ABSTRACT
Food preservation is a big challenge for the industries. The mechanisms of preservation involved microorganisms such as lactic acid bacteria. Modeling the micro-organisms growth is a very useful information to well-understand their behavior and to propose the best conditions for food preservation. In this way, this study was to propose a mathematical model of the dynamical growth of Lactococcus lactis CWBI-B1410 which play a main role for the bio-conservation of fish. This study is conducted by varying the concentration of glucose and nitrogenized matter. The results showed that the mathematical model predicted the growth of L. lactis CWBI-B1410. The curves that are predicted are exactly those that can be observed experimentally. The results showed that the production of biomass by L. lactis CWBI-B1410 is obtained on the MRS medium composed of 15 g of glucose per liter and 40 g of nitrogenous material per liter. From the obtained results, it could be clearly advocated that for two tests with the same amount of nitrogenous matter, it is possible to achieve economies of scale in glucose while maintaining the same biomass performance.
Key words: Mathematical models, lactic bacteria, Lactococcus lactis CWBI-B1410, glucose, nitrogenous matter.
Quality control and microbiological safety of fish products are a permanent challenge for fishery industry. They always consider fish products degradation due to conservation issues (Fabinyi et al., 2017). Fish conservation is very sensitive to environmental conditions that can increase the risk to undesirable micro-organisms growth under certain conditions and then constitute a threat for public health. This system for food production and distribution in fishery industries has to ensure that undesirable microorganisms growth is controlled. Then, understanding the conditions leading to micro- organisms growth is important (Nikolaev, 2010). Different optimization approaches to determining appropriate levels of metabolic enzymes have been used in a number of purely simulation-based studies for various aspects of microbial metabolism, including the production of serine (Nikolaev, 2010), tryptophan (Chen and Zeng, 2017), and bacteriocin produced by a strain of Streptococcus gallolyticus (Abdu et al., 2020). The simultaneous production of serine and tryptophan in E. coli has also been considered using a multi-objective optimization strategy (Lee et al., 2010). According to Alfredo et al. (2020), silage technology, with the use of lactic acid bacteria, could help extend the shelf-life of orange residue by up to 10 days by keeping it fresh. In order to describe the behavior of microorganisms in foods and particularly their growth based on key ecological food characteristics, many mathematical models have been proposed in the literature during the recent years (Alfredo et al., 2020). Predictive microbiology based on mathematical models is very useful and it helps to understand microbial hazards in a food chain. It has been showed that it is relatively easy and inexpensive to assess and to validate theoretical models that can be used to predict and to quantify the effects of experimental conditions on microorganism growth (Delhalle et al., 2012). These modeling activities result to link the growth parameters to the environmental factors through mathematical equations (Maoura et al., 2006). Comparing the adjustment of primary models on laboratory data has been the subject of several studies (Pal et al., 2008). Currently, it is not possible to select a particular model to represent the most appropriate bacterial growth. The simplest models can often be sufficient to properly represent the basic parameters of growth. The first growth model in predictive microbiology described by Buchanan (1918) described the exponential phase, but it does not take into account the latent phase or the stationary phase. However, Buchanan’s model can be used in a first approach to assess the evolution bacteria population and growth rate. An alternative exponential model has been proposed by Brillet et al. (2016). This exponential model takes into account the reaction time and the deceleration phase. Delhalle et al. (2012) described the exponential growth phase which appeared in the form of a linear portion when the evolution of microbial population is represented by the time. For a given bacterium, the value of this maximum growth rate depended on the characteristics of the medium culture (Kyu et al., 2009). When the microbial concentrations are expressed in semi logarithmic mathematical form the maximum specific growth rate can be determined by calculating (Fahimi, 2012). Senegal is known today for its high level of production of sea products (Diop et al., 2008). However, their fishery industry is not efficient due to the weak conservation methods (Diei-Ouadi, 2005). The insufficient safety and toxic substances affected the organoleptic quality and health products reducing the fish production (Diop et al., 2008). Through their work’s, Diop et al. (2008) developed a bio-conservation method for fishery industry by using a strain of L. lactis CWBI-B1410 isolated from cereals in Senegal. This important discover is very important for fishery industry to increase their productivity by improving the conservation process. However, the biological characteristic of L. lactis CWBI-B1410 expressed into mathematical growth model is still not available in the literature. Then, the main objective of this study was to develop a mathematical model to predict the growth of the strain of lactic acid bacterium L. lactis CWBI-B1410 in two components of MRS culture medium, glucose and nitrogenous matter.
