ABSTRACT
The objective of this study was to degrade the phytic acid content in composite wheat/cassava/ sorghum bread by activating intrinsic cereal phytases during the baking process. The aim was to reach a phytate:iron molar ratio <1 in order to achieve an enhanced iron absorption in humans. Means to activate the phytase included dough preparation at different pH values and temperature as well as pre-soaking of the sorghum flour prior tobefore baking. The phytic acid and mineral content was measured by high high-performance ion chromatography. In the composite bread without pH adjustment of the dough, the phytate content was 1.58 µmol/g. After adjustment of the dough pH to 4.3, the phytate content in the composite bread decreased to 0.86 µmol/g. Soaking of the sorghum flour at 22°C for 3 h at pH 4.3 prior tobefore baking, further decreased the phytate content to 0.58 µmol/g. Increasing the soaking temperature to 37°C and addition of 10% wheat flour resulted in a phytate content of 0.14 µmol/g in the composite bread, that is a reduction by 97% of the initial phytate content. The phytate:iron molar ratio was then 0.70 and the phytate:zinc molar ratio was 1.1 that is expected to have a positive effect on the absorption of both minerals in humans.
Key words: Phytic acid, wheat flour, cassava flour, sorghum flour, bread making, soaking, pH.
Bread has become the most widely consumed non-indigenous food product within Mozambique. However, wheat flour, the most suitable cereal flour for bread making, is produced in less than 3% of the needs (FAOstat, 2018) and substantial quantities must be imported at a high cost (Akubor and Badifu, 2004). Replacing wheat flour with locally produced cereal and root-tuber flours would help to reduce the need for expensive wheat imports and promote the agriculture sector in Mozambique.
Wholemeal cereal flours provide significant amounts of nutrients such as carbohydrate, protein, vitamin, minerals including the trace elements, but also contain high levels of phytic acid (myo-inositolhexakisphosphate, InsP6) that inhibits the bioavailability of essential minerals such as iron and zinc (Hallberg et al., 1989; Nävert et al., 1985).
As a result, iron deficiency anaemia is highly prevalent in many low-income countries where cereals constitute the major staple food (Taylor et al., 1995; Tatala et al., 1998; Hurrell et al., 2002). Cereals do also contain phytase, an enzyme that degrades the phytate content and as a result may free the minerals making them available for absorption by humans (Hurrell, 2004). However, the inhibiting effect of phytate occurs at very low phytate concentrations and to improve iron absorption, the phytate content needs to be degraded to a phytate:iron molar ratio <1 (Hurrell et al., 2002), and for an improved zinc absorption the phytate:zinc molar ratio needs to be <15 (Nävert et al., 1985; Hunt, 2003). The phytase activity of cereal grains varies greatly with rye and wheat showing high activities while maize, millet and sorghum have low activities (Egli et al., 2002; Azeke et al., 2011). With optimal baking conditions for intrinsic phytase activity, prolonged proofing time (120 min) at 37oC and pH adjusted to pH 4.5, Türk et al. (1996) succeeded to degrade up to 96% of the phytate content in whole wheat bread. Adding commercial exogenous free phytase from Aspergillus niger has been shown to completely degrade phytic acid in infant formulas based on soy and pea protein isolates (Davidsson et al., 1994 & 2001). However, adding to exogenous phytase in the baking of whole wheat bread has been less successful in reducing the molar ratio of phytate:iron is lower than one (Haros et al., 2001; Porres et al., 2001; Penella et al., 2008; Rosell et al., 2009). The reduction of sorghum phytate content by genetic modification in combination with preparation into a lactic fermented porridge resulted in a 90% lower phytate content, however, still with a phytate:iron molar ratio >1 (Kruger et al., 2012).
The present study aimed to degrade the phytate content in whole flour sorghum by activating the intrinsic phytase during soaking of the sorghum flour with and without the addition of wheat flour at different pH values, time and temperature. The soaked sorghum flour was then included as a component in the baking of composite wheat/cassava/sorghum bread with the assumption to achieve a phytate:iron molar ratio <1; thus resulting in a bread with improved iron absorption in humans.
