Full Length Research Paper
ABSTRACT
Two traditional treatments for shea (Vitellaria paradoxa) butter processing namely storage of fresh nuts and duration of subsequent boiling were studied by using the response surface methodology (RSM) to determine best treatment. Experimental treatments influenced several kernel parameters, such as fat content (38-52% dw), redness (a* values between 6.3 and 11.7), and butter parameters, viz. yield (24 to 36% wet weight of kernel mass), brightness (L* values between 70-80), yellowness (b* values between 16-23), and free fatty acid (FFA) percentage (0.5-2%). On the other hand, the moisture content (6-8%) of the kernels and the peroxide values (2.3 - 3.8 meq O2/kg) of the butter were not affected. Storage for 3 days and boiling for 28 ± 3 min gave the best results, that is, kernels with a moisture content of 7% and a fat content of 50% dw. Butter extracted by traditional technique from these kernels yielded 32% on wet weight of kernel mass with 0.8% of FFA, and 2.5 meq O2/kg of peroxide. This butter can be used for food and cosmetic purposes without refining. Furthermore, the microstructure of fresh shea nuts, studied with Laser Scanning Confocal Microscopy, showed large and small fat globules with some free spaces inside.
Key words: Vitellaria paradoxa, kernels, storage, boiling, physico-chemical characteristics, shea butter.
INTRODUCTION
The shea tree (Vitellaria paradoxa) is a forest food resource with a significant contribution to the diet of local people in extensive parts of Africa (Honfo et al., 2014). Shea kernels are also an important export commodity at international level due to a great percentage of butter that it contained (Ajala et al., 2015; Bello-Bravo et al., 2015). Shea butter is one of the most important vegetable fats due to its various uses over the years: Locally, the butter is used for human consumption, in soap, pomade and traditional pharmacology. At global level it is in great demand among chocolate, cosmetic and pharmaceutical industries as well as biodiesel production (Bello-Bravo et al., 2015). Shea fat is the main component of the kernel and it is principally composed of triglycerides and a large fraction (5-17%) of unsaponifiable compounds that make this fat useful for cosmetic purposes (Maranz et al., 2004; Nahm, 2011). Among shea fatty acids, stearic fatty acid and oleic fatty acid represent 90% of the total fatty acids (Honfo et al., 2014; Maranz et al., 2004). The unsaponifiable fraction of shea butter is mainly composed of triterpene alcohols, tocopherol, phenols, and sterols (Maranz et al., 2003; Nahm, 2011). Shea fat distribution and structure in the kernel are important in determining its physical and chemical properties as well as its extractability. Hence, knowledge of the microstructure, in relation to the macroscopic properties, may offer options to improve the existing processing methods and to design new ones (Borchers et al., 2003).
The harvesting period of shea fruits often coincides with the start of the rainy season, a period of many competing farm activities. Consequently, the processors, women only, have limited time, and therefore, need to store the fresh nuts for several days (commonly 3 to 15 days) before further processing (Honfo et al., 2012). In general, storage conditions are inappropriate and not suited to keep the nuts fresh. All these conditions often lead to the germination of the nuts and exposed the nuts to external agents such as microorganisms, moisture and insects, which affected the quality of the final products (kernel and butter). Germination of nuts also led to the reduction of butter yield, affected the quality and gave the butter a bitter taste (Nahm, 2011).
The preservation and processing of fresh shea nuts varied across shea regions and generally involved fruit gathering, depulping, and boiling of fresh nuts, followed by sun drying, shelling, roasting, milling, churning, and oil separation. Some processing unit operations, such as boiling and sun drying, have been reported as critical operations for kernel quality (Womeni, 2004; Kapseu et al., 2007; Jasaw et al., 2015). The boiling operation during shea processing facilitates the shelling of kernels and inactivates the enzymes, lipases, responsible for triglyceride hydrolysis (Womeni et al., 2006; Abdul-Mumeen et al., 2013). In Benin, boiling is traditionally done for 15 to 60 min and the end of this unit operation is generally determined by a colour change of the boiling water (Honfo et al., 2012). Variations in the duration of boiling could influence shea butter quality. For instance, excessive boiling results in cellular damage, leading sometimes to discolouration of the shea nut (Aculey et al., 2012), while improper lipase inactivation may be occurred due to insufficient boiling, leading to a high free fatty acid (FFA) content in the kernels (Bup et al., 2011). In addition, mouldy kernels may be come from nuts that have been boiled and were not dried well. Among many other challenges with this traditional method are also the technological limits of the process, which did not enable a full separation of liquid and solid phases, with no adequate monitoring of the extraction conditions (Alenyorega et al., 2015).
