ABSTRACT
Camu-camu fruits have high functional importance and great potential as food. Knowing and determining the nutrients present in its fruitsis crucial, in order to understand the relationship between consumption and human health. For this reason, the objective of this work was to evaluate qualitative attributes, such as organic acids content, antioxidant capacity and functional potential of different parts of camu-camu fruit. The fruit used in the present study were collected in the state of Amazonas, Brazil, and analyzed at the University of Florida, Gainesville, U.S.A. Seed, peel, pulp, pulp+peel, and whole fruit were evaluated regarding pH, soluble solids, titratable acidity, and levels of ascorbic acid, phenolic compounds, anthocyanins, and flavonoids. Antioxidant activity was evaluated by the Ferric Reducing Antioxidant Power (FRAP) and Diphenyl-1-picrylhydrazyl (DPPH) methods. Based on the results, camu-camu fruit proved to be a good source of acids, and the pulp is the part that is most concentrated in organic acids. Also, the pulp presents the highest amount of phenolic compounds and highest antioxidant activity. The peel had relatively higher amount of pigments, anthocyanins and flavonoids, and higher concentration of ascorbic acid, proving that it can also be used as a source of bioactive compounds.
Key words: Functional food, antioxidant activity, Ferric Reducing Antioxidant Power (FRAP), Diphenyl-1-picrylhydrazyl (DPPH), pigments, Caçari.
Camu-camu (Myrciaria dubia (H.B.K.) McVaugh), also known as ‘caçari’, ‘araçád'água’, or ‘sarão’, belongs to the family Myrtaceae, and is native to the Amazonian floodplains and lake (Maeda et al., 2007). For being a fruit species with great nutraceutical and technological potential, the interest of the scientific community on this species has increased in recent years.
Fruits are important nutritional sources, since they are rich in vitamins, minerals and carbohydrates. Some species have higher content of a specific nutrient when compared with others. The increasing interest in camu-camu fruit is due to its remarkable ascorbic acid (vitamin C) content, and it is known as "King of Vitamin C" or "Super Fruit". In the state of Roraima, Brazil, the fruit is known as ‘caçari’, presenting mean values of 3,571 to 7,355 mg ascorbic acid 100 g-1 pulp (Aguiar and Souza, 2016; Chagas et al., 2015; Grigio et al., 2015; Grigio et al., 2016).
In addition to vitamin C, these fruits contain other anti-oxidant compounds, such as carotenoids, anthocyanins and other phenolic compounds which are valuable in human nutrition (Silva, 2012). These compounds help in the fight against and prevention of free radicals, increasing immune resistance and delaying the early or natural aging of cells (Santos et al., 2009).
However, the in natura consumption of camu-camu fruits is low due to the high content of acids (Azevedo et al., 2014), limiting its current consumption to the producing regions. The rest of the production is exported in the form of pulp, mainly to the United States, Europe and Japan (Akter et al., 2011), for the development of products that are considered natural sources of biocompounds or antioxidants.
Several studies have been carried out with the by-products obtained from fruit pulping, and they denote that the peel has great potential as promising sources of bioactive compounds, even though it is underutilized. These compounds can be used as functional food, not only in the Amazon region, but also in other parts of the world (Fracassetti et al., 2013; Azevedo et al., 2014; Azevedo et al., 2015). Moreover, other by-products that are considered waste could have great economic, nutritional, and functional potential. The seed, for instance, has also proved to be excellent source of minerals, with potential to be used in biotechnological development (Sousa et al., 2015).
Vitamin C has vital effect on the brain, since neurodegenerative diseases usually involve high levels of oxidative stress. Moreover, ascorbic acid has positive therapeutic implications against Alzheimer's disease, Parkinson's disease, Huntington's disease and stroke (Harrison and May, 2009). Thus, the potential of camu-camu to minimize the risks of these diseases is extremely significant.
Camu-camu has great functional and bioactive potential. Besides being rich in vitamin C, it has high levels of phenolic compounds, such as flavonoids. Some of these phenolic compounds also have antioxidant and anti-inflammatory properties, with potential to combat chronic diseases induced by the stress, when the fruits are consumed as part of the diet (Fujita et al., 2015). Antioxidants are chemical compounds that can prevent or reduce the oxidative damage of lipids, proteins and nucleic acids caused by reactive oxygen. Antioxidants have the ability to react with free radicals and thus restrict their effects on the organism (Couto and Canniatti-Brazaca, 2010).