Composite flours
The ingredients used for the composite flour bread were wheat (Triticum aestivum) flour (Frebago 1050 72% extraction rate, Sweden), cassava (Manihot esculenta Crantz) flour and non-tannin white whole sorghum (Sorghum bicolor L. Moench) flour from Inhambane province, in the districts Inharrime and Massinga, Mozambique. The cassava roots were peeled, washed, cut in pieces and sun-dried for 4 days. During the drying period, the cassava pieces were flipped from time to time to ensure no mould contamination and then milled, packed and stored. The sorghum grains were harvested, then picked or sorted after that the grains were washed, sun-dried for 2 days, milled to 100% extraction rate, and finally packed and stored.
Bread making procedure
The basic dough formula consisted of wheat flour (100 g), cassava flour (50 g), whole sorghum flour (50 g), sugar (4 g), salt (2 g), baking yeast (4 g) (Saccharomyces cerevisiae), margarine (6 g) and ascorbic acid (0.1 g).The composite bread was prepared by mixing the dry ingredients for 1 min in a kitchen aid (Artisan, Model 5KSM 150, USA). The rest of the ingredients and 135 mL of water (determined in pre-experiments to achieve a manageable dough consistency) was then added and blended for 2 min at speed level 2, and 3 min at speed level 4. The pH was adjusted by adding between 5 and 20 ml of a lactic acid solution (20%) at the mixing stage. The dough was then covered and left to ferment for 1 h at ambient temperature. After weighing, the dough was then divided in four roundshaped 80 g pieces and placed into baking pans to ferment for another 1 to 2 h and then baked as rolls for 8 min at 250°C in a kitchen oven. Each recipe was baked in triplicate. Dough samples were taken after the mixing and the fermentation step, weighed and placed into a flask tube and put into a freezer (-20°C), and then lyophilized for 3 days. Pre-treatment of the sorghum flour was done by soaking in 50 mL of water for 1 to 3 h with and without 10% wheat flour either at ambient temperature 22oC or 37°C, an optimal temperature for intrinsic sorghum and wheat phytase activity (Engelen et al., 2001; Shen et al., 2005).
Phytate analysis
The phytate content was determined using an HPIC method developed by Carlsson et al. (2001). A milled freeze-dried sample (dough and composite bread) of 0.5 g was extracted with 10 ml of 0.5 M HCl for 3 h at ambient temperature (22°C) under magnetic stirring. The extracts were frozen overnight, thawed and centrifuged at 12000 rpm corresponding to 13400 g for 5 min, and the supernatants were then decanted and 50 µl of supernatants injected and analyzed by HPIC with an Omni Pac PAX-100 (4 mm x 250 mm) analytical column and equipped with a PAX-100 (4 mm x 50 mm) guard-column (Dionex Corp., Sunnyvale, CA, USA). The inositol phosphates were detected and quantified after a post-column reaction with Fe(NO3)3x9H2O (Sigma–Aldrich Co, St. Louis, MO, USA), and the absorbance was monitored at 290 nm, using UV detection (Waters 486, tunable absorbance detector, Massachusetts, USA). All the reagents were of analytical grade (Sigma-Aldrich Co, St. Louis, MO, USA), and de-ionized water was used. The concentrations are presented on dry weight (DW) basis as the mean ± SD µmol/g.
Mineral analysis
Minerals (Fe, and Zn) were determined according to the method by Fredrikson et al. (2002). Approximately 250 mg of freeze-dried and ground sample was digested to a transparent solution with concentrated nitric and hydrochloric acid in Teflon vessels in an Ethos Plus microwave oven (model Multiwave PRO, Anton Paar Co., USA). After digestion, samples were diluted to 10 mL with de-ionized water and a volume of 50 µL was injected and analysed using ion chromatography coupled with UV – Vis detection at 500 nm. The mobile phase was composed of 1 g of pyridine-2.6-dicarboxylic acid (PDCA), 20 g of sodium acetate (C2H3NaO2)xH2O), 8.5 g of acetic acid (C2H4O2), and 0.2 g of ascorbic acid in 1 L of H2O, pH 4.3 ± 0.1. The post-column reaction solution comprised 240 mL of 25% ammonium (NH3 aq), 77 mg of 4-(2-pyridylazo) resorcinol (PAR) and 57 mL of acetic acid diluted to 1 L, pH 10.2.
Determination of dry matter
The dry matter content was determined using a moisture balance device 310M and a HA300 dryer (Precisa, Dietikon, Switzerland). Approximately, 0.5 g of food material was weighed in the vacuum oven device and heated to a temperature of 70°C under reduced pressure (900 mbar) until constant weight.