However, several studies on shea fruits assessed the impact of processing on quality indicators like the FFA, peroxide value, unsaponifiable fraction, and tocopherol content of kernels and butter (Kapseu et al., 2007; Bup et al., 2011; Aculey et al., 2012; Alenyorega et al., 2015; Honfo et al., 2017). To date, the impact of the storage duration of fresh shea nuts on kernel and butter quality characteristics is not well documented. Such an investigation combined with assessing the effect of the duration of boiling is important to determine how long the fresh nuts can be stored and how long they should be boiled to obtain shea products with optimal quality attributes. The main objective of this study was therefore to assess the effect of storage of fresh nuts and boiling time on some physico-chemical quality attributes of shea kernels and butter. Additionally, the microstructure of fat distribution in shea kernels was visualized to improve the understanding of the processes used for butter extraction.
MATERIALS AND METHODS
Experimental design
The response surface methodology (RSM) is a statistical method that used quantitative data derived from an appropriate experimental design with quantitative factors to estimate the relationship between a response and the factors in order to optimize processes or products (Giovanni, 1983; Stroescu et al., 2013). In this study, a central composite face-centered design (CCFD) with two factors was used to assess the simultaneous effect of storage periods (3-21 days) and boiling time (10-60 min) of fresh shea nuts on certain quality characteristics of derived kernels and butter and to determine the optimum storage period and boiling time that will give kernels and butter that comply to export requirements. This design is usually used to study linear interactions and the quadratic effects between factors (Montogomery, 2001; Akinoso et al., 2011). Ranges of storage duration and boiling time were chosen according to the processing practices in Benin (Honfo et al., 2012). The design generated 13 combinations (Table 1) and each of them was duplicated, giving a total of 26 combinations. For each parameter investigated, the design gave the following regression formula:
Y= I + aX1 + bX2 + cX12 + dX22+ eX1X2 (1)
Where: Y is the response, I is a constant; a and b are linear effect coefficients; c and d are quadratic effect coefficients; and e is an interaction effect coefficient. X1 and X2 are the variables storage duration and boiling time, respectively.
Experimental processing
Fresh shea fruits were collected from different shea trees in Arbonga village at Banikoara (11° 4’N and 2° 25’E), a location in Alibori Department, North-Benin. The fruits were depulped on the same day. After-day the fresh nuts were transported to the University of Abomey-Calavi, where the experiments took place.
Three kilogram of fresh shea nuts were used for each treatment. The fresh shea nuts were stored in a room at 28 ± 1°C with a relative humidity of 81 ± 2%. The nuts were just piled on the floor until the end of storage period according to treatment. Boiling was done in water that was three times the volume of the nuts (Honfo et al., 2012) during the experimental time for each treatment. At the end of the boiling time, the nuts were placed in a basket for draining. The drained nuts were subsequently oven-dried at 38-40°C for 5 days, within the temperature range commonly used for sun-drying in Benin. The drying reduced the moisture content of the nuts from 75 to 10%, which facilitated the shelling operation. The dried nuts were shelled manually using a metal rod and the kernels were further oven dried at the same temperature for another 5 days. Next, the butter was extracted from the dried kernels according to the traditional process by grinding with electric grinder machine, roasting for 20 min at 130°C in an oven, milling with an electric milling machine (Kenwood blender, UK), manual churning and heating. The oil was washed and heated to remove particles and mucilage from the first stage of heating. The resulting oil was then filtered and left to cool. Samples of dried kernels and butters were packed in plastic containers and stored at 4°C until analyses.
Microstructural observation of shea kernels
Cross-sections of fresh kernels were studied using a Laser Scanning Confocal Microscopy (LSCM) (510 META Carl Zeiss Germany). The excitation wavelength was 543 nm, and the emission was recorded between 420 and 590 nm. For LSCM observations, pieces of fresh kernels (that is, kernels that had not been treated) were cut into slices of 10 µm thickness by a Reichert-Jung cryostat (Microtome) at -15°C. The samples were then mounted on glass slides, coloured with deionized water containing lipid-soluble Nile Red to stain the fat and covered for observation. Samples were also directly observed under the lenses of the digital microscope (Nikon Eclipse 80i, Nikon Corporation, Tokyo, Japan).