Several studies have reported camu-camu as a food that helps spermatogenesis. The species also present antimutagenic potential, and helps treat diseases such as Alzheimer's and Parkinson's, diabetes, hypertension and obesity (Schwertz et al., 2012; Gonzales et al., 2013; Borges et al., 2014; Carvalho-Silva et al., 2014; Gonçalves et al., 2014; Azevedo et al., 2015; Fujita et al., 2015; Langrey et al., 2015).For this reason camu-camu has attracted great interest in the scientific environment, in order to exploit all its functional potential, nutraceutical and nutritional, which are still unknown and under-exploited.
Due to the high functional importance and to the great potential of camu-camu fruit, the knowledge and determination of its nutrients is imperative in order to understand the relationship between consumption and human health. And mainly phytochemical concentration differences between different parts of the camu-camu fruit are not known. For this reason, the objective of this work was to evaluate qualitative attributes, antioxidant capacity and functional potential, of different parts of camu-camu fruit.
The camu-camu fruit used in the experiment were collected froma private property,in planted area, in Amazonas, located on Highway AM 010, km 98, Rio Preto da Eva-Brazil.
The fruit were collected at a mature stage, which was established in previous studies (Grigio et al., 2015). After harvest, fruit were taken to the Post-Harvest Laboratory of Embrapa Roraima, where they were cleaned and selected for the absence of damages, washed in running water, and sanitized with 0.02% sodium hypochlorite (NaClO) for 30 min, following Brazilian National Health Surveillance Agency (ANVISA) recommendations. After the hygienization, fruits were processed according to the treatments. Some fruits were properly pulped in an industrial pulper, with no water, by separating the pulp from the peel and the seeds; other fruit were kept intact; and some other fruit had only the seeds removed, keeping the pulp+peel for the treatments.
The treatments consisted of: peel+pulp, pulp, peel, whole fruit and seed. Totaling five treatments composed of four replicates each, where each replicate was from a sample of 30 fruits.
After the separation of the material according to the treatments, samples were lyophilized, stored in aluminized bags, and transported to the University of Florida, Gainesville, FL, U.S.A. for analysis. The material was rehydrated according to the previously calculated moisture content equivalent to each treatment, until obtaining 10 g of fresh sample. The material was centrifuged in a refrigerated centrifuge at 4°C, 12,000 rpm, for 20 min, and the supernatant was removed for the following analyses:
pH
This was determined by reading 5 mL aliquot of the sample, using an 814 USB sample processor.
Soluble solids (SS)
Soluble solids were estimated by the reading, using a portable refractometer (SOLOESTE, model RT-30ATC), with automatic temperature compensation (10 to 30°C). Results were expressed in °Brix (Institute Adolf Lutz, 2008).
Titratable acidity (TA)
This was determined by titration, following the methodology described by the Institute Adolf Lutz (2008). Results were expressed in mg of citric acid 100 g-1 sample.
Ratio (SS/TA)
This was calculated by the solid soluble:titratable acidity ratio.
Ascorbic acid
The samples were extracted using 0.5% oxalic acid and titrated with 2,6-dichlorophenolindophenol (Ranganna, 1986). Results were expressed in mg of ascorbic acid 100 g-1sample.
Total flavonoid content
This was determined by the aluminum chloride colorimetric assay (Zhishen et al., 1999), using quercetin as standard. For the extraction, methanol solution and 5% aluminum chloride were added. After 30 min, spectrophotometer readings were performed at 441 nm. For each sample, a blank containing only sample and methanol was made. Results were expressed in μg of quercetin equivalent g-1 sample.
Total anthocyanins content
This was determined according to the method described by Lee and Francis (1972), using cyanidin as standard. An acidified solution of methanol (methanol:HCl, 85:15, v:v) was added to the samples. Once being homogenized, samples were stored in the dark. After 24 h, samples were read in a spectrophotometer at 520 nm. Results were expressed in μg of cyanidin equivalent g-1 sample.