This study is conducted in the Analysis and Testing Laboratory at École Supérieure Polytechnique de Dakar (University Cheikh Anta Diop), Senegal.
Materials
Two types of substrates were used: glucose and nitrogenous matter. The nitrogenized matter was provided by the culture medium composed of casein peptone (Sharlau, Spain).
The laboratory equipment’s were glassware oven at 30°C, Petri dishes, a spectrophotometer Helios TM Gamma UV-VIS, Thermo Fisher Scientific, (UVD 134502, England).
Preparation of the culture medium
The culture medium used in this work was the MRS agar (Man Rogosa Sharpe) with different concentrations of glucose and nitrogenous matter. The pH of the culture medium was adjusted to 6.6 by adding hydrochloric acid (1 N) in order to obtain an optimal pH for growth of L. lactis CWBI-B1410. The medium was stored into tubes slants (10 ml/tube). These tubes containing the medium were put in a autoclave for 15 min at 120°C.
Preparation of the inoculum
The strain used in this study was L. lactis CWBI-B1410. It is a strain of the laboratory collection in Belgium (the Bio-industries Wallonia Center of Industrial Biology). It was revived by two subcultures Man Rogosa Sharpe liquid medium and stored at a temperature of 4°C in conservation tubes containing MRS agar substrate.
The re-activation of the strain was done by extracting some colonies from these subcultures and transferred into 10 ml tube containing MRS medium liquid. This tube was then incubated at 30°C during 24 h to get our pre-culture.
After 24 h of incubation, 1 ml of this pre-culture was introduced into an Erlenmeyer flask with a capacity of 250 ml containing 100 ml of MRS liquid medium and incubated at 30°C for 40 hours under constant mixing condition by agitation. The all septical conditions were respected to avoid any external contamination.
Determination methods
The total biomass concentration of L. lactis CWBI-B1410 is determined by measuring the optical density (OD) by using a spectrophotometer Helios TM Gamma UV-VIS at 620 nm.
Dieng et al. (2013) showed that the best growth of L. lactis CWBI-B1410 are obtained with combinations of different concentrations of glucose and nitrogenized substrate as shown in Table 1.
These associations of glucose (G) and nitrogenous matter (NM) were then tested and mathematical models were proposed by determining the equation for each association. These mathematical models allowed a variation of the biomass according to the quantity of glucose and the nitrogenous matter.
The evolution of the biomass concentration was evaluated by measuring the optical density at 620 nm as a function of time. The data was analyzed with the help of the Excel software. To understand biological phenomena more precisely, the equation parameters have been identified after many simulations in excel to get the best results.
The effect of different combinations of glucose and nitrogenous matter on the growth of L. lactis CWBI-B1410 was evaluated and to study if the concentrations of glucose and nitrogenous material vary, number of bacteria varies from one association (Table 1) to another.