Determination of pH
The pH was measured using a Mettler Toledo MA 235 pH/Ion Analyzer. A 16 g dough piece was weighed and put into a flask tube containing a magnetic stirrer and then 8 g of water was added. The content was stirred for 5 min using an electromagnetic plate (Retsch, Rühra mag type Mo12 and no 33533). Finally, the pH was read using the pH-meter probe.
Statistical analysis
Results are expressed as mean values of duplicate analyses of at least 3 replications. Multiple sample comparison of the means and Fisher's least significances (LSD was applied to establish significant differences between treatments. All statistical analysis were carried out with SPSS version 15 software and significance was tested at 95%.
Phytic acid and mineral composition of the composite flours
The phytate, minerals and moisture content of the flours are shown in Table 1. The phytate concentration in the whole sorghum flour was higher (12.0 µmol/g) than in the white wheat flour (3.3 µmol/g) and cassava flour (2.4 µmol/g). The incorporation of 25% whole sorghum flour in the composite flour mixture resulted in a higher phytate content (5.26 µmol/g) as well as iron (12.1 µg/g) and zinc content (8.4 µg/g), compared with the corresponding values of 3.3 µmol/g, 7.7 µg/g, and 4.7 µg/g in the wheat flour. The phytate:mineral molar ratios in the composite flour ranged from 24 for iron to 41 for zinc. The moisture content of the flours ranged from 9.1% in the whole sorghum flour to 11.5% in the cassava flour.
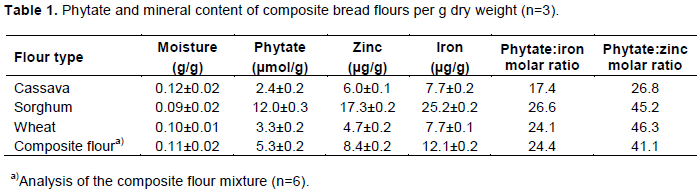
Effect of bread making process on the phytate content
During the bread-making process phytate content was significantly reduced, from the mixing stage to the final composite bread (Figure 1). The composite bread had a phytate mean value of 1.58 µmol/g with a phytate:iron molar ratio of 6.8. After the mixing stage, defined as the 5 min period with the mechanical blending of dry and wet ingredients, about 24% of the initial phytate content was degraded. A further reduction of about 60% was obtained after the fermentation step and reached a final reduction of about 70% in the composite bread. The pH during the fermentation stage varied between 6.2 and 6.0.
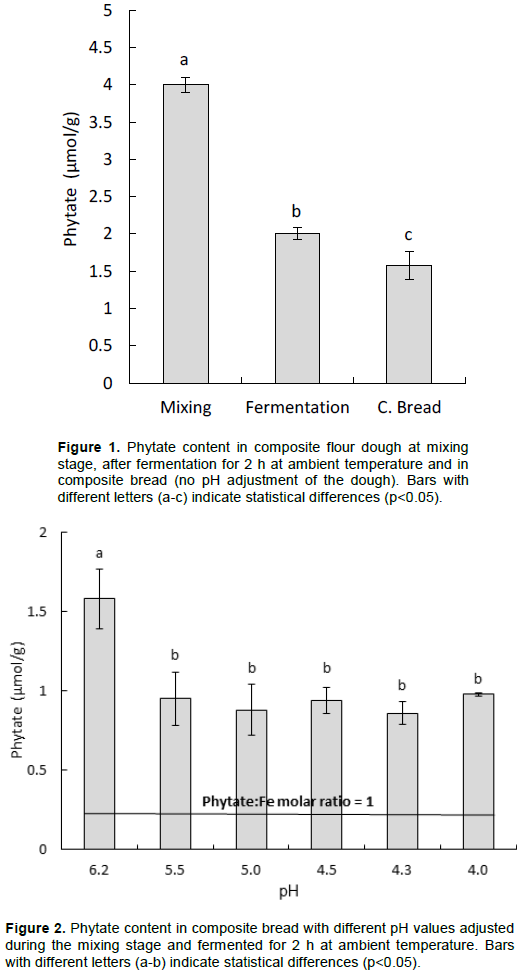
Effect of pH adjustment
Figure 2 shows the phytate content in composite bread with the dough adjusted to different pH values during the mixing stage. The highest value, 1.58 µmol/g, was obtained in the composite bread without pH adjustment of the dough (pH 6.2), that was significantly higher (p<0.05) than the values in the composite breads with pH adjusted to between 4 and 5.5. These values were between 0.86 – 0.98 µmol/g and not significantly different from each other. However, the composite bread with the lowest phytate content still had a phytate:iron molar ratio >1 (Figure 2).
