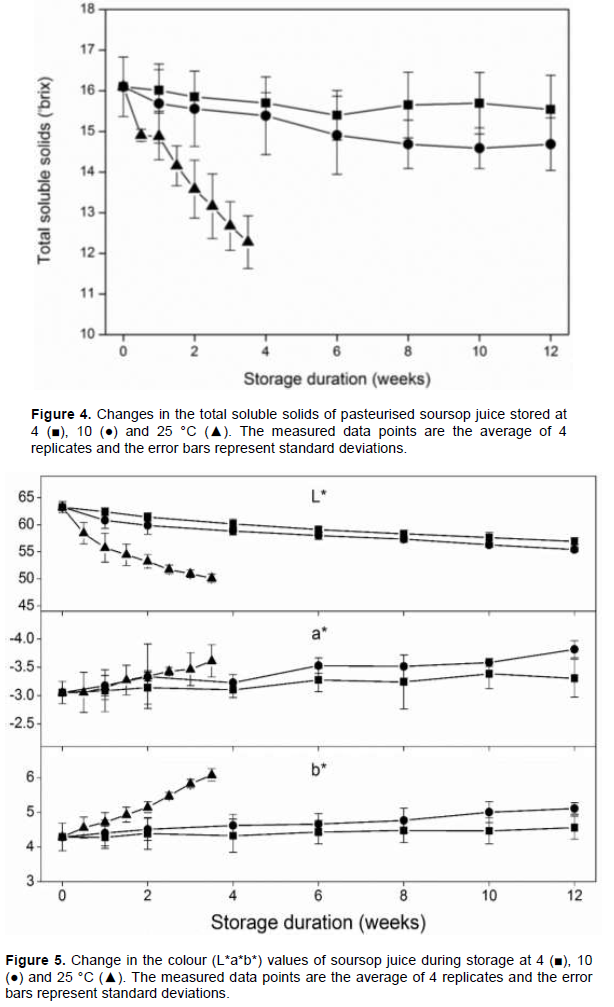
Though extracted soursop juice is white in color, it can gradually change to creamy white and eventually yellowish depending on the storage condition. The L*a*b* values of soursop juice in this study did not change significantly at the storage temperature of 4°C. This observation is supported by the observations in other studies where soursop nectar retained its color at 4°C for 45 days and again after pasteurization and storage at 4°C and evaluated during sensory analysis. The retention of color at the low storage temperature can be attributed to a reduction in browning reactions. At higher storage temperatures, browning reactions can occur thus reducing the whiteness of soursop juice. This is reflected in the decreasing L* values and the changes in a* and b* values.
Effect of storage temperature on nutritional quality of soursop juice
The effect of storage temperature on the total phenolic content and total antioxidant capacity of soursop juice is shown in Table 2. There was a general reduction in the total phenolic content of soursop juice within the first few weeks of storage, irrespective of the storage temperature. Afterwards, the total phenolic content remained relatively constant throughout the storage period. The total phenolic content of the stored soursop juice were no significantly different compared to the control. Storage at 4 and 10°C resulted in a reduction in total antioxidant capacity of soursop juice until week 4. However, at week 6 the total antioxidant capacity increased and generally decreased again until the end of the storage period. At 25°C storage, the reduction in total antioxidant capacity occurred until week 1.5, increased at week 2 and remained constant until the end of storage (Table 2). Similar to the total phenolic content, the total antioxidant capacity of the stored soursop juice were no significantly different compared to the control.
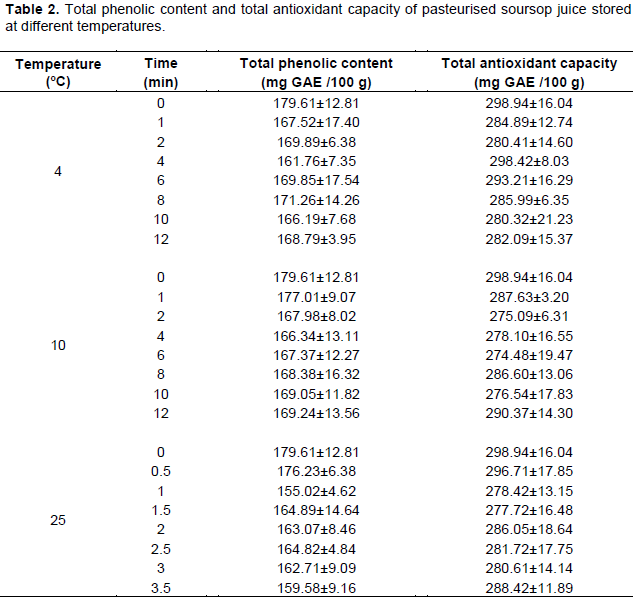
Storage temperature have been observed to have a variable effect on the total phenolic content and total antioxidant capacity of fruit juices. The phenolic content of pineapple juice remained relatively unchanged during 13 weeks of storage (Chia et al., 2012); however, decreases were observed in soursop nectar (Anaya-Esparza et al., 2017). It is possible that the total phenolic content of the soursop juice remained relatively unchanged due to the inactivation of peroxidase during pasteurization (Odriozola-Serrano et al., 2008). In other fruit juices, no changes in antioxidant capacity have been observed during storage (Anaya-Esparza et al., 2017; Mgaya-Kilima et al., 2014).