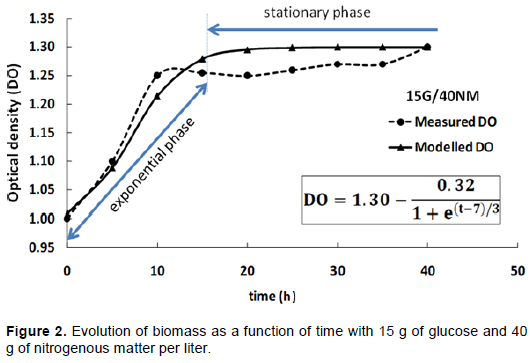
The biomass measurements performed showed very similar trends for different situations. Figure 1 clearly shows a startup culture at the fifth hour. The four different biomass obtained have almost super imposed curves. It was also noted that the MRS media with a concentration of 40 g/ l crude protein have a higher biomass compared with the media that contain 20 g / l. At the 10th hour, the curves corresponding to associations 15 G / 20 N.M. and 20 G / 20 N.M. had OD600nm close to 1.2 while the curves corresponding to 15 g/40 N.M. and 20 G / 40 N.M., which look better, have an OD600nm of about 1.30 despite the fact that all cultures are seeded with cells in the same physiological state and at the same time. Moreover, from the 10th hour of culture, bacteria L. lactis CWBI-B1410 seemed to start the stationary phase for all tests. At the 40th hour, Figure 1 shows that the best growth is presented by the MRS medium 15G/40 N.M. followed by MRS medium 20 G/40 N.M. reaching respectively an OD600nm value of 1.32 and 1.30.
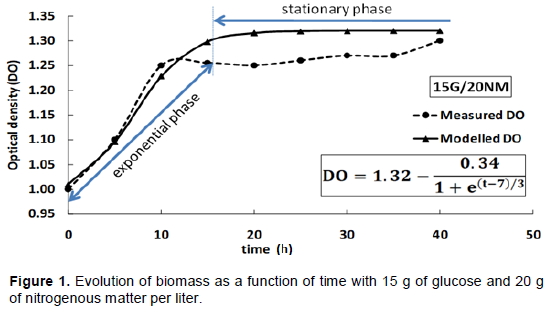
These findings are consistent with those of Yaov et al. (2019), which validated a mathematical approach using different experiments with Escherichia coli. The approach in this study provided an effective way to predict growth of L. lactis CWBI B-1410 by the variation of glucose and nitrogen. The results also shows that for two tests with the same quantity of nitrogenous matter (Figure 1), it is possible to achieve economies of glucose while maintaining the same performance biomass (Figures 2, 3 and 4),. Mataragas et al. (2003) showed that continuous presence of carbon in the MRS medium during culture is necessary but the amount of glucose is not proportional to the amount of biomass in the culture medium. Moreover, increasing the amount of glucose does not necessarily produce a high level of bacteriocin
(Abbasiliasi et al., 2017). The study showed that the variation in the amount of glucose and nitrogen influenced the growth of L. lactis. This is consistent with the results of Thiago et al. (2019), which showed that the biosynthesis of bacteriocins can be influenced by various culture conditions, such as the composition of the medium, pH, temperature and growth kinetics of the microorganisms.
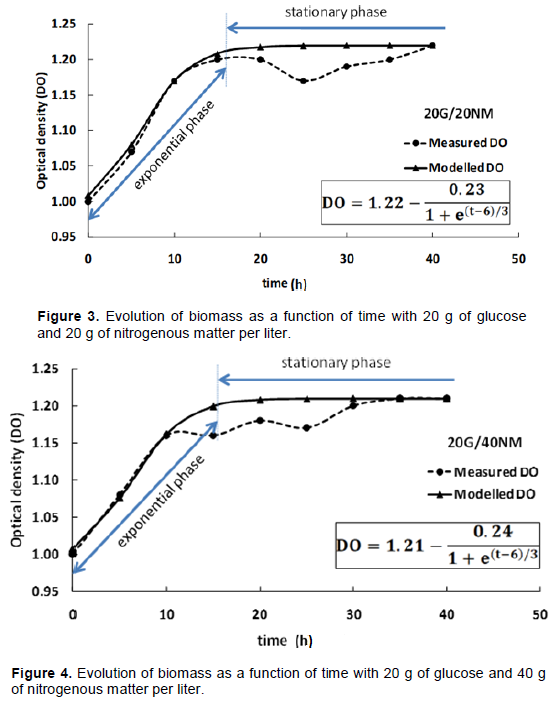
These results are in agreement with the work of Pemmaraju et al. (2016) who showed that Candida albicans formed more biofilms in the presence of an important carbon source. Moryl et al. (2013) have been shown that the composition and quantity of the polysaccharide matrix of a biofilm depend on a carbon source such as glucose. According to studies, stressors (salt, oxygen, microbial competition, etc.) can positively influence the production of bacteriocin in lactic acid bacteria grown in MRS broth (Neysens and DE-Vuyst, 2005). The kinetics of growth and the increase in nitrogenous matter present a best fit by a single function called Boltzmann function with the correlation coefficient of R2≥0.97. This value expresses a little error between reality and experimentation. According to the results, it was concluded that there was biomass production by L. lactis CWBI-B1410 on the MRS 15 G / 40 N.M.