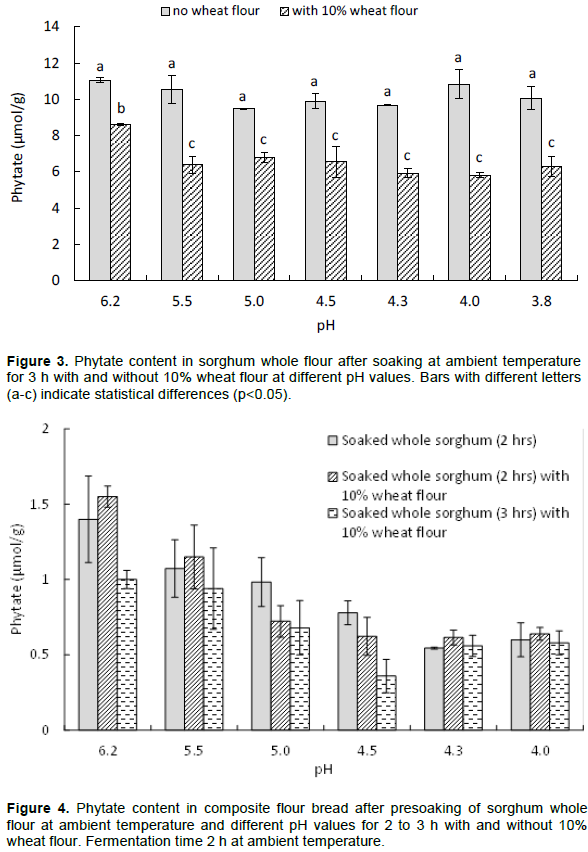
Effect of soaking the whole sorghum flour
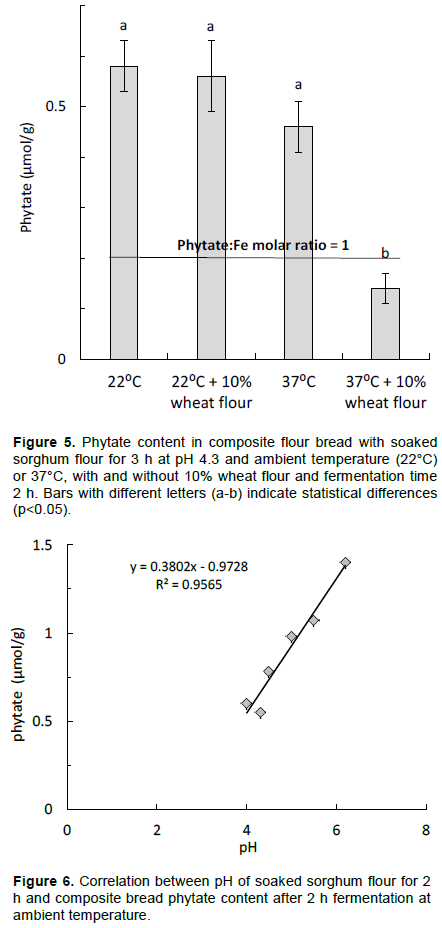
To reduce the phytate content of the sorghum flour before the mixing of the composite flour, the whole sorghum flour was soaked with and without 10% wheat flour for 3 h and at pH values ranging from 6.2 to 3.8. Figure 3 shows that by adding wheat flour in the soaking process a significantly higher reduction took place and an optimal effect was obtained at pH 4.3 to 4.0. The phytate content was reduced by about 50% with wheat flour added while only about 10% phytate reduction was obtained without added wheat flour. However, the phytate content in the composite bread baked with soaked sorghum flour with and without wheat flour (2 to 3 h) at pH 4.3 was similar, about 0.57 µmol/g (Figure 4). However a significantly (p<0.05) lower phytate content, 0.36 µmol/g, was obtained in the composite bread with soaked sorghum and wheat flour for 3 h at pH 4.5.