Phenolic compounds
This was determined according to the Folin-Ciocateau spectro-photometric method described by Singleton et al. (1999). A 20 μl aliquot of the sample was diluted in 1.58 mL water. Afterwards, 100 µL of the Folin-Ciocalteu reagent was added and wellmixed. After 30 s to 8 min, 300 μl of the sodium carbonate solution was added and stirred. Solutions were allowed to stand at 20°C for 2 h, and the absorbance of each solution was determined at 765 nm. Results were expressed in mg of gallic acid g-1 sample.
Antioxidant activity (DPPH)
Total antioxidant activity was determined by the oxidation inhibition potential, using the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) as reference (Brand-Williams et al., 1995). One gram of the sample was weighed, 10 mL of ethyl alcohol was added and homogenized, and the mixture was taken to centrifuge at 6000 rpm for 50 min. Subsequently, the supernatant was separated using a pipette, and the solution was stored in a dark bottle in an ice-bath, added to 3 mL ethanol. The standard curve was made with ascorbic acid. Absorbance was read using a spectrophotometer at 517 nm, in 500 μL of the sample extract, added to 300 μL of the DPPH stock solution. Results were expressed in mg of ascorbic acid equivalent g-1sample.
Antioxidant activity (FRAP)
Total antioxidant activity was also estimated by the iron reduction method (FRAP), following the procedure adapted by Rufino et al. (2006). One gram of sample was added to 40 mL 50% methanol, which was homogenized and allowed to stand for 60 min at room temperature. Afterwards, samples were centrifuged (15,000 rpm) for 15 min, and the supernatant was transferred to 100 mL volumetric flask. Forty millimeter 70% acetone was added to the residue of the first extraction, which were then homogenized and allowed to stand for 60 min at room temperature.
One hour later, samples were centrifuged again (15,000 rpm) for 15 min, and the supernatant was transferred to the volumetric flask containing the first supernatant, and the volume was completed with distilled water. The extract and the FRAP reagent (Acetate buffer, 0.3 mol/L, 2,4,6-Triz(2-pyridil)-s-triazine (TPTZ) solution, 8.0 mmol/L and ferric Chloride solution, 20 mmol/L) were brought to a water bath at 37°C.The standard curve was made with ascorbic acid. Absorbance reading was performed at 595 nm. Results were expressed in mg of ferrous sulfate g-1 fruit.
Data were subjected to analysis of variance, and the means were tested by the Tukey test at 5% probability using the SISVAR software (Ferreira, 2011).
Results show statistically significant difference for all the treatments tested. When comparing the pH, only the seed was different from the others, with mean value higher than that of the other treatments (Table 1). Thus, the seed presented lower acidity when compared with the other parts of the fruit. Values lower than those observed in the present study have been reported in the literature (Akter et al., 2011; Barreto et al., 2013; Rufino et al., 2009).
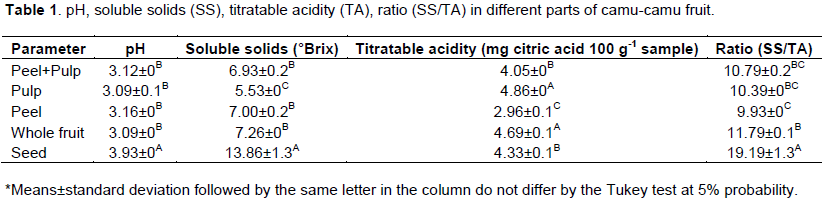
The seed had the highest content of soluble solids, followed by whole fruit, peel, and peel+pulp, which did not show statistically significant difference between them. Finally, the pulp had the lowest soluble solids, with mean value of 5.53 °Brix, similar to the reports found in the literature regarding the pulp (Barreto et al., 2013; Fujita et al., 2013; Grigio et al., 2016) and the fruit (Rufino et al., 2009). Thus, it can be inferred that camu-camu seeds likely have the highest sugar content. Similar behavior was observed by Daiuto et al. (2014), who detected higher sugar contents when evaluating avocado seed. This greater amount of sugar is possibly the necessary source for seed germination.The highest titratable acidity index was observed in the pulp, according to the method of the Instituto Adolf Lutz, and it did not statistically differ from the whole fruit, followed by the seed and the peel + pulp, which also did not have statistically significant difference between them. The peel had the lowest acidity index. These results are consistent with those found in the literature (Rufino et al., 2009; Akter et al., 2011; Barreto et al., 2013; Fujita et al., 2013).