The main objective of this study was to develop a mathematical model able to predict the evolution of a strain of lactic acid bacterium Lactococcus lactis CWBI- B1410 by varying two components of MRS culture medium, glucose and nitrogenous substrate. It was found, according to the type of MRS medium used (standard MRS and modified MRS), from the 1st to 6th hour of the tests showed that the average number of bacteria varies considerably from one medium to another. The application of negative binomial regression showed that the model is globally significant. The model shows a significant statistical difference between the standard MRS medium and the modified MRS medium. This approach has also stimulated the interest of studying a mathematical model for modeling the growth dynamics L. lactis CWBI-B1410. To increase the interest of such a model, the effect of pH conditions should be integrated. In future work, the significance of the effect of inoculum size on the length of the lag phase will be also assessed. Finally, for economic reasons, it is important to consider the use of another less expensive source of nitrogenous material such as certain wastes from the food industry as constituents of culture media for the reformulation of the MRS medium in the production of bacteriocins.
The authors have not declared any conflict of interests.
REFERENCES
|
Abbasiliasi S, Tan JS, Tengku ITA, Bashokouh F, Ramakrishnan NR, Mustafa S, Ariff AB (2017). Fermentation factors influencing the production of bacteriocins by lactic acid bacteria. Royal Society of Chemistry 7:29395-29420.
Crossref
|
|
|
|
Abdu A, Arif F, Vikram S, Mathew U (2020). Purification and characterization of nisin produced by a strain of Streptococcus gallolyticus. Journal of Medical Microbiology 69:605-616.
Crossref
|
|
|
|
|
Alfredo IGG, Janeth VS, Carolina FG, Mónica CG, Liliana LH, Cristina RDT, German B, Daniel BV, Miguel AAG, Raúl RH, CristÓbal NA (2020). Ensiling as bioprocess for bioconservation of citrus peels. Microbial Services in Restoration Ecology 20:297-314.
Crossref
|
|
|
|
|
Brillet VA, Pilet MF, Courcoux PPH, Leroi F (2016). Optimization of growth and bacteriocin activity of the food bioprotective Carnobacterium divergens V41 in an animal origin protein free medium. Frontiers Marine Science 3:128.
Crossref
|
|
|
|
|
Buchanan RE (1918). Life phases in a bacterial culture. Journal of Infectious Diseases 23:109-125.
Crossref
|
|
|
|
|
Chen L, Zeng A (2017). Rational design and metabolic analysis of Escherichia coli for effective production of L-tryptophan at high concentration. Applied Microbiology and Biotechnology 101:559-568.