To further improve the phytate degradation, the soaking temperature was increased to 37°C at pH 4.3. Figure 5 shows a significant effect of increasing the soaking temperature and especially when soaking whole sorghum flour with 10% wheat flour. Phytate content of 0.14 µmol/g was obtained in the composite bread, which means that about 97% of the initial phytate content was degraded. The phytate:iron molar ratio was then as low as 0.70 and the phytate:zinc molar ratio was 1.1. Figure 6 shows the high correlation between phytate content in the composite bread and the pH-values of presoaked sorghum (for 2 h) before mixing into the dough and fermentation for 2 h at ambient temperature.
Phytic acid and minerals in the composite flours
Due to the inclusion of the bran fraction in the whole sorghum flour, the phytate content was high (11.99 µmol/g) as well as the mineral content. Similar findings in whole cereal products have been reported by others (Egli et al., 2002; Kruger et al., 2012). The phytate content of the wheat flour and the cassava flour was at the same level as that reported by Lazarte et al. (2015) as well as the iron content of the cassava flour. The wheat iron content was low and expected, as the wheat flour was not fortified.
Phytate degradation during bread making
The results from the present study indicate that the fermentation stage was the most important in bread phytate degradation and is in line with other studies (Qazi et al., 2003; Frontela et al., 2011). However, as much as 24% of the initial phytate content was degraded after the 5 min mixing stage and a similar effect was obtained by Buddrick et al. (2014) who showed a 45% phytate reduction after 10 min mixing of a whole flour wheat dough. The short time for the mixing stage (5 min) was obviously enough to release some of the intrinsic phytases from the cereal flour ingredients to be able to degrade a minor part of the phytate content although the pH was not optimal for the phytase activity. The reason for the lower degradation of phytate in the final composite bread without pH adjustment can be explained by the high pH of the dough, about 6.0, a pH that will not favour phytase activity, neither in the wheat flour nor the sorghum flour. The optimum pH for wheat phytase has earlier been reported to be about 5.0 (Türk et al., 1996).
Effect of pH and temperature on composite bread phytate
The lowest phytate value for composite bread adjusted to pH 4.3 at the mixing stage was 0.85 µmol/g, most likely as a result of having a pH for optimal activity of both wheat and sorghum flour phytases (Azeke et al., 2011). The pH optimum for sorghum phytases has not been reported; however, most plant phytases belong to the acidic phytases with a pH optimum in the range of 6.0 to 4.4 (Brinch-Pedersen et al., 2014). Figure 4 shows that the lowest phytate content is obtained in the composite bread at low pH and long soaking time, both with and without 10% wheat flour. However, the results imply that there is a need for further phytate degradation, as the phytate:iron molar ratio is still greater than one. Presoaking of whole sorghum flour with 10% wheat flour for 3 h resulted in a significant reduction (p<0.05) of the phytate content (Figure 3). The addition of 10% wheat flour was expected to increase the degradation of the phytate content in the whole sorghum flour as the phytase in wheat flour has been shown to have an optimal phytase activity around pH 5 (Egli et al., 2003).
Optimization of intrinsic sorghum phytase
Based on the results in this study, the pH during the soaking process had a major influence on phytate degradation (p<0.05), while the soaking time had only a minor influence. The best phytate degradation was obtained in the range of pH 5.0-4.0. Besides, the results obtained from soaking whole sorghum flour plus 10% wheat flour showed that there might be a combined effect of wheat and sorghum phytases. However, the effect of sorghum phytase is probably dominant due to the high amount of sorghum flour in the soaking process. The use of 10% wheat flour and increased soaking temperature gave the same results to that reported by Koréissi-Dembéle et al. (2013) who used whole wheat flour to increase the intrinsic phytase activity in preparation of whole millet flour (fonio) porridge. Reale et al. (2007) reported a reduction of phytate content during fermentation of whole wheat flour that was explained by an activation of the intrinsic cereal phytase as a consequence of a decreased pH during fermentation. They further incubated the wheat flour at different pH and found an optimum phytate degradation within the pH range of 4.0 to 5.5 and similar findings were reported by Leenhardt et al. (2005). Egli et al. (2003) succeeded to completely degrade the phytate content in a wheat-soy flour mixture after incubation in water for 2 h at pH 5.1 and 50°C and addition of whole wheat flour as the phytase source. The phytase activity of whole wheat flour has been measured to be about 30 times higher than in whole sorghum flour (Egli et al., 2002). Soaking of non-tannin sorghum flour for 1 h at around pH 6.0 resulted in about 25% reduction of the phytate content as a result of phytate degradation and phytate solubilized in the soaking water (Kruger et al., 2014). Also, Fretzdorff and Brümmer (1992) obtained optimal phytate degradation in whole wheat flour bread with a pH between 4.3 and 4.6 and a fermentation temperature of 30oC. These results support our findings of phytate:iron molar ratio less than 1 after increasing the incubation temperature to 37oC and pH adjusted to 4.3. The effect of this optimization resulted in an improved phytate degradation to obtain a phytate:iron molar ratio of 0.70, a ratio considered to have a positive impact on the iron absorption in humans (Hurrell, 2004). Koréissi-Dembéle et al. (2013) showed an increased iron absorption in women (3.2 times) from a millet flour porridge with a reduced phytate:iron molar ratio from 1.9 to 0.3 when whole wheat flour was added as a phytase source.