The pulp and the whole fruit had higher values of acid. Results indicated that a large number of acids are present in the pulp of camu-camu fruits, corroborating the literature that reports camu-camu fruit to be an extremely acidic one (Rufino et al.,2009; Grigio et al., 2015; Maeda et al., 2006). On the other hand, the lowest values were observed in the peel. This fact is consistent with the fact that camu-camu pulp is the part of the fruit that contains the highest contents of acids, since in the treatments with pulp these values were always higher than in the treatments with peel or seed.
Regarding the acceptability index, or ratio, higher values were observed for the seed, since it contained a greater quantity of Brix, a proxy for soluble sugars, consequently denoting more expressive values to this ratio, followed by the whole fruit, peel+pulp, and pulp, which did not differ statistically between treatments. The lowest soluble solids/titratable acidity ratio was observed peel+pulp and pulp.
When analyzing the total anthocyanins content, statistically significant difference was observed among all the treatments. As expected, the content was higher in the peel of camu-camu fruits, due to the greater purplish pigment, with mean value of 107.19 μg g-1sample (Table 2), followed by peel + pulp, and whole fruit, with mean values of 50.70 and 29.90 μg g-1sample, respectively. Lower incidence of this pigment was observed in the pulp, and no traces of anthocyanins were detected in the seed. Similar behavior was observed by Solis et al. (2009), when evaluating the pulp, peel and seed of camu-camu fruits. The more mature the fruits, the higher was the anthocyanin content (Pinto et al., 2013).
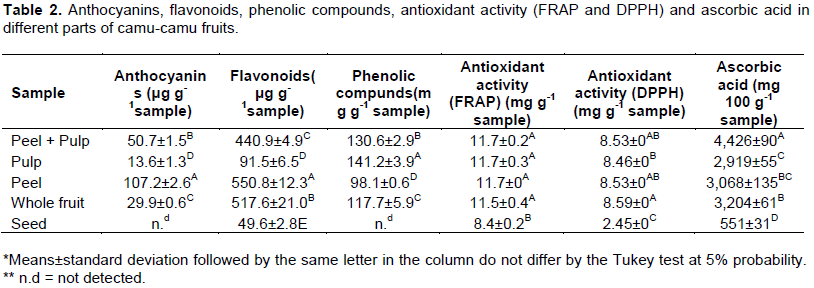
In relation to the total flavonoids content, statistically significant difference was observed among all treatments. The highest values were: Peel (550.80), whole fruit (517.65), peel+pulp (440.92), pulp (91.54), and seed (49.56 μg g-1sample). The peel presented the highest concentration of these pigments, such as anthocyanins and flavonoids, which have antioxidant potential. Anthocyanins were responsible for the color of the peel, corroborating with other studies that evaluated the pulp, the peel and seed of camu-camu fruits (Solis et al., 2009).
Currently, camu-camu has been considered one of the richest foods, mainly due to its great amount of phenolic compounds, and consequently to its great antioxidant potential. The highest amount of phenolic compounds was observed in the pulp, followed by pulp+peel, whole fruit, and peel, with mean values of 141.18, 130.57, 117.66 and 98.15 mg of gallic acid g-1 sample. Phenolic compounds were not observed in the seed. Similar results have reported the pulp with the greatest amount of phenolic compounds (Solis et al., 2009). In particular this fruit is known for its content of ellagic acid derivatives (Francassetti et al., 2013), which we assume are preserved in our dried powdered samples.
Several studies have been carried out in order to evaluate the amount of phenolic compounds present in the fruits, and a great difference on the mean values had been observed between different studies. This difference may be due to genetic diversity or even to conservation methods and maturation stage of the fruit, resulting in the divergent data found in the literature (Bataglion et al., 2015; Fracassetti et al., 2013; Fujita et al., 2013; Neves et al., 2015; Solis et al., 2009; Rufino et al., 2010; Villanueva et al., 2010).