Physico-chemical characteristics of shea kernels and butter
Moisture contents of kernels were determined according to
AOAC (2002) as well as the fat content of kernels using a Soxhlet apparatus with petroleum ether as a solvent for 4 h at 70°C. The yield of shea butter was expressed on wet weight as the percentage of the mass of filtered oil (butter) on the mass of the kernels used. Colour was determined by measuring the Hunter parameters L*, a* and b* values using a chromameter (Konica Minolta CR 410, Japan). The parameter a* explains the redness of kernels, while L* and b* express the brightness and the yellowness of butters, respectively. FFA percentages were determined by titration and calculated as the oleic acid percentage (NB ISO 660, 2006) and the peroxide values of butter samples were determined by titration (NB ISO 3960, 2006). All reagents used for laboratory analysis were from Sigma-Aldrich, St. Louis, USA.
Statistical analyses
The experimental design and the different statistical analyses as well as the optimization conditions were determined with Minitab 16.0 software as well as the different contour plots for each treatment. Analysis of variance (ANOVA) tables were generated and the effect of independent variables and regression coefficients of individual linear, quadratic and interaction terms were calculated. Based on sequential and lack-of-fit P values the best fitting significant model (p<0.05) was selected.
RESULTS AND DISCUSSION
Microstructure of shea kernel
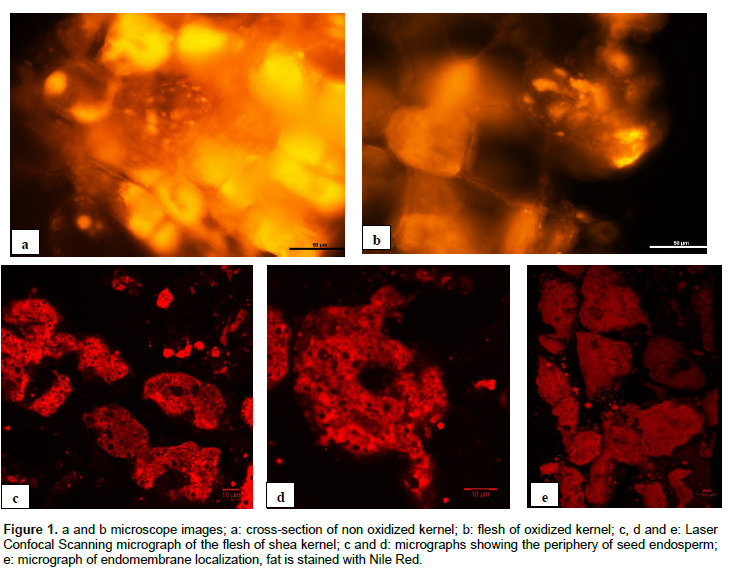
Figures 1a and b showed the micrographs of non-oxidized and oxidized kernel sections taken by the digital microscope. Both figures showed similar cell sizes and cell structures. However, some cells of the cross-section of the oxidized kernel are black, showing the absence of lipid inside. In the micrograph of the non-oxidized kernels, all cells contained lipid. Micrographs 1c and 1d showed the fat structure of the periphery of the seed endosperm and Figure 1e presented the endoplasmic reticulum containing lipid fraction in cells. As the figures illustrated, fat can be recognized under LSCM as oval, polygonal or irregular shapes with various sizes. The dark areas represented the serum pores including water, protein and others nutrients. Most fat globules were large with some free space inside; they were organized into aggregates. However, small fat globules were also dispersed in the serum pores. Lopez et al. (2007) found that small fat globules in milk had a higher stability against rupture of the milk fat globule membrane and a greater resistance to deformation and coalescence under pressure than large fat globules. A similar behaviour might be occurred in plant fat. In addition, rupture of the fat globule membrane to release fat could be enhanced by the closer proximity of the fat globules and may also be depended on several parameters including the temperature and time during different processing operations (Lopez et al., 2007). Investigation of changes in the microstructure in relation to storage and boiling treatments is recommended to gain a better insight in the way in which processing affects the quality of shea products.
Moisture content and colour characteristics of shea kernels
Moisture content (MC) gave the following regression equation for the effect of storage period (X1) and boiling time (X2) of nuts:
MC (%) = 6.9117 + 0.113X1 - 0.0254X2 - 0.0058X12 + 0.0003X22 + 0.0012X1X2
This model explained only 36% of the variation in moisture content (6.3-7.7%) of shea kernels (Table 1). Both factors did not significantly influence the moisture content of the kernels (Table 2). The linear term of the model had a positive value whereas its quadratic term expresses negative influence on moisture content. The contour plots show the variations of moisture content with the storage period and the boiling time (Figure 2a). These different variations could be due to the integrity of the nuts shell as well as their permeability to water. Shells are sometimes cracked and this might facilitate the water absorption by kernel. However, the moisture content of shea kernels complies with the export requirements for this parameter (7-8%) (UEMOA, 2011). In general, moisture content represented one of the most important characteristics of agricultural materials as it affects their physical, mechanical and chemical properties (Sitkei, 1986). This assumption is also valid for shea kernels; besides, some studies found that the moisture content of shea kernels influenced their storability, handling and processing (Aviara et al., 2005; Jasaw et al., 2015).