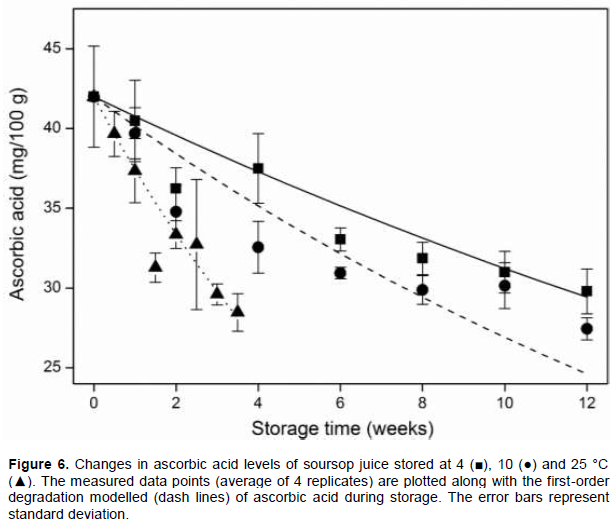
Effect of storage temperature on ascorbic acid level
Figure 6 shows the changes in ascorbic acid levels of soursop juice as well as the modelled first-order degradation kinetics of ascorbic acid during storage. There was a general decrease in ascorbic acid levels during storage. No significant differences in ascorbic acid levels were observed after one week of storage at the different temperatures. During storage at 4 and 10°C, significant differences in ascorbic acid levels were observed between the control and stored samples after 2 weeks. During storage at 25°C, however, significant differences in ascorbic acid levels were observed after 1.5 weeks of storage. Comparing the different storage temperatures, significant differences in ascorbic acid levels were observed between the juice samples stored at 4 and 10°C only on week 12. The estimated model parameters for the degradation of ascorbic acid in soursop juice gave a degradation rate constant and activation energy of 0.087 min-1 and 43.98 kJ/mol with standard deviations of 0.004 and 2.99, respectively. The degradation of ascorbic acid during storage of fruit juices is one of the most important factors affecting quality (Davey et al., 2000). In most fruit juices, ascorbic acid is the most important factor influencing nutritional quality (Franke et al., 2004) The degradation of ascorbic acid have been observed in other fruit juices (Ajibola et al., 2009; Polydera et al., 2003; Roig et al., 1995). Different levels of degradation of ascorbic acid have been reported in the literature. In the soursop juice, ascorbic acid levels reduced by 29.04 and 34.65% during storage at 4 and 10°C, respectively. However, during storage a 25°C, ascorbic acid levels decreased by 32.93% within 3.5 weeks. This shows that the degradation of ascorbic acid in soursop juice is temperature dependent. In several fruit juices, the temperature dependence of ascorbic acid degradation during storage have been confirmed (Burdurlu et al., 2006; Polydera et al., 2003; Roig et al., 1995; Sapei and Hwa, 2014; Uddin et al., 2002). The first-order model was adequate to explain the degradation of ascorbic acid in soursop juice. Similar rate constant and activation energy has been reported in the literature for the degradation of ascorbic acid in other fruit juices (Zheng and Lu, 2011; Wibowo et al., 2015; Polydera et al., 2003; Sapei and Hwa, 2014).
The physicochemical, nutritional and microbial quality of soursop juice is affected by the temperature of storage. Storage at 4°C can be used to achieve shelf life in excess of 12 weeks without changing the quality of soursop juice. At higher storage temperatures, the growth of acidophilic microorganisms such as lactic acid bacteria, and yeasts and moulds enhances the spoilage of soursop juice resulting in changes in the quality of soursop quality. Loss of ascorbic acid occurs during storage of soursop juice. However, the temperature of storage influences this loss with higher losses occurring at the high storage temperatures.