One important advantage of using the water intended for the dough preparation is that any leakage of minerals to the soaking media will not be discarded as the soaked flour will be prepared into dough after addition of the cassava flour and the rest of the baking ingredients. Traditional soaking processes of whole cereal grains and cereal flours usually includes a subsequent removal of the soaking water by decanting thereby resulting in losses of soluble phytate and minerals (Hotz and Gibson, 2001). Up to 39% of the phytate was solubilized after 1 h soaking of milled whole grain white sorghum and 57% in maize flour (Kruger et al., 2014). The pH of the soaking water was in the range of 6.2 to 6.7 so any degradation of the phytate content due to the activity of intrinsic phytase is not likely. Any losses of minerals into the soaking water was only observed for zinc (17%). However, the iron content was significantly reduced (~50%) in the soaking process (9 hrs) of red sorghum for beer production and the phytate content by ~20% explained by leaching into the soaking medium (Kayodé et al., 2007). In a village-based study in Malawi, the phytate reduction in soaked pounded maize flour was about 40% after soaking for 1 h and decanting the soaking water (Hotz et al., 2001).
Soaking whole sorghum flour at optimal pH for intrinsic phytase activity prior to baking of composite flour bread resulted in enhanced phytate degradation. The composite bread with pH 4.3 adjusted at mixing stage had the lowest phytate content of 0.85 µmol/g. The inclusion of 10% wheat flour positively affected the phytate degradation. The combined effect of soaking whole sorghum flour at optimal pH 4.3 and at higher temperature further reduced the residual phytate content to a molar ratio of phytate to iron to be less than one and a molar ration of phytate to zinc about one; thus resulting in a composite flour bread with expected improved bioavailability of these two minerals.
Degradation of phytic acid in whole flour cereals has important implications for human health, as phytate binds minerals especially iron and zinc making them unavailable for absorption in humans. In low-income countries, cereal-based diets are related to a high prevalence of iron deficiency, especially in preschool children and women. Soaking of cereals at optimal conditions to activate intrinsic phytase prior to bread making has the potential to decrease the phytate content to levels that would increase mineral bioavailability.
The authors have not declared any conflict of interests.
REFERENCES
|
Akubor PI, Badifu GIO (2004). Chemical composition, functional properties and baking potential of African breadfruit kernel and wheat flour blends. International Journal of Food Science and Technology 39(2):223-229.
Crossref
|
|
|
|
Azeke MA, Egielewa SJ, Eigbogbo MU, Ihimire IG (2011). Effect of germination on the phytase activity, phytate and total phosphorus contents of rice (Oryza sativa), maize (Zea mays), millet (Panicum miliaceum), sorghum (Sorghum bicolor) and wheat (Triticum aestivum). Journal of Food Science and Technology 48(6):724-729.
Crossref
|
|
|
|
|
Brinch-Pedersen H, Madsen CK, Holme IB, Dionisio G (2014). Increased understanding of the cereal phytase complement for better mineral bioavailability and resource management. Journal of Cereal Science 59(3):371-381.
Crossref
|
|
|
|
|
Buddrick O, Jones OAH, Cornell HJ, Small DM (2014) The influence of fermentation processes and cereal grains in wholegrain bread on reducing phytate content. Journal of Cereal Science 59(1):3-8.