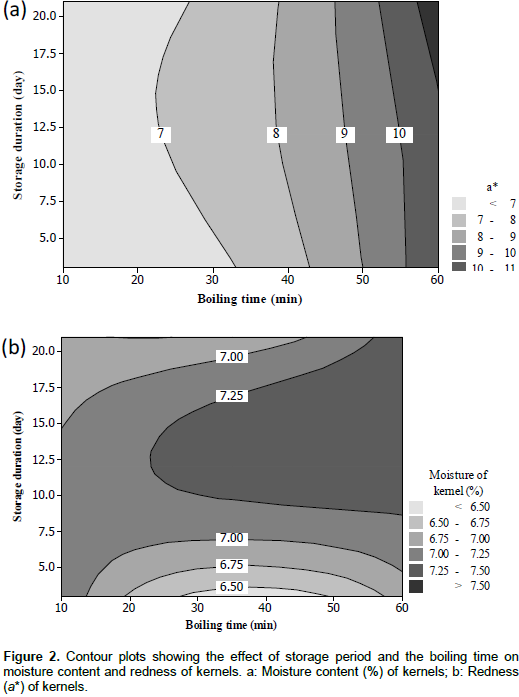
Colour is one of the quality characteristics of shea kernels that is often taken into account during purchase, and the desired colour is red/brown (Lovett, 2004). Values of a* indicated the red colour of kernels. Values of a* were significantly influenced by the boiling time and the model explained 97% of these variations (Table 2); they varied from 6.3 to 11.7 (Table 1). The contour plots of the variations of a* showed an increase of a* values with increasing boiling time, irrespective of the storage duration (Figure 2b). Additionally, long boiling times resulted in darker nuts and kernels. The darker colour of nuts might be due to the release of tannin of the shell with the increasing of boiling time. However, it was noticed that a long storage duration of shea nuts resulted in more infested, black and germinated nuts (Figure 3), than when stored for short times.

Fat content of shea kernels and butter yield
The regression model explained 85% of the variations in fat content of the kernels, which ranged from 38 to 52% dw (Table 1). The linear terms of both factors as well as their quadratic terms were significant for the fat contents of the shea kernels (Table 2). This effect can be described as a decrease in the fat content with increasing storage duration, which might be due to the germination occurring during prolonged storage (Figure 4a). However, as shown in Figure 4a, fat content increased with boiling time until it reached a maximum of 48 to 52% around 32 min after 3 days of storage. This could be caused by the rupture of the membranes of fat globules membrane to release fat with the increasing of boiling time and the size of the fat globules.
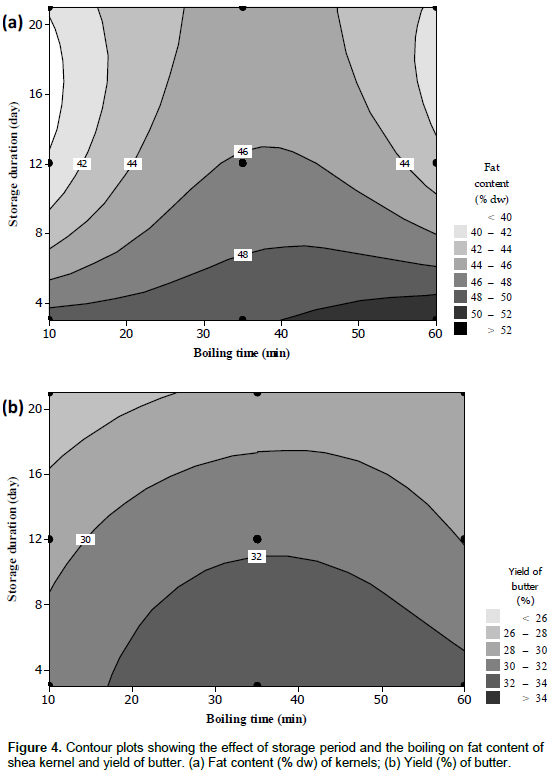
During boiling, small fat globules coagulated, promoting fat released as it does in the case of milk (Lopez, 2002). Additionally, the coagulation of proteins during cooking, resulting in free space for the diffusion of oil, may be increased the amount of oil extracted as cooking time increases (Board, 2002).