Crossref
|
|
|
|
|
Delhalle L, Daube G, Adolphe Y, Crevecoeur S, Clinquart A (2012). Les modèles de croissance en microbiologie prévisionnelle pour la maitrise de la sécurité des aliments. Biotechnology Agronomy Society Environment 16(3):369-381.
|
|
|
|
|
Diei-Ouadi Y (2005). Minced sardinella fillets in fish landing and marketing sites in Senegal. In: FAO fisheries Circular no 999: FIIU/C999 (EN). Roma: FAO.
|
|
|
|
|
Dieng M, Mbengue M, Kone PS, Ndiaye K, Tine E, Balde M, Kane NCT (2013). Bioconservation des filets de sole limande frais (Syacium guineensis) au Sénégal par utilisation de la souche de Lactococcus lactis CWBI-B1410. Revue Africaine de Santé et de Production Animale 11(1):31-36.
|
|
|
|
|
Diop MB, Dubois DR, Dortu C, Destain J, Tine E, Thonart P (2008). In vitro detection and characterization of bacteriocin-like inhibitory activity of lactic acid bacteria (LAB) isolated from Senegalese traditional food products. African Journal of Microbiology Research 2:206-216.
|
|
|
|
|
Fabinyi M, Dressler WH, Pido MD (2017). Fish, trade and food security: Moving beyond 'availability' Discourse in Marine Conservation. Human Ecology 45:177-188.
Crossref
|
|
|
|
|
Fahimi N (2012). Study of the interactions between oenological lactic bacteria Enococcus oeni. Kinetic analyzes and modeling. Doctoral thesis. University of Toulouse.
|
|
|
|
|
Kyu J, Chae MJ, Choi JW, Lee KYK (2009). Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresources Technology 100(14):3518-3525.
Crossref
|
|
|
|
|
Lee FC, Pandu RG, Lee DY (2010). Modeling and optimization of a multiproduct biosynthesis factory for multiple objectives. Metabolic Engineering 12:251-267.
Crossref
|
|
|
|
|
Maoura N, Mbaiguinam M, Gaillardin C, Pourquie J (2006). Suivi technique, analytique et microbiologique de la «bilibili», bière traditionnelle tchadienne». Afrique Science 02(1):69-82.
|
|
|
|
|
Mataragas M, Metaxopolous J, Galiotou M, Drosinos EH (2003). Influence of pH and temperature on growth and bacteriocin production by Leuconostoc mesenteroides L124 and Lactobacillus curvatus L442. Meat Science 64:265-271.
Crossref
|
|
|
|
|
Moryl M, Torzewska A, JaÅ‚mużna P, Różalski A (2013). Analysis of Proteus mirabilis distribution in multi-species biofilms on urinary catheters and determination of bacteria resistance to antimicrobial agents. Journal Microbiology 62:377-384.
Crossref
|
|
|
|
|
Neysens P, DE Vuyst L (2005). Carbon dioxide stimulates the production of amylovorin L by Lactobacillus amylovorus DCE 471, while enhanced aeration causes biphasic kinetics of growth and bacteriocin production. International Journal of Food Microbiology 105:191-202.
Crossref
|
|
|
|
|
Nikolaev EV (2010). The elucidation of metabolic pathways and their improvements using stable optimization of large-scale kinetic models of cellular systems. Metabolic Engineering 12:26-38.
Crossref
|
|
|
|
|
Pal A, Labuza TP, Diez-Gonzalez F (2008). Comparison of primary predictive models to study the growth of Listeria monocytogenes at low temperatures in liquid cultures and selection of fastest growing ribotypes in meat and turkey product slurries. Food Microbiology 25(3):460-470.
Crossref
|
|
|
|
|
Pemmaraju SC, Parul A, Pruthi R (2016). Modulation of Candida albicans biofilm by different carbon sources. Mycopathologia 181:341-352.
Crossref
|
|
|
|
|
Thiago S, Adriano B, Sávio LB, Carolina KS, Lisiane FC (2019). Physical and nutritional conditions for optimized production of bacteriocins by lactic acid bacteria - A review. Critical Reviews in Food Science and Nutrition 59(17):2839-2849.
Crossref
|
|
|
|
|
Yaov R, Eynat DG, Maayan B, Kedar K, Uri O, Marcus WF, Tim FC, Judith B, Lilach H (2019). Predicting microbial growth in a mixed culture from growth curve data. Proceedings of the National Academy of Sciences 116(29):14698-14707.
Crossref
|
|