Crossref
|
|
|
|
|
Carlsson NG, Bergman EL, Skoglund E, Hasselblad K, Sandberg AS (2001). Rapid Analysis of Inositol phosphate. Journal of Agricultural and Food Chemistry 49(4):1695-1701.
Crossref
|
|
|
|
|
Davidsson L, Galan P, Kastenmayer P, Cherouvrier F, Juillerat MA, Hercberg S, Hurrel RF (1994). Iron bioavailability studied in infants - the influence of phytic acid and ascorbic acid in infant formulas based on soy isolate. Pediatric Research 36(6):816-822.
Crossref
|
|
|
|
|
Davidsson L, Dimitriou T, Walczyk T, Hurrell RF (2001). Iron absorption from experimental infant formulas based on pea (Pisum sativum)-protein isolates: the effect of phytic acid and ascorbic acid. British Journal of Nutrition 85(1):59-63.
Crossref
|
|
|
|
|
Egli I, Davidsson L, Juillerat MA, Barclay D, Hurrell RF (2002). The influence of soaking and germination on the phytase activity and phytic acid content of grains and seeds potentially useful for complementary feeding. Journal of Food Science 67(9):3484-3488.
Crossref
|
|
|
|
|
Egli I, Davidsson L, Juillerat MA, Barclay D, Hurrell RF (2003). Phytic acid degradation in complementary foods using phytase naturally occurring in whole grains cereals. Journal of Food Science 68(5):1855-1859.
Crossref
|
|
|
|
|
Engelen JA, van der Heeft FC, Randsdorp PHG, Somers WAC, Schaefer J, van der Vat BJC (2001). Determination of phytase activity in feed by a colorimetric enzymatic method: Collaborative interlaboratory study. Journal of AOAC International 84(3):629-633.
Crossref
|
|
|
|
|
FAOStat (2018). Food and Agriculture Organization of the United Nations. Available at:
View
|
|
|
|
|
Fredrikson M, Carlsson NG, Almgren A, Sandberg AS (2002). Simultaneous and Sensitive Analysis of Cu, Ni, Zn, Co, Mn, and Fe in Food and Biological Samples by Ion Chromatography. Journal of Agricultural and Food Chemistry 50(1):59-65.
Crossref
|
|
|
|
|
Frontela C, Ros G, Martinez C (2011). Phytic acid content and "in vitro" iron, calcium and zinc bioavailability in bakery products: The effect of processing. Journal of Cereal Science 54(1):173-179.
Crossref
|
|
|
|
|
Fretzdorff B, Brümmer JM (1992). Reduction of phytic acid during breadmaking of whole-meal bread. Cereal Chemistry 69(3):266-270.
|
|
|
|
|
Hallberg L, Brune M, Rossander L (1989). Iron absorption in man: ascorbic acid and dose-dependent inhibition by phytate. American Journal of Clinical Nutrition 49(1):140-144.
Crossref
|
|
|
|
|
Haros M, Rosell CM, Benedito C (2001). Fungal phytase as a potential bread-making additive. European Food Research and Technology 213(4-5):317-322.
Crossref
|
|
|
|
|
Hotz C, Gibson RS (2001). Assessment of home-based processing methods to reduce the phytate content and phytate/zinc molar ratio of white maize (Zea mays). Journal of Agricultural and Food Chemistry 49(2):692-698.
Crossref
|
|
|
|
|
Hotz C, Gibson RS, Temple L (2001). A home-based method to reduce phytate content and increase zinc bioavailability in maize-based complementary diets. International Journal of Food Sciences and Nutrition 52(2):133-142.
Crossref
|
|
|
|
|
Hunt JR (2003). Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. American Journal of Clinical Nutrition 78(3):633S-639S.
Crossref
|
|
|
|
|
Hurrell RF, Reddy, MB, Burri J, Cook JD (2002). Phytate degradation determines the effect of industrial processing and home cooking on iron absorption from cereal-based foods. British Journal of Nutrition 88(2):117-123.
Crossref
|
|
|
|
|
Hurrell RF (2004). Phytic acid degradation as a means of improving iron absorption. International Journal for Vitamin and Nutrition Research 74:445-452.
Crossref
|
|
|
|
|
Kayodé APP, Hounhouigan JD, Nout MJR (2007). Impact of brewing process operations on phytate, phenolic compounds and in vitro solubility of iron and zinc in opaque sorghum beer. LWT-Food Science and Technology 40(5):834-841.