The butter yield varied from 24 to 36% on wet weight of kernel mass (Table 1); the highest values were obtained for nuts stored for 3 days and boiled for 30 to 35 min. Compared with the yields (25-30%) generally found for traditional processing (CNUCED, 2006), most butter yields were relatively high. Butter yield was significantly and positively linked to the linear effect of boiling time; for its quadratic term, significant negative effects were also observed (Table 2). This result is corroborated by the variation in the crude fat content of the kernels. The linear and quadratic effects of storage period as well as the interaction effect of the two factors were not significant. The regression model explained 73% of the variation and the contour plots showed the same trend as observed for the fat content (Figure 2b). Thus, irrespective of the boiling time, the butter yield decreases with increasing storage duration. Besides, Ajala and others found that extraction methods and extraction time have significant effect on the yield of shea butter (Ajala et al., 2015).
Colour characteristics of butter
Brightness (L*) values express the level of lightness or brightness. L* values for the butter were significantly and negatively affected by the storage period of nuts (Table 2). L* values varied from 70.5 to 80.1 (Table 1) and the regression model based on the storage period and boiling time explained 62% of the variation. The contour plots show a decrease of L* values due to the storage, irrespective of the boiling time (Figure 5a). This decrease might be due to the damage of fat by nut germination during the storage, resulting in a change of butter colour, in particular, the brightest of butter.
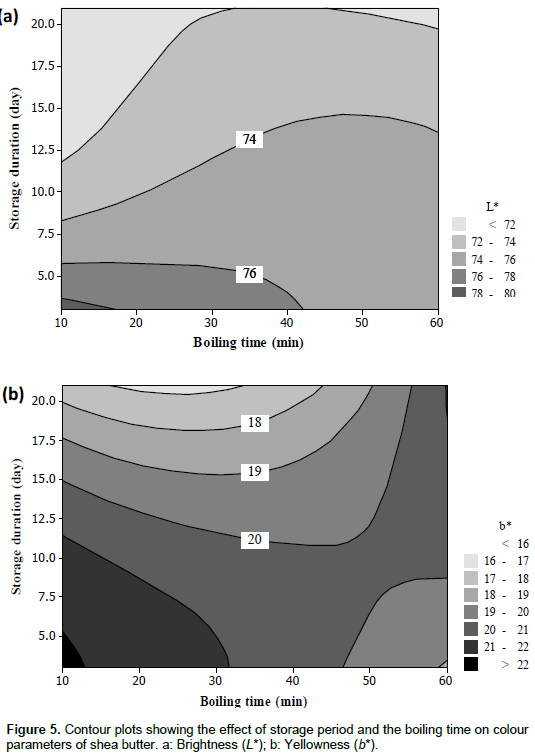
Yellowness (b*) was positively related to the interactive effect of both storage period and boiling time (Table 2). The parameter b* expressed the intensity of the yellow colour of the butter and varied from 15.8 to 22.7 (Table 1). Only 43% of the variation was explained by the regression model related to the two factors. The contour plots showed a drop of the b* values with the increasing of storage duration from the beginning until 47 min of boiling time (Figure 5b). A decrease of b* values was also noticed with an increase of the boiling time at the beginning of storage period.
The highest values for L* and b* were found for the shortest storage period in combination with the shortest boiling treatment, namely 10 min. Colour is of great importance in many food products, including shea butter, as consumers use it as a cue to determine product attributes such as safety, texture and flavour (Clydesdale, 1998). A yellow colour is based on the pigments such as carotenoids and corresponds to the natural state of shea butter; when the butter is also bright, it is most attractive (Lovett, 2004; Megnanou and Niamke, 2015).