The FRAP method detected greater antioxidant activity than the DPPH method, and thus, the former is considered one of the most indicated to evaluate camu-camu fruits (Rufino et al., 2010). Higher antioxidant activity was observed in both methods when both pulp and peel were evaluated. However, antioxidant activity is very low in the seed. Much information is found regarding the antioxidant activity of camu-camu fruits, and several methods are being tested. However, all of them lead to the same result, proving that camu-camu is one of the fruits with the highest antioxidant activity, and with great potential for application and development as a functional food (Baldeon et al., 2015; Barreto et al., 2013; Bataglion et al., 2015; Fracassetti et al., 2013; Fujita et al., 2013; Neves et al., 2015; Rufino et al., 2010; Solis et al., 2009; Villanueva et al., 2010).
The highest concentration of ascorbic acid was observed for the peel+pulp, with mean value of 4.426 mg acid 100 g-1 sample, followed by the whole fruit and the peel, which did not present statistical difference between them. On the other hand, the lowest values were also observed for the seed. The highest values were always observed in the treatments that contained the peel, which is in agreement with other results found in the literature (Zamudio, 2007; Imán-Correa et al., 2011). According to Fujita et al. (2013), the lyophilization process can generate losses of ascorbic acid and of other polyphenols. However, our study shows that the products can still retain considerable amounts of vitamin C, phenols, anthocyanins and antioxidants, even after being dried.
Results show that camu-camu fruit has good qualitative attributes. The pulp had the greatest amount of phenolic compounds and antioxidant activity. Higher amounts of pigments (such as anthocyanins and flavonoids), and higher concentration of ascorbic acid were found in the peel, making it useful as a source of bioactive compounds as a food colorant with antioxidant qualities.
The authors have not declared any conflict of interests.
REFERENCES
|
Aguiar JPL, Souza FCA (2016). Camu-Camu super fruit Myrciaria dubia (H.B.K) Mc Vaugh) at different maturity stages. Afr. J. Agric. Res.11:2519-2523.
Crossref
|
|
|
|
Akter MOh S, Eun J, Ahmed M (2011). Nutritional compositions and health promoting phytochemicals of camu-camu (Myrciaria dubia) fruit: A review. Food Res. Int. 44:1728-1732.
Crossref
|
|
|
|
|
Azevêdo JCS, Fujita A, Oliveira EL, Genovese MI, Correia RTP (2014). Dried camu-camu (Myrciaria dubia H.B.K. McVaugh) industrial residue: A bioactive-rich Amazonian powder with functional attributes. Food Res. Int. 62:934-940.
Crossref
|
|
|
|
|
Azevêdo JCS, Fujita A, Oliveira EL, Genovese MI, Correia RTP (2015). Neuroprotective effects of dried camu-camu (Myrciaria dubia HBK McVaugh) residue in C. elegans. Food Res. Int. 73:135-141.
Crossref
|
|
|
|
|
Baldeon EO, Alcaniz M, Masot R, Fuentes EM, Barat JM, Grau R (2015). Voltammetry pulse array developed to determine the antioxidant activity of camu-camu (Myrciaria dubia (H.B.K.) McVaug) and tumbo (Passiflora mollisima (Kunth) L.H. Bailey) juices employing voltammetric electronic tongues. Food Control 54:181-187.
Crossref
|
|
|
|
|
Barreto AG, Cabral LMC, Matta VM, Freitas SP (2013). Clarificação de polpa de camu-camu por microfiltração. Brazilian J. Food Technol. 16:207-215.
Crossref
|
|
|
|
|
Bataglion GA, Silva FMA, Eberlin MN, Koolen HHF (2015). Determination of the phenolic composition from Brazilian tropical fruits by UHPLC–MS/MS. Food Chem. 180:280-287.
Crossref
|
|
|
|
|
Borges LL, Conceição EC, Silveira D (2014). Active compounds and medicinal properties of Myrciaria genus. Food Chem. 153:224-233.
Crossref
|
|
|
|
|
Brand-Williams W, Cuvelier ME, Berset C (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28:25-30.
|
|
|
|
|
Carvalho-Silva LB, Dionísio AP, Pereira ACS, Wurlitzer NJ, Brito ES, Bataglion GA, Brasil IM, Eberlin MN, Liu RH (2014). Antiproliferative, antimutagenic and antioxidant activities of a Brazilian tropical fruit juice. Food Sci. Technol. 59:1319 -1324.