Crossref
|
|
|
|
|
Koréissi-Dembéle Y, Fanou-Fogny N, Moretti D, Schuth S, Dossa RAM, Egli I, Zimmermann MB, Brouwer ID (2013). Dephytinisation with intrinsic wheat phytase and iron fortification significantly increase iron absorption from fonio (Digitaria exilis) meals in West African women. Plos One 8(10):e70613.
Crossref
|
|
|
|
|
Kruger J, Taylor JRN, Oelofse A (2012). Effects of reducing phytate content in sorghum through genetic modification and fermentation on in vitro iron availability in whole grain porridges. Food Chemistry 131(1):220-224.
Crossref
|
|
|
|
|
Kruger J, Oelofse A, Taylor JRN (2014). Effects of aqueous soaking on the phytate and mineral contents and phytate:mineral ratios of wholegrain normal sorghum and maize and low phytate sorghum. International Journal of Food Sciences and Nutrition 65(5):539-546.
Crossref
|
|
|
|
|
Lazarte CE, Carlsson NG, Almgren A, Sandberg AS, Granfeldt Y (2015). Phytate, zinc, iron and calcium content of common Bolivian food, and implications for mineral bioavailability. Journal of Food Composition and Analysis 39:111-119.
Crossref
|
|
|
|
|
Leenhardt F, Levrat-Verny MA, Chanliaud E, Remesy C (2005). Moderate decrease of pH by sourdough fermentation is sufficient to reduce phytate content of whole wheat flour through endogenous phytase activity. Journal of Agricultural and Food Chemistry 53(1):98-102.
Crossref
|
|
|
|
|
Nävert B, Sandström B, Cederblad A (1985). Reduction of the phytate content of bran by leavening in bread and its effect on zinc absorption in man. British Journal of Nutrition 53(1):47-53.
Crossref
|
|
|
|
|
Penella JMS, Collar C, Haros M (2008). Effect of Wheat bran and enzyme addition on dough functional performance and phytic acid levels in bread. Journal of Cereal Science 48(3):715-721.
Crossref
|
|
|
|
|
Porres JM, Etcheverry P, Miller DD, Lei XG (2001). Phytase and citric acid supplementation in whole - wheat bread improves phytate - phosphorus release and iron dialyzability. Journal of Food Science 66(4):614-619.
Crossref
|
|
|
|
|
Qazi IM, Wahab S, Shad AA, Zeb A, Ayuab M (2003). Effect of different fermentation time and baking on phytic acid content of whole-wheat flour bread. Asian Journal of Plant Sciences 2(8):597-601.
Crossref
|
|
|
|
|
Reale A, Konietzny U, Coppola R, Sorrentino E, Greiner R (2007). The importance of lactic acid bacteria for phytate degradation during cereal dough fermentation. Journal of Agricultural and Food Chemistry 55:2993-2997.
Crossref
|
|
|
|
|
Rosell CM, Santos E, Sanz-Penella JM, Haros M (2009). Wholemeal wheat bread: A comparison of different breadmaking processes and fungal addition. Journal of Cereal Science 50(2):272-277.
Crossref
|
|
|
|
|
Shen Y, Yin Y, Chavez ER, Fan MZ (2005). Methodological aspects of measuring phytase activity and phytate phosphorus content in selected cereal grains and digest of faeces of pigs. Journal of Agricultural and Food Chemistry 53(4):853-859.
Crossref
|
|
|
|
|
Tatala S, Svanberg U, Mduma B (1998). Low dietary iron availability is a major cause of anemia: a nutrition survey in the Lindi District of Tanzania. American Journal of Clinical Nutrition 68(1):171-178.
Crossref
|
|
|
|
|
Taylor PG, Méndez-Castellanos H, Martínez-Torres C, Jaffe W, López de Blanco M, Landaeta-Jiménez M, Leets I, Tropper E, Ramírez J, Casal MG, Layrisse M (1995). Iron bioavailability from diets consumed by different socioeconomic strata of the Venezuelan population. Journal of Nutrition 125(7):1860-1868.
Crossref
|
|
|
|
|
Türk M, Carlsson NG, Sandberg AS (1996). Reduction in the levels of phytate during wholemeal bread making; Effect of yeast and wheat phytases. Journal of Cereal Science 23(3):257-264.
Crossref
|
|