FFA percentage and peroxide value of shea butter
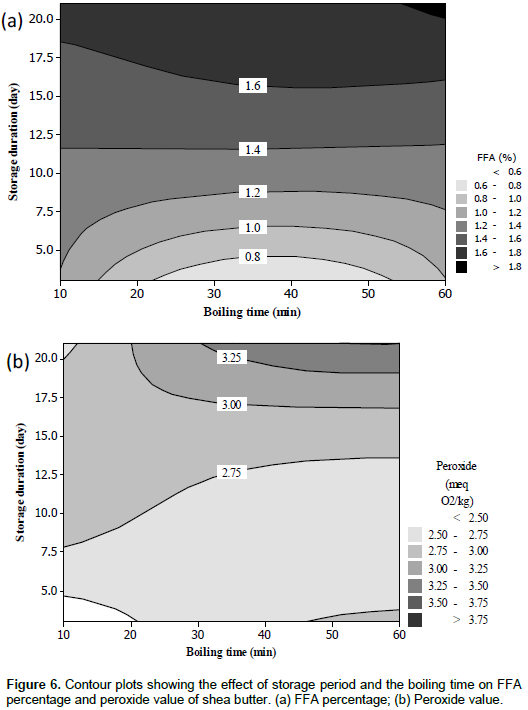
The FFA percentages varied from 0.5 to 2% (Table 1) and were significantly and positively related to the linear effect of storage period while the opposite was found for the boiling time (Table 2). The regression model explained 74% of the variation. Low FFA values were found at the beginning of the storage period. However, some of the FFA percentages were higher than the threshold of 1% tolerated for cosmetic purposes (NB 04 02 001, 2006), but all of them were lower than the maximum value of 3 and 4% approved by shea production countries for international trade and by the Codex Alimentarius, respectively for food purposes (Codex Alimentarius, 2006; NB 04 02 001, 2006; UEMOA, 2011). An increase in FFA is commonly associated with a decrease in the hardness of the butter, thereby reducing the commercial value in the case of cocoa butter (Calliauw et al., 2008). This phenomenon is likely to apply to shea butter too as both butters are similar in terms of composition and uses. The contour plots presented in Figure 4a showed a gradual increase of the FFA percentage during the storage of nuts, irrespective of the boiling time. This increase may be due to the germination of the nuts during storage. Indeed, shea fruits fall from the tree and may start to germinate, which led to the activation of the lipases, responsible for triglyceride hydrolysis (Kim and Min, 2008; Megnanou and Niamke, 2015) as FFA are carboxylic acids released from triglycerides through the effect of a lipase or oxidation. Many studies have shown detectable lipase activity, mostly during seed germination (Wanasundara et al., 2001). Furthermore, storage conditions may be promoted the growth of micro-organisms particularly moulds which could be extended the FFA percentage (Allal et al., 2013).
The different peroxide values obtained 2.3 to 3.8 meq O2/kg (Table 1) were lower than the maximum value of 10 meq O2/kg set for cosmetic uses (NB 04 02 001, 2006). The regression model of the peroxide values explained 48% of the variation (Table 2). The simultaneous effects of the two factors on the peroxide value were not significant. The contour plots showed a gradual increase of the peroxide value with increasing storage period and boiling time (Figure 6b). The increase in the amount of peroxide with the boiling time was also found by Womeni et al. (2006), when they investigated the effect of cooking time and oven temperature on the peroxide value. The peroxide value is usually used to quantify the primary oxidative products in oils and fat. The rise in the peroxide value during prolonged cooking of shea nuts may be due to the breakdown of water soluble anti-oxidants such as the monomers and polymers of catechins during the process (Maranz et al., 2004; Honfo et al., 2014). This led to oxidation of fat inside the nuts and subsequently increased the peroxide value.
Since the models have shown non-significant lack-of-fit P values for all investigated parameters, the different regression equations sufficiently explained most of the variability of the responses. In general, a lack-of-fit is used to check the fitness of the regression models and a non-significant lack-of-fit P value indicates the adequacy of the model and the accuracy of the predictions (Womeni et al., 2006).
Optimisation of storage period and boiling time
Optimum conditions for the storage period and boiling time are predicted by the models. A condition is considered optimal if the desirability value associated to the response is 1 or close to 1. The desirability value is generally between 0 and 1 and explained the level of validity of the predicted optimum condition. From the results, optimum conditions were obtained for each parameter investigated. Since, the desirability was not the same for all responses with a unique optimal condition to get kernels with low moisture and high fat content, and butter with low FFA and peroxide values, a range of desirability (0.8-1) was then used. This resulted in an optimum storage period of 3 days and a boiling time of 28 ± 3 min at which the following values were obtained: A moisture content of the kernels of 7%, a fat content of 50% dw, a butter yield of 32%; butter extracted from these kernels may have a FFA content of 0.8% and a peroxide value of 2.5 meq O2/kg and could be used for cosmetic and food purposes without refining (NB 04 02 001, 2006; USAID/WATH, 2005).
CONCLUSION
In shea kernels, the fat is organized in large globules with some free spaces inside, and in small globules. The preservation of shea nuts for further use is very critical for end product quality. Handling alternatives of shea nuts such as storage and boiling applied separately or in combination had significant effects on colour and fat content of kernels and the FFA percentage of butter. Longer storage reduced the fat content of the kernels and increased the FFA percentage of the butter. Increasing the boiling time might allow the extraction of more fat from the kernels. The optimum value was found around 32 min. Based on the predicted optimal conditions, the storage of fresh nuts 3 days and their boiling for 28 ± 3 min should be recommended for shea nuts processing. In addition to this study, further investigations should be taken up on other critical processing operations (viz. roasting, churning) to upgrade each step of the traditional technique and consequently to improve the yield and quality of butter.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors appreciate the Netherlands Universities’ Foundation for International Cooperation (NUFFIC) for supporting the research with a scholarship through the NPT/BEN/263 project, hosted at the University of Abomey-Calavi in Benin, Prof. Romain Glele Kakai for his help in statistical analysis and Elias Noumatekpo for his assistance during the experiment.