Crossref
|
|
|
|
|
Chagas EA, Lozano RMB, Chagas PC, Bacelar-Lima CG, Garcia MIR, Oliveira JV, Souza OM, Morais BS, Araújo MCR (2015). Intraspecific variability of camu-camu fruit in native populations of northern Amazonia. Crop Breed. Appl. Biotechnol.15:265-271.
Crossref
|
|
|
|
|
Couto MAL, Canniatti-Brazaca SG (2010). Quantificação de vitamina C e capacidade antioxidante de variedades cítricas. Ciência e Tecnologia de Alimentos 30:15-19.
Crossref
|
|
|
|
|
Daiuto ER, Tremocoldi MA, Alencar SM, Vieites RL, Minarelli PH (2014). Composição química e atividade antioxidante da polpa e resíduos de abacate 'Hass'. Revista Brasileira de Fruticultura 36:417-424.
Crossref
|
|
|
|
|
Fracassetti D, Costa C, Moulay L, Tomás-Barberán FA (2013). Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food Chem. 139:578-588.
Crossref
|
|
|
|
|
Fujita A, Borges K, Correia R, Franco B, Genovese M (2013). Impact of spouted beddrying on bioactive compounds, antimicrobial and antioxidant activities of commercial frozen pulp of camu-camu (Myrciaria dubia Mc. Vaugh). Food Res. Int.54: 495-500.
Crossref
|
|
|
|
|
Ferreira DF (2011). Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia 35:1039-1042.
Crossref
|
|
|
|
|
Gonçalves AESS, Lellis-Santos C, Curi R, Lajolo FM, Genovese MI (2014). Frozen pulp extracts of camu-camu (Myrciaria dubia McVaugh) attenuate the hyperlipidemia and lipid peroxidation of Type 1 diabetic rats. Food Res. Int. 64:1-8.
Crossref
|
|
|
|
|
Gonzales GF, Vasquez VB, Gasco M (2013). The transillumination technique as a method for the assessment of spermatogenesis using medicinal plants: the effect of extracts of black maca (Lepidium meyenii) and camucamu (Myrciaria dubia) on stages of the spermatogenic cycle in male rats. Toxicol. Mechanisms Methods 23:559-565.
Crossref
|
|
|
|
|
Grigio ML, Chagas EC, Durigan MFB, Sousa AA, Mota-Filho AB, Chagas PC (2016). Determination of harvest time and quality of native camu-camu fruits (Myrciaria dubia (Kunth) Mc Vaugh) during storage. Fruits 71:373-378.
Crossref
|
|
|
|
|
Grigio ML, Durigan MFB, Chagas EA, Chagas PC, Nascimento CR, Almeida MS (2015). Post-harvest conservation of camu–camu fruits (Myrciaria dubia (Kunth) Mc Vaugh) using different temperatures and packages. Food Sci. Technol. 35: 652-658.
Crossref
|
|
|
|
|
Harrison FH, May JM (2009). Vitamin C function in the brain: Vital role of the ascorbate transporter (SVCT2). Free Radical Biol. Med. 46:719-730.
Crossref
|
|
|
|
|
Imán-Correa S, Zamudio LB, Sotero-Solís V, Cruz CO (2011). Contenido de vitamina C en frutos de camu camu Myrciaria dubia (H.B.K) Mc Vaugh, en cuatro estados de maduración, procedentes de la Colección de Germoplasma del INIA Loreto, Perú, Scientia Agropecuaria 2:123-130.
Crossref
|
|
|
|
|
Instituto Adolfo Lutz (2008). Métodos físico-químicos para análise de alimentos. São Paulo: Instituto Adolfo Lutz, 2008. 1020 p
|
|
|
|
|
Langley P, Pergolizzi J, Taylor R, Ridgway C (2015). Antioxidant and associated capacities of camu-camu (Myrciaria dubia): A systematic review. Journal of Alternative and Complementary Medicine 21: 8-14.