REFERENCES
|
Abdul-Mumeen I, Zakpaa HD, Mills-Robertson FC (2013). Proximate and bio-phytochemical properties of shea nut cake. Journal of Chemical and Pharmacy Resources 5:961-970. |
|
|
Aculey PC, Lowor ST, Kumi WO, Assuah MK (2012). The effect of traditional primary processing of the shea fruit on the kernel butter yield and quality. American Journal of Food Technology 7:73-81. |
|
|
Ajala EO, Aberuagba F, Olaniyan AM, Onifade RK (2015). Optimization of solvent extraction of shea butter (Vitellaria paradoxa) using response surface methodology and its characterization. Journal of Food Science and Technology 53:730-738. |
|
|
Akinoso R, Aboaba SA, Olajide WO (2011). Optimization of roasting temperature and time during oil extraction from orange (Citrus sinensis) seeds: a response surface methodology approach. African Journal of Food and Agricultural Nutrition and Development 11:5300-5317. |
|
|
Allal F, Piombo G, Kelly BA, Okullo JBL, Thiam M, Diallo OB, Nyarko G, Davrieux F, Lovett PN, Bouvet JM (2013). Fatty acid and tocopherol patterns of variation within the natural range of the shea tree (Vitellaria paradoxa). Agroforestry and System 87:1065-1082. |
|
|
Alenyorega EA, Hussein YA, Adongo TA (2015). Extraction yield, efficiency, and loss of the traditional hot water floatation (HWF) method of oil extraction from the seeds of Allanblackia floribunda. International Journal of Science and Technology Resource 4:92-95. |
|
|
AOAC (2002). Official Methods of Analysis.16th edition (Association of Official Analytical Chemists) Washington DC. |
|
|
Aviara NA, Oluwole FA, Haque MA (2005). Effect of moisture content on some physical properties of shea nut. International Agrophysics 19:193-198. |
|
|
Bello-Bravo J, Lovett NP, Pittendrigh RB (2015). The evolution of shea butter's "Paradox of paradoxa" and the potential opportunity for information and communication technology (ICT) to improve quality, market access and women's livelihoods across rural Africa. Sustainability 7:5752-5772. |
|
|
Board N (2002). Modern technology of oils, fats & its derivatives. NIIR Project Consultancy Services, Asia Pacific Business Press Incorporated, Delhi, India. |
|
|
Borchers C, Gärtner F, Stoltenhoff T, Assadi H, Kreye H (2003). Microstructural and macroscopic properties of cold sprayed copper coatings. Journal of Applied Physics 93(12):1064-1067. |
|
|
Bup ND, Kapseu C, Matos L, Mabiala B, Mouloungui Z (2011). Influence of physical pretreatments of sheanuts (Vitellaria paradoxa Gaertn.) on butter quality. European Journal of Lipid Science and Technology 113:1152-1160. |
|
|
Calliauw G, Vila Ayala J, Gibon V, Wouters J, De Greyt W, Foubert I, Dewettinck K (2008). Models for FFA-removal and changes in phase behavior of cocoa butter by packed column steam refining. Journal of Food Engineering 89:274-284. |
|
|
CNUCED (2006). Le karité: production, consommation et marché, Accessed 22/09/2017 |
|
|
Codex Alimentarius (2006). Programme mixte FAO/OMS sur les normes alimentaires. FAO, Rome. |
|
|
Honfo FG, Linnemann A, Akissoe N, Soumanou M, van Boekel MAJS (2012). Indigenous knowledge of shea processing and quality perception of shea products in Benin. Ecology of Food and Nutrition 51:505-525. |
|
|
Honfo FG, Akissoe N, Linnemann AR, Soumanou M, van Boekel MAJS (2014). Nutritional composition of shea products and chemical properties of shea butter: a review. Critical Review of Food and Nutrition 54(5):673-686. |
|
|
Honfo FG, Linnemann AR, Guo M, Akissoe N, Soumanou MM, van Boekel MAJS (2017). Influence of roasting of shea kernels on their fat content and some quality characteristics of shea butter. Journal of Food Studies 6(1):2377-1356. |
|
|
Jasaw SG, Osamu Saito O, Kazuhiko Takeuchi K (2015). Shea (Vitellaria paradoxa) butter production and resource use by urban and rural processors in Northern Ghana. Sustainability 7:3592-3614. |
|
|
Kapseu C, Bup ND, Tchiegang C, Fon Abi C, Broto F, Parmentier M (2007). Effect of particle size and drying temperature on drying rate and oil extracted yields of Buccholzia coriacea (MVAN) and Butyrospermum parkii ENGL. International Journal of Food Science and Technology 42:573-578. |
|
|
Kim HJ, Min, DB (2008). Chemistry of Lipid Oxidation. In: Akoh, C.C. and Min, D.B. (eds.), Food lipids, chemistry, nutrition and biotechnology. Boca Rotan, Florida: CRC Press, pp. 299-320. |
|
|
Lopez C Camier, B Gassi JY (2007). Development of the milk fat microstructure during the manufacture and ripening of emmental cheese observed by confocal laser scanning microscopy. International Dairy Journal 17:235-247 |
|
|
Lopez C, Bourgaux C, Lesieur P, Bernadou S, Keller G, Ollivon M (2002). Thermal and structural behavior of milk fat: In?uence of cooling rate and droplet size on cream crystallisation. Journal of Colloid and Interface Science 254:64-78. |
|
|
Lovett PN (2004). The shea butter value chain: Production, transformation and marketing in West Africa. Technical Report Nº 2, West Africa Trade Hub, 52p. |
|
|
Maranz S, Wiesman,Z, Garti N (2003). Phenolic constituents of shea (Vitellaria paradoxa) kernels. Journal of Agricutural and Food Chemistry 51:6268-6273. |
|
|
Maranz S, Wiesman Z, Bisgaard J, Bianchi G (2004). Germplasm resources of Vitellaria paradoxa based on variations in fat composition across the species distribution range. Agroforestry System 60:71-76. |
|
|
Megnanou RM, Niamke S (2015). Improving the optimized shea butter quality: a great potential of utilization for common consumers and industrials. SpringerPlus 4:665-667. |
|
|
Montogomery DC (2001). Design and analysis of experiments, 5th edition. John Wiley and Sons, New York. |
|
|
Nahm HS (2011). Quality characteristics of West African shea butter (Vitellaria paradoxa) and approaches to extend shelf-life. Graduate School-New Brunswick Rutgers, The State University of New Jersey, New Brunswick Rutgers, pp. 1-133. |
|
|
NB ISO 660 (2006). Normes Béninoises pour les corps gras d'origine animale et végétale: détermination de l'indice d'acide et de l'acidité. CEBENOR (Centre Béninois de Normalisation) Cotonou 13p. |
|
|
NB ISO 3960 (2006). Normes Béninoises pour les corps gras d'origine animale et végétale: détermination de l'indice de peroxyde. CEBENOR (Centre Béninois de Normalisation) Cotonou 9p. |
|
|
NB 04.02.001 (2006). Beurre de karité non raffiné: spécification. CEBENOR (Centre Béninois de Normalisation et de la Gestion de la Qualité) Cotonou 9p. |
|
|
Sitkei G (1986). Mechanics of Agricultural Materials. Elsevier Amsterdam. |
|
|
Stroescu M, Stoica-Guzun A, Ghergu S, Chira N, Jipa I (2013). Optimization of fatty acids extraction from Portulaca oleracea seed using response surface methodology. Industrial Crops and Products 43:405-411. |
|
|
UEMOA (2011). Spécification du beurre de karité non raffiné. PN UEMOA ICS-67. |
|
|
USAID/WATH (2005). Guide à l'exportation du beurre de karité, West Africa Trade Hub (WATH) Accra. |
|
|
Wanasundara PD, Wanasundara UN, Shahidi F (2001). Lipolytic activity of enzymes from germinating seeds of sesame (Sesamum incidcum L.). Journal of Food Lipids 8:75-84. |
|
|
Womeni HM (2004). Identification et analyse des opérations critiques de préparation des fruits, graines et amandes de karité (Butyrospermum parkii (G. Don) Kotschy): étude de leur influence sur la qualité du beurre.Thèse de Doctorat/Ph.D en technologie alimentaire, ENSAI, Université de Ngaoundéré 170p. |
|
|
Womeni HM, Ndjouenkeu R, Kapseu C, Tchouanguep Mbiapo F, Parmentier M, Fanni J (2006). Effet de la cuisson et du séchage des noix de karité (Butyrospermum parkii (G. Don) Kostchy) sur la qualité du beurre. Tropicultura 24:175-182. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0