Crossref
|
|
|
|
|
Lee DH, Francis FJ (1972). Standardization of pigment analyses in Cranberries. Hort. Sci. 7:83-84.
|
|
|
|
|
Liu TZ, Chin N, Kiser MD, Bigler WN (1982). Specific spectrophotometry of ascorbic acid in serum or plasma by use of ascorbate oxidase. Clin. Chem. 28:2225-2228.
|
|
|
|
|
Maeda RN, Pantoja L, Yuyama LKO, Chaar JM (2006). Determinação da formulação e caracterização do néctar de camu-camu (Myrciaria dúbia McVaugh). Ciência e Tecnologia de Alimentos 26:70-74.
Crossref
|
|
|
|
|
Maeda RN, Pantoja L, Yuyama LKO, Chaar JM (2007). Estabilidade de ácido ascórbico e antocianinas em néctar de camu-camu (Myrciaria dubia (H. B. K.) McVaugh). Ciência e Tecnologia de Alimentos 27:313-316.
Crossref
|
|
|
|
|
Neves LC, Silva VX, Pontis JA, Flach A, Roberto SR (2015). Bioactive compounds and antioxidant activity in pre-harvest camu-camu [Myrciaria dubia (H.B.K.) Mc Vaugh] fruits. Sci. Horticulturae 186:223-229.
Crossref
|
|
|
|
|
Pinto PM, Jacomino AP, Silva SR, Andrade CAW (2013). Ponto de colheita e maturação de frutos de camu‑camu colhidos em diferentes estádios. Pesquisa Agropecuária Brasileira, 48:605-612.
Crossref
|
|
|
|
|
Ranganna S (1986). Analysis and quality control for fruit and vegetable products. Tata McGraw-Hill Publishing, 1112 p.
|
|
|
|
|
Rufino MSM, Alves RE, Brito ES, Jiménez JP, Calixto FS, Filho JM (2010). Bioactive compounds and oxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 121:996-1002.
Crossref
|
|
|
|
|
Rufino MSM, Alves RE, Brito ES, Silveira MRS, Moura CFH (2009). Quality for fresh consumption and processing of some non-traditional tropical fruits from Brazil. Fruits 64:361-370.
Crossref
|
|
|
|
|
Rufino MSM, Alves RE, Brito ES, Morais SM, Sampaio CG, Jiménez JP, Saura-Calixto FD (2006). Metodologia Científica: Determinação da atividade antioxidante total em frutas pelo método de redução do ferro (FRAP). Comunicado Técnico, Embrapa Agroindústria Tropical. 4 p.
|
|
|
|
|
Santos JC, Santos AP, Rocha CIL (2009).Estrutura da cadeia produtiva de camu-camu no Brasil. Relatório Final de projeto. Belém: CPATU:. 35 p.
|
|
|
|
|
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu Reagent. Methods Enzymol. 299:152-178.
Crossref
|
|
|
|
|
Solis VS, Doza LS, Sotero DG, Correa SI (2009). Evaluacion de la actividad antioxidante de la pulpa, cascara y semilla del fruto del camu camu (Myrciaria dubia H.B.K.). Revista Sociedad Química Perú. 75:293-299.
|
|
|
|
|
Sousa RCP, Chagas EA, Guimaraes PVP, Nascimento Filho WB, Melo Filho AA (2015). Sais minerais em extrato aquoso de coprodutos de Myrciaria dubia (Kunth.) McVaugh, Myrtaceae. Revista Virtual de Quimica. 7:1299-1305.
Crossref
|
|
|
|
|
Villanueva-Tiburcio JE, Condezo-Hoyos LA, Asquieri ER (2010). Antocianinas, ácido ascórbico, polifenolestotales y actividad antioxidante, em lacáscara de camu-camu (Myrciaria dubia (H.B.K) McVaugh). Ciência e Tecnologia de Alimentos 30:151-160.
Crossref
|
|
|
|
|
Zamudio LHB (2007). Caracterização de vitamina C em frutos de camu-camu (Myrciaria dúbia (H.B.K.)) em diferentes estágios de maturação do banco ativo de germoplasma de Embrapa. 121f. Monografia (Especialização em Nutrição Humana). Brasília: Faculdade de Ciências da Saúde, Universidade de Brasília. 2007.
|
|
|
|
|
Zhishen J, Mengcheng T, Jianming W (1999). The determination of flavonoid contents in mulberry and their scavenging effects on Superoxide radicals, Food Chem. 64:555-559.
Crossref
